BCMA-Directed CAR T-Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma and Renal Impairment
Simple Summary
Abstract
1. Introduction
2. Methods
Study Population
3. Results
3.1. Patient and Disease Characteristics
3.2. Post-BCMA CAR-T Toxicities and Adverse Events
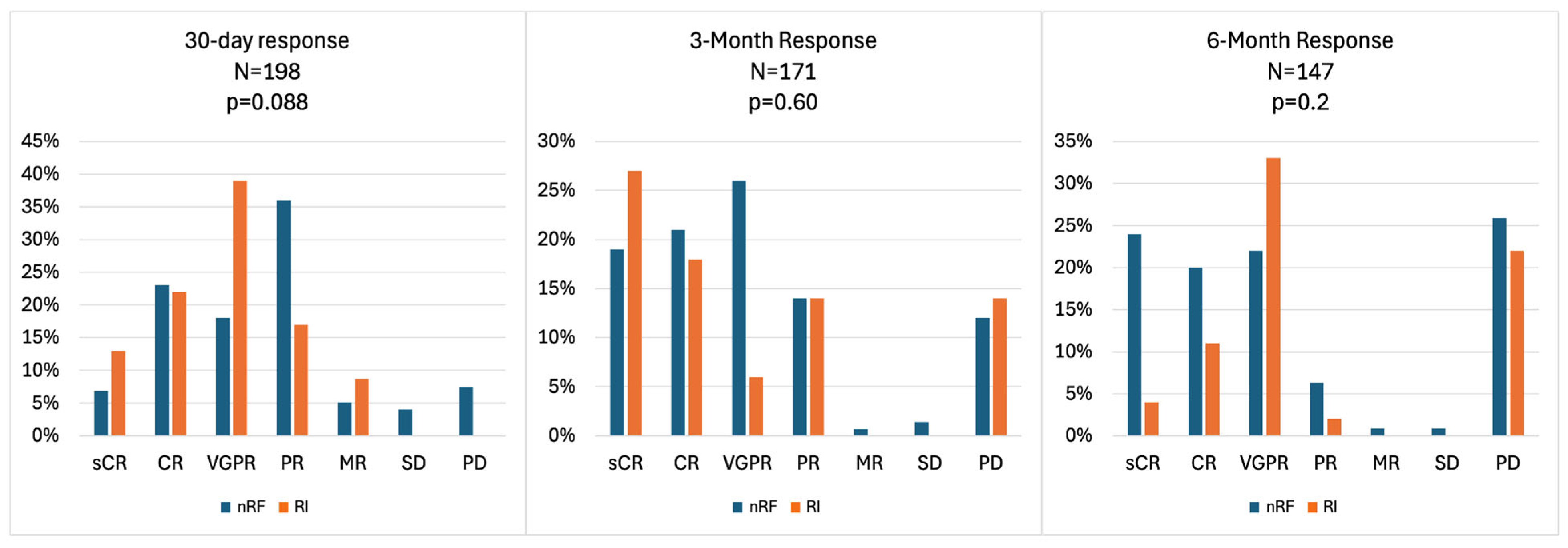
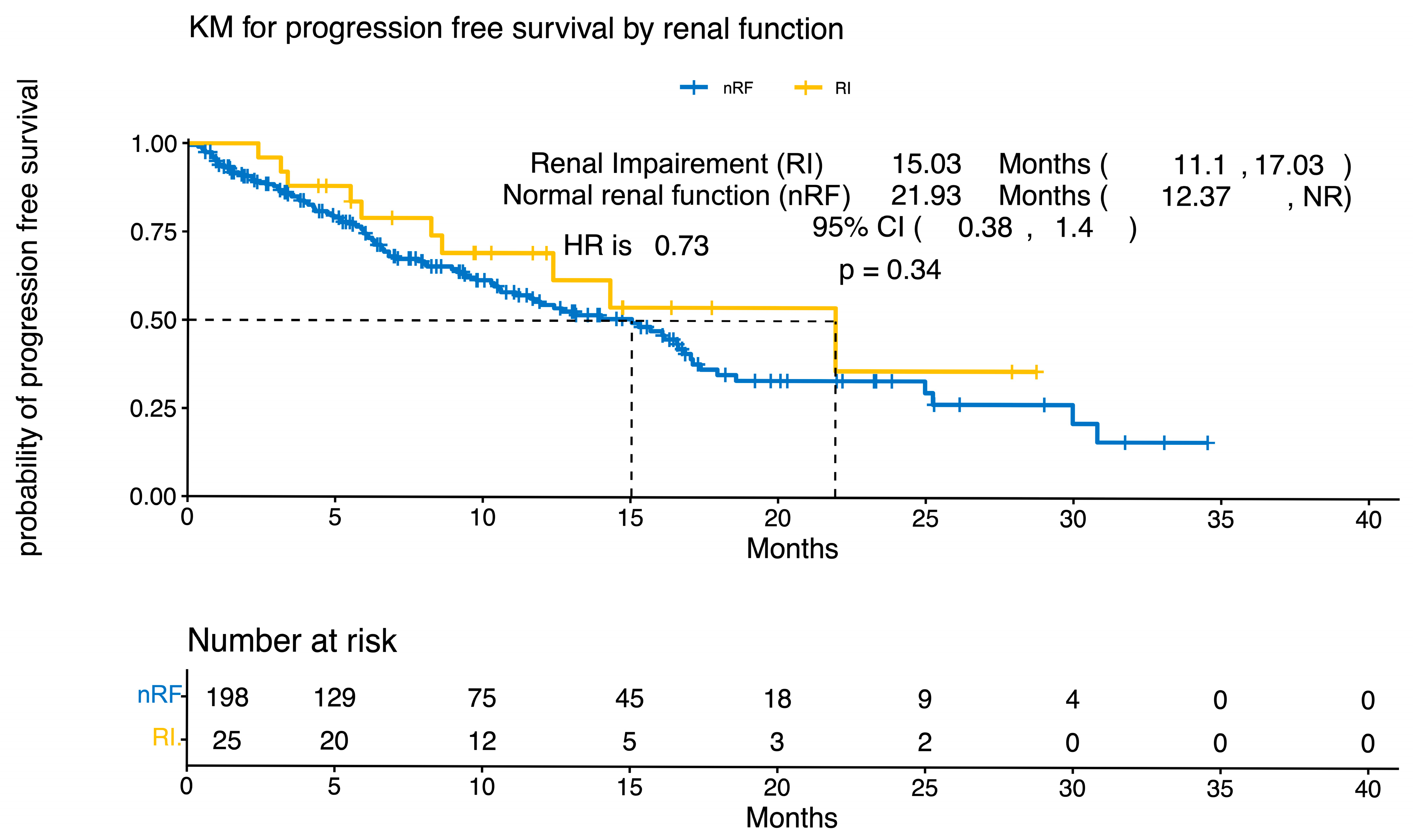
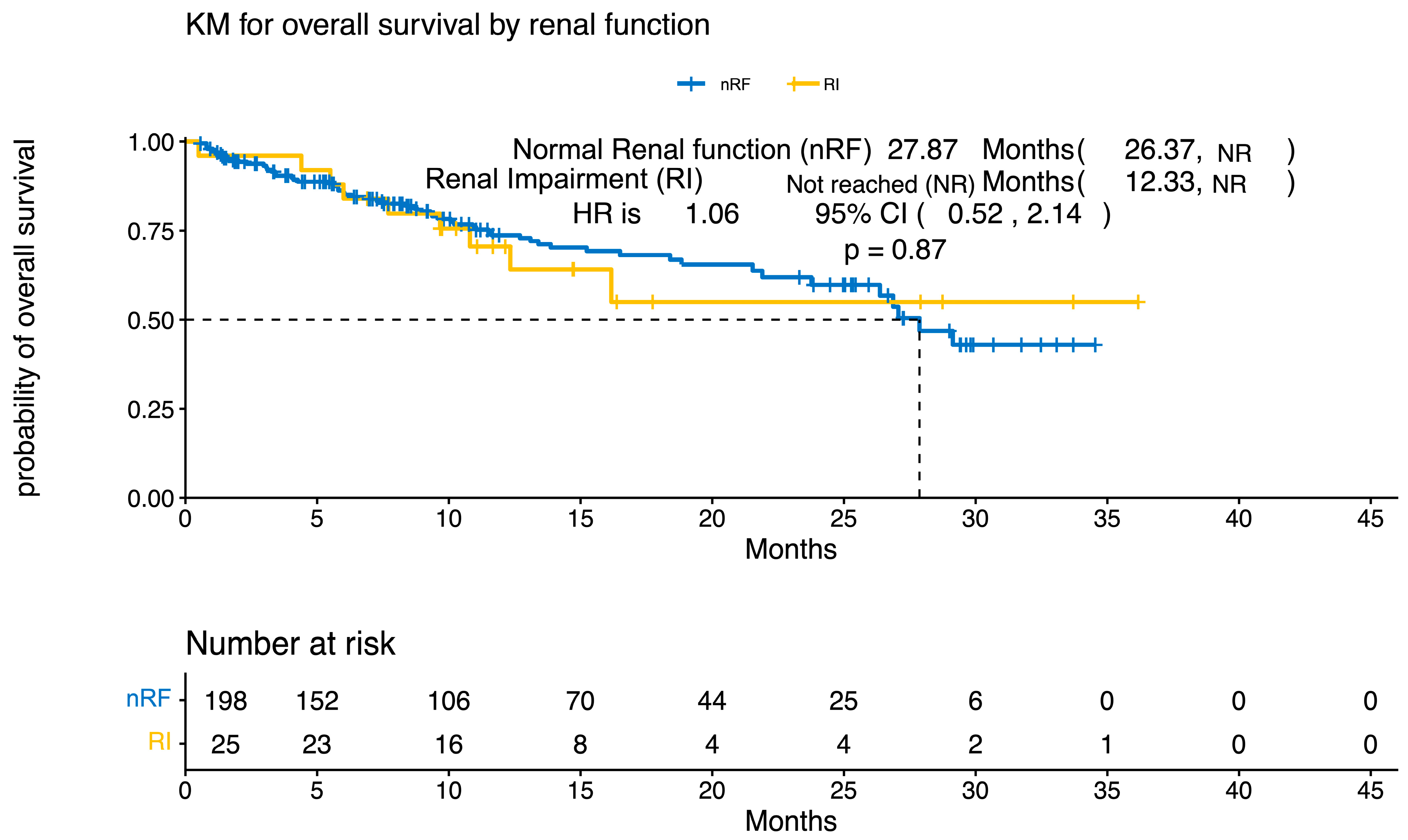
3.3. Response Rates and Survival
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyle, R.A.; Rajkumar, S.V. Multiple Myeloma. N. Engl. J. Med. 2004, 351, 1860–1873. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Dimopoulos, M.A.; Terpos, E.; Bernhard, S.; Theodorakakou, F.; Beksac, M.; Cengiz-Seval, G.; Leung, N.; Arcaini, L.; Mangiacavalli, S.; et al. Parameters Associated With Renal Recovery and Survival in Myeloma Patients With Acute Renal Failure to Cast Nephropathy. Am. J. Hematol. 2026, 101, 56–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hutchison, C.A.; Cockwell, P.; Stringer, S.; Bradwell, A.; Cook, M.; Gertz, M.A.; Dispenzieri, A.; Winters, J.L.; Kumar, S.; Rajkumar, S.V.; et al. Early reduction of serum-free light chains associates with renal recovery in myeloma kidney. J. Am. Soc. Nephrol. 2011, 22, 1129–1136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, Y.-T.; Anderson, K.C. B cell maturation antigen (BCMA)-based immunotherapy for multiple myeloma. Expert Opin. Biol. Ther. 2019, 19, 1143–1156. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegal, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients With Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; Ailawadhi, S.; Arnulf, B.; Patel, K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.; Costa, L.J.; Vij, R.; et al. Ide-cel or Standard Regimens in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 388, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.; Dhakal, B.; Yong, K.; Spencer, A.; Anguille, S.; Mateos, M.-V.; de Larrea, C.F.; Martinez-Lopez, J.; Moreau, P.; Touzeau, C.; et al. Ciltacabtagene Autoleucel versus Standard Care in Lenalidomide-Refractory Multiple Myeloma. N. Engl. J. Med. 2023, 389, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Otero, P.; San Miguel, J.F. Post-CAR-T Cell Therapy (Consolidation and Relapse): Multiple Myeloma. In The EBMT/EHA CAR-T Cell Handbook; Springer: Cham, Switzerland, 2022; pp. 173–176. [Google Scholar] [CrossRef]
- Li, C.; Cao, W.; Que, Y.; Wang, Q.; Xiao, Y.; Gu, C.; Wang, D.; Wang, J.; Jiang, L.; Xu, H.; et al. A phase I study of anti-BCMA CAR T cell therapy in relapsed/refractory multiple myeloma and plasma cell leukemia. Clin. Transl. Med. 2021, 11, e346. [Google Scholar] [CrossRef] [PubMed]
- Ailawadhi, S.; Arnulf, B.; Patel, K.K.; Cavo, M.; Nooka, A.K.; Manier, S.; Callander, N.S.; Costa, L.J.; Vij, R.; Bahlis, N.J.; et al. Ide-cel vs standard regimens in triple-class-exposed relapsed and refractory multiple myeloma: Updated KarMMa-3 analyses. Blood 2024, 144, 2389–2401. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Yong, K.; Harrison, S.J.; Mateos, M.-V.; Moreau, P.; van de Donk, N.W.C.J.; Sidana, S.; Popat, R.; Lendvai, N.; Lonardi, C.; et al. First phase 3 results from CARTITUDE-4: Cilta-cel versus standard of care (PVd or DPd) in lenalidomide-refractory multiple myeloma. J. Clin. Oncol. 2023, 41, LBA106. [Google Scholar] [CrossRef]
- Russo, E.; Gambella, M.; Raiola, A.M.; Beltrametti, E.; Zanetti, V.; Chirco, G.; Viazzi, F.; Angelucci, E.; Esposito, P. Acute kidney injury in hematological patients treated with CAR-T cells: Risk factors, clinical presentation and impact on outcomes. Dent. Sci. Rep. 2024, 14, 26886. [Google Scholar] [CrossRef] [PubMed]
- Chishinga, N.; Mapalo, M.T.; Bondine, C.D., Jr. Risk stratification for immune effector cell-associated neurotoxicity syndrome in patients receiving chimeric antigen receptor T cell therapy. J. Clin. Oncol. 2024, 42, e14534. [Google Scholar] [CrossRef]
- Abid, M.B.; Rubin, M.; Szabó, A.; Fenske, T.S.; Abedin, S.; D’Souza, A.; Dhakal, B.; Shah, N.N.; Hamadani, M. Infectious Complications in Patients with Hematologic Malignancies Receiving CD19 Vs. BCMA Targeted CAR-T Therapy. Transplant. Cell. Ther. 2024, 30, S210–S211. [Google Scholar] [CrossRef]
- Garner, W.; Samanta, P.; Dorritie, K.A.; Sehgal, A.R.; Winfield, D.; Agha, M.; Boudreau, R.M.; Nguyen, M.H.; Haidar, G. 1105. The Burden of Infections Prior to Chimeric Antigen Receptor (CAR) Modified T-cell Therapy Predicts Post-CAR T-cell Infectious Complications. Open Forum Infect. Dis. 2020, 7, S583. [Google Scholar] [CrossRef]
- Bae, S.; Kook, M.S.; Chang, E.; Jung, J.; Kim, M.J.; Chong, Y.P.; Kim, S.; Choi, S.; Lee, S.-O.; Kim, Y.S. Risk Factors for Infection-Attributable Mortality in Patients with Staphylococcus aureus Bacteremia: A Competing Risk Analysis. Open Forum Infect. Dis. 2024, 12, ofae734. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, K.; Wang, Y.; Albanyan, O.; Munoz, J.; Sesques, P.; Iacoboni, G.; López-Corral, L.; Ries, I.; Bücklein, V.; Mohty, R.; et al. The CAR-HEMATOTOX score identifies patients at high risk for hematological toxicity, infectious complications, and poor treatment outcomes following brexucabtagene autoleucel for relapsed or refractory MCL. Am. J. Hematol. 2023, 98, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.; Mehdi, A. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J. Immunother. Cancer 2022, 10, e004475. [Google Scholar] [CrossRef]
- Davis, J.A.; Sborov, D.W.; Wesson, W.; Julian, K.; Abdallah, A.O.; McGuirk, J.P.; Ahmed, N.; Hashmi, H. Efficacy and Safety of CD34+ Stem Cell Boost for Delayed Hematopoietic Recovery After BCMA Directed CAR T-cell Therapy. Transplant. Cell. Ther. 2023, 29, 567–571. [Google Scholar] [CrossRef] [PubMed]
| Patient and Disease Characteristics | Normal Renal Function (n = 198) | Baseline Renal Impairment (CrCl < 45 mL/min; n = 25) | p-Value |
|---|---|---|---|
| Sex, male/female | 117:81 | 8:17 | |
| Age, years, median (range) | 65 (34–84) | 67 (50–80) | 0.2 |
| Race, no. of patients (%) | 0.043 | ||
| Caucasian | 162 (82%) | 15 (60%) | |
| African American | 30 (15%) | 9 (36%) | |
| Asian | 2 (1%) | 0 | |
| Hispanic | 1 (0.5%) | 1 (4%) | |
| Other | 3 (1.5%) | 0 | |
| ECOG | 0.8 | ||
| PS: 0–1 | 162 (81.5%) | 22 (88%) | |
| PS: 2 | 16 (8%) | 3 (12%) | |
| PS: 3 | 1 (0.5%) | 0 | |
| Unknown | 19 (10%) | 0 | |
| MM paraprotein, no. of patients (%) | |||
| IgG | 122 (61%) | 11 (44%) | 0.3 |
| Non-IgG | 35 (18%) | 7 (28%) | |
| Light chain | 41 (21%) | 7 (28%) | |
| Baseline ISS stage, no. of patients (%) | 0.03 | ||
| Stage III | 39 (20%) | 1 (4%) | |
| Stage II | 74 (37%) | 8 (32%) | |
| Stage I | 38 (19%) | 10 (40%) | |
| Unknown | 47 (24%) | 6 (24%) | |
| Cytogenetics, no. of patients (%) | |||
| High-risk disease | |||
| Deletion 17 p | 41 (21%) | 4 (16%) | 0.6 |
| t(4;14) | 18 (9%) | 4 (16%) | 0.3 |
| t(14;16) | 8 (4%) | 2 (8%) | 0.3 |
| Extramedullary disease | 75 (38%) | 8 (32%) | 0.6 |
| Median no. of previous lines of therapy | 6 (3–13) | 6 (3–11) | |
| Cilta-Cel | 69 (35%) | 4 (16%) | 0.058 |
| Ide-Cel | 129 (65%) | 21 (84%) | 0.058 |
| Prior Treatment Characteristics | Normal Renal Function (n = 198) | Baseline Renal Impairment (CrCl < 45 mL/min; n = 25) | p-Value 1 |
|---|---|---|---|
| Bortezomib exposed | 193 (97%) | 25 (100%) | >0.9 |
| Bortezomib refractory | 106 (54%) | 17 (68%) | 0.2 |
| Carfilzomib exposed | 180 (91%) | 23 (92%) | >0.9 |
| Carfilzomib refractory | 139 (70%) | 19 (76%) | 0.5 |
| Lenalidomide exposed | 196 (99%) | 25 (100%) | >0.9 |
| Lenalidomide refractory | 152 (77%) | 21 (84%) | 0.4 |
| Pomalidomide exposed | 183 (92%) | 24 (96%) | >0.9 |
| Pomalidomide refractory | 150 (76%) | 19 (76%) | 0.4 |
| Anti-CD38 monoclonal antibody exposed | 196 (99%) | 24 (96%) | 0.3 |
| Anti-CD38 monoclonal antibody refractory | 183 (92%) | 23 (84%) | >0.9 |
| Double refractory | 163(82%) | 20 (80%) | 0.8 |
| Triple exposed | 195 (98%) | 24 (96%) | 0.4 |
| Triple refractory | 154 (78%) | 20 (80%) | >0.9 |
| Penta exposed | 161 (81%) | 21 (84%) | 0.3 |
| Penta refractory | 58 (29%) | 11(44%) | 0.3 |
| Number of patients with prior ASCT | 167 (84%) | 18 (72%) | 0.2 |
| Prior BCMA-directed therapies * | 25 (13%) | 3 (12%) | >0.9 |
| Normal Renal Function (n = 198) | Baseline Renal Impairment (CrCl < 45 mL/min; n = 25) | p-Value | |||
|---|---|---|---|---|---|
| All Grades | Grade 3/4 | All Grades | Grade 3/4 | ||
| CRS | 158 (80%) | 2 (1%) | 24 (96%) | 0 | 0.055 |
| ICANS | 37 (19%) | 4 (2%) | 15 (60%) | 3 (12%) * | 0.044 |
| Parkinsonism | 1 (0.5%) | 0 | 0 | 0 | >0.9 |
| Infection | 40 (20%) | 11 (44%) | 0.008 | ||
| Neutropenia | 151 (76%) | 101 (51%) | 21 (84%) | 14 (56%) | |
| Anemia | 158 (80%) | 46 (23%) | 22 (88%) | 7 (28%) | |
| Thrombocytopenia | 146 (74%) | 95 (48%) | 21 (84%) | 12 (48%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Habib, A.; Ahmed, N.; Khan, A.M.; Chang, D.; Paul, B.; Shaikh, H.; Strouse, C.; Struble, E.; Vegel, A.; Mahmoudjafari, Z.; et al. BCMA-Directed CAR T-Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma and Renal Impairment. Curr. Oncol. 2026, 33, 80. https://doi.org/10.3390/curroncol33020080
Habib A, Ahmed N, Khan AM, Chang D, Paul B, Shaikh H, Strouse C, Struble E, Vegel A, Mahmoudjafari Z, et al. BCMA-Directed CAR T-Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma and Renal Impairment. Current Oncology. 2026; 33(2):80. https://doi.org/10.3390/curroncol33020080
Chicago/Turabian StyleHabib, Alma, Nausheen Ahmed, Abdullah Mohammad Khan, Darryl Chang, Barry Paul, Hira Shaikh, Christopher Strouse, Emily Struble, Andrew Vegel, Zahra Mahmoudjafari, and et al. 2026. "BCMA-Directed CAR T-Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma and Renal Impairment" Current Oncology 33, no. 2: 80. https://doi.org/10.3390/curroncol33020080
APA StyleHabib, A., Ahmed, N., Khan, A. M., Chang, D., Paul, B., Shaikh, H., Strouse, C., Struble, E., Vegel, A., Mahmoudjafari, Z., Mushtaq, M. U., McGuirk, J. P., Abdallah, A.-O., Atrash, S., & Friend, R. (2026). BCMA-Directed CAR T-Cell Therapy in Patients with Relapsed/Refractory Multiple Myeloma and Renal Impairment. Current Oncology, 33(2), 80. https://doi.org/10.3390/curroncol33020080






