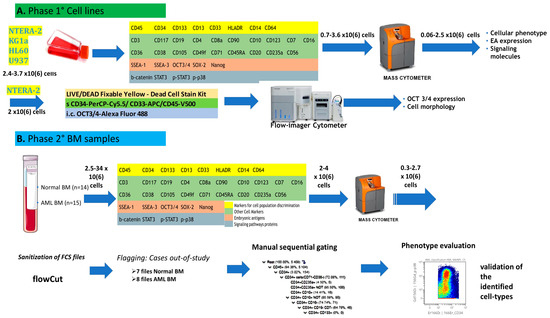
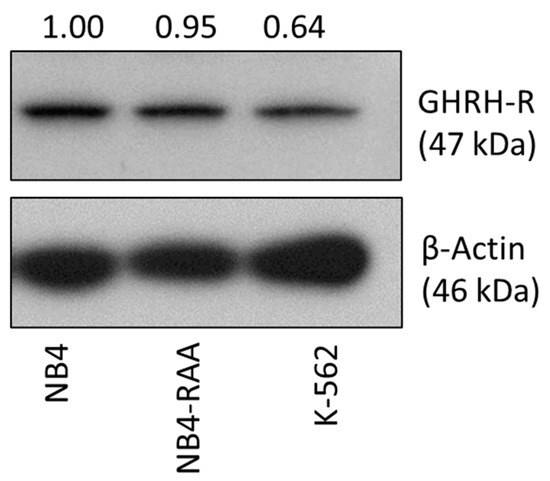
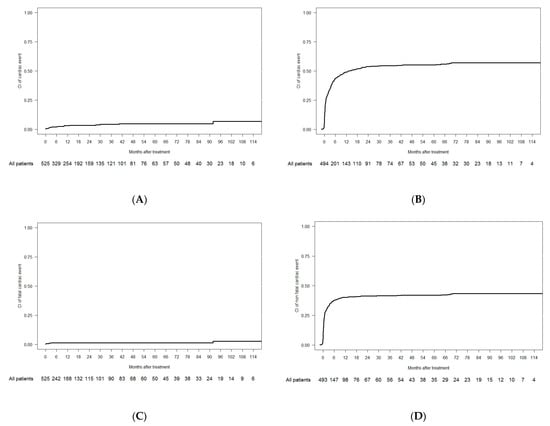
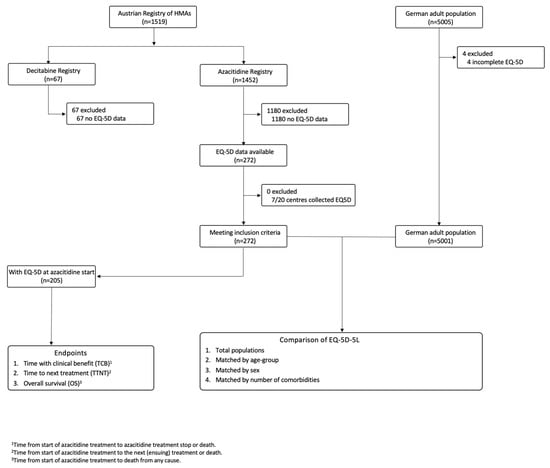
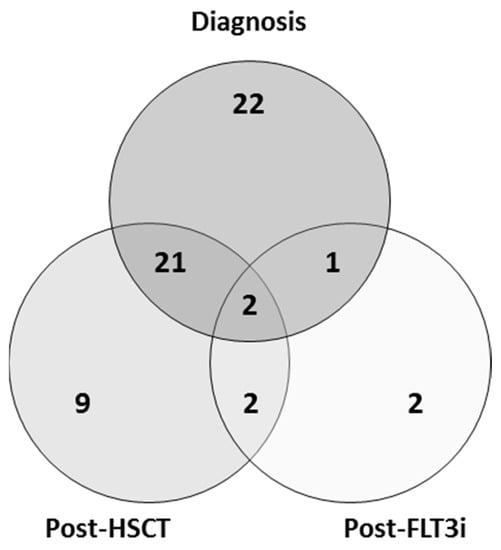
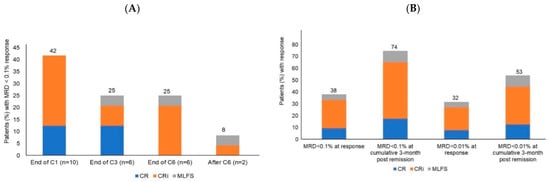
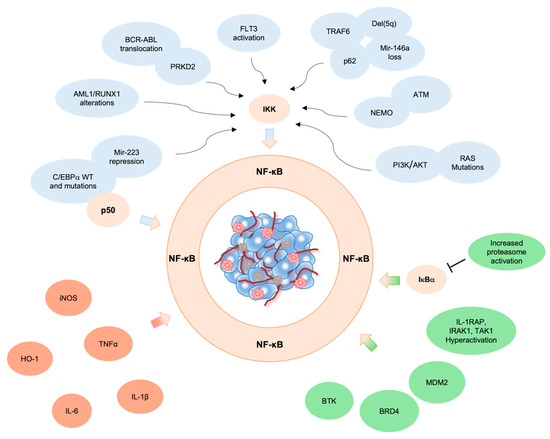
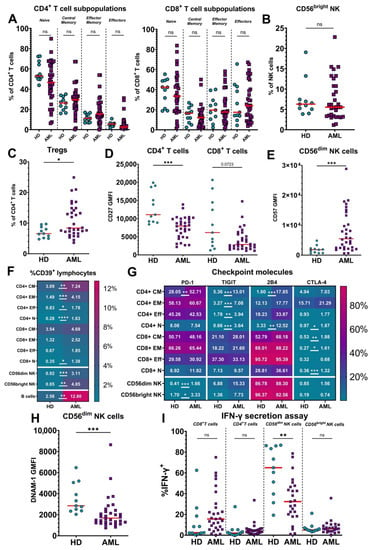
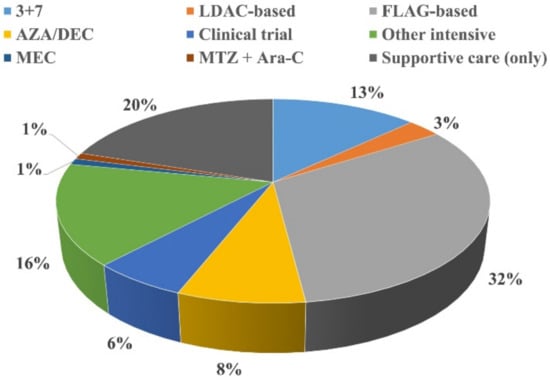
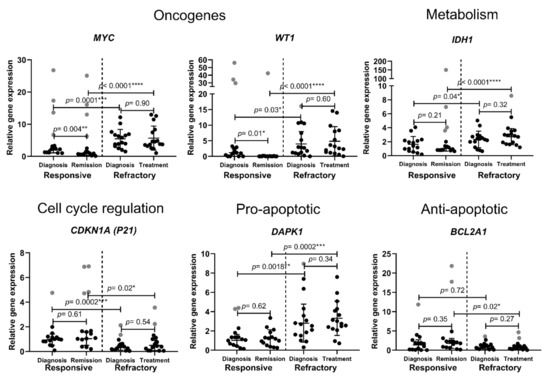
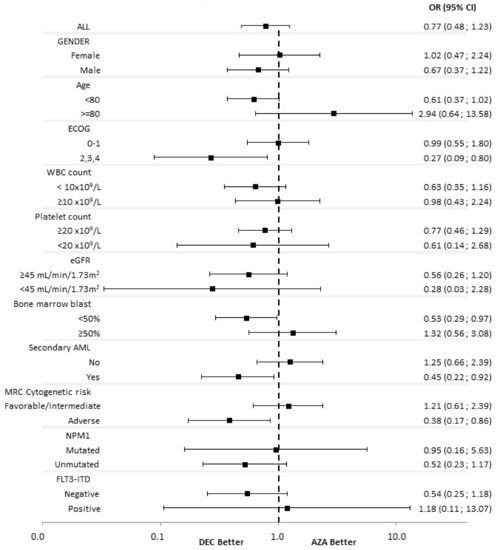
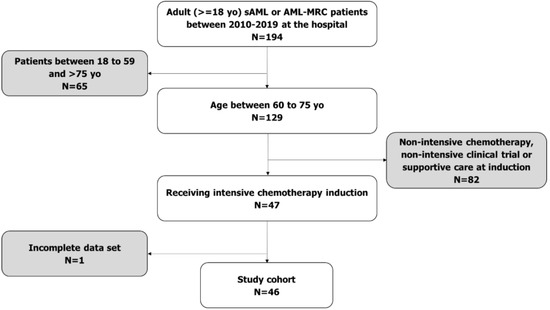
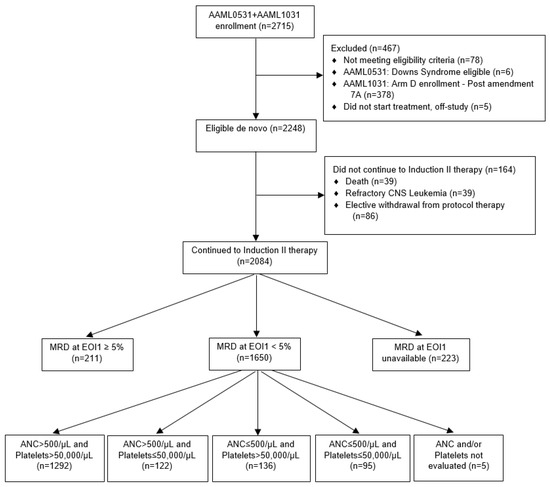
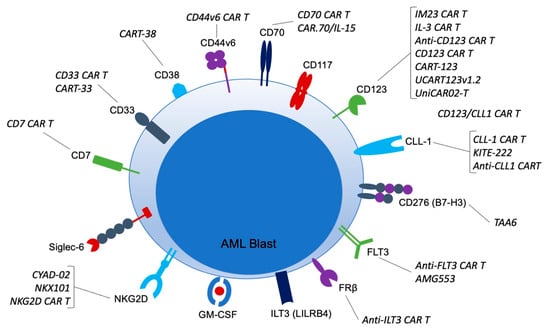
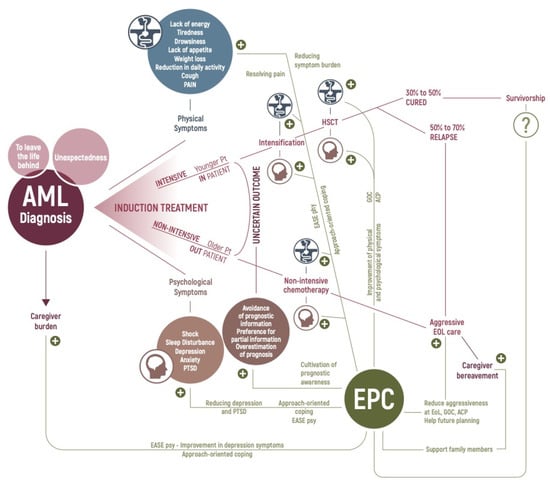
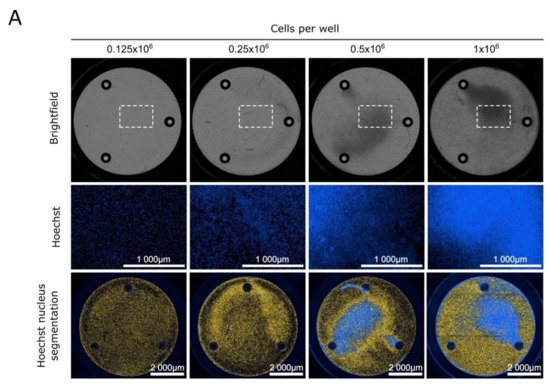
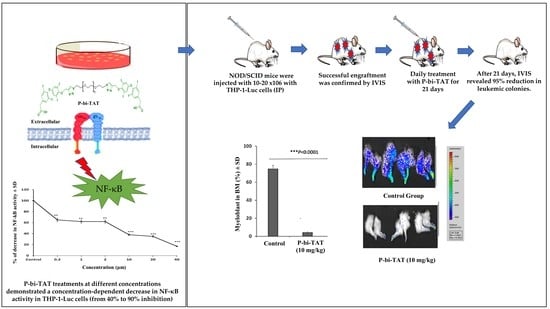
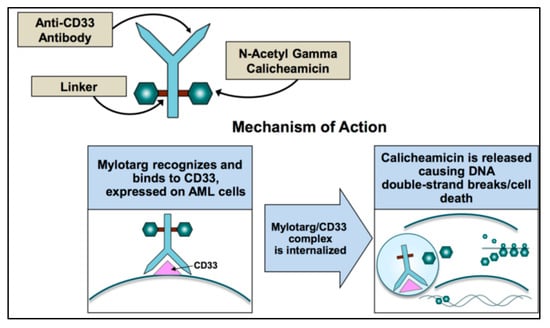
Acute Myeloid Leukemia (AML)
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Cancer Epidemiology and Prevention".
Viewed by 228853Editor
Interests: acute myeloid leukemia; cancer; hematology
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Precision medicine has opened the door to personalized therapy for specific Acute Myeloid Leukemia (AML) patient populations with promising results. Several targeted therapies have been approved or are being tested for specific mutations (i.e., FLT3, IDH, BCL-2, and TP53), obtaining improvements in clinical outcomes and less toxicity as compared with some intensive or non-intensive regimens, potentially allowing for combination therapy. Unfit patients, especially older patients, have higher disease progression and a lower tolerance than fit patients, and are treated with non-intensive schemes (i.e., low-dose cytarabine (LDAC) or hypomethylating agents (HMAs)). This subset of patients could potentially benefit from emerging therapies focused on specific poor prognosis features (FLT3, secondary AML). HMAs in combination with small molecule inhibitors (unspecific such as glasdegib or venetoclax or specific such as FLT3 or IDH inhibitors) could increase survival and quality of life in older/unfit patients, which represent the most difficult-to-treat AML population. Venetoclax is an oral selective inhibitor of broad-spectrum B-cell lymphoma 2 (BCL-2), and therefore is not considered to be a targeted therapy. The promising results of phase 1b trials exploring the combination of venetoclax plus HMAs or LDAC led to FDA approval and commercialization of this new drug for AML. The recent positive results of the phase 3 trial of azacitidine plus venetoclax could open the door to further indications of venetoclax in AML. Appropriate clinical development and use of non-approved combinations in the context of clinical trials should be recommended in order to avoid unexpected adverse events in AML patients.
This Special Issue will highlight the critical role of supportive care and appropriate management of new drugs and combinations to ensure therapeutic success in AML cases. In addition, we will critically assess epidemiological and real-life evidence of AML outcomes beyond the clinical trial setting.
Dr. Pau Montesinos
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.