Simple Summary
Cytokine release syndrome is a potentially life-threatening complication of therapy with T-cell engaging bispecific antibodies. Here we evaluated the risk, characteristics and biomarkers of treatment-emergent cytokine release syndrome in patients with relapsed/refractory acute myeloid leukemia or myelodysplastic syndrome who received weekly intravenous infusions of the CD3xCD123 bispecific antibody APVO436. Cytokine release syndrome was encountered in 10 of 46 patients (21.7%) treated with APVO436 with a cumulative Grade 3/4 cytokine release syndrome incidence of 8.7%. Cytokine profiling in patients who developed cytokine release syndrome after APVO436 infusion indicated that the predominant cytokine in this inflammatory cytokine response was IL-6. The findings from this research provide new insights regarding the biology and effective management of cytokine release syndrome in leukemia patients treated with T-cell redirecting bispecific antibodies.
Abstract
We evaluate the risk, characteristics and biomarkers of treatment-emergent cytokine release syndrome (CRS) in patients with relapsed/refractory acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) who received APVO436 during the dose-escalation phase of a Phase 1B study (ClinicalTrials.gov, identifier: NCT03647800). Of four patients who developed Grade ≥ 3 CRS, two received steroid prophylaxis. The dose level, gender, race, obesity, or baseline hematologic parameters in peripheral blood did not predict the risk of CRS. Patients with a higher leukemia burden as determined by a higher total WBC, higher percentage of blasts in bone marrow, or higher percentage of blasts in peripheral blood (by hematopathology or immunophenotyping) did not have a higher incidence of CRS. There was an age difference between patients who did versus patients who did not develop CRS (72.9 ± 1.6 years (Median 73.5 years) vs. 63.3 ± 2.3 years (Median: 65.0 years), which was borderline significant (p = 0.04). Premedication with steroids did not eliminate the risk of CRS. Cytokine profiling in patients who developed CRS after APVO436 infusion indicates that the predominant cytokine in this inflammatory cytokine response was IL-6. APVO436-associated CRS was generally manageable with tocilizumab with or without dexamethasone. Notably, the development of CRS after APVO436 therapy did not appear to be associated with a response. The prolonged stabilization of disease, partial remissions and complete remissions were achieved in both patients who experienced CRS, as well as patients who did not experience CRS after APVO436 infusions.
1. Introduction
An urgent unmet medical need in acute myelogenous leukemia (AML), the most common form of adult acute leukemia, is to salvage relapsed or refractory R//R patients who have a dismal prognosis with <10% surviving five years [1,2,3,4,5,6,7,8,9,10]. Biotherapeutic agents, including CD3-engaging bispecific antibodies (BiAb), may provide the foundation for new and effective treatments against R/R AML [9,10]. CD3-engaging BiAb recruit cytotoxic T-cells (CTL) to the close vicinity of AML cells to create “cytolytic synapses” which triggers a CTL-mediated destruction of AML cells [11,12,13,14,15,16]. AML-directed CD3-engaging BiAb act as agonists and activate T-cells in the presence of tumor cells expressing the target tumor-associated antigen, which can lead to an excessive T-cell activation with the release of inflammatory cytokines and the development of potentially life-threatening systemic inflammation, known as cytokine release syndrome (CRS) [17,18,19,20,21].
The α-chain of the interleukin-3 (IL-3) receptor, also known as the CD123 antigen, is broadly expressed on AML cells [22,23,24,25]. APVO436 is a recombinant CD3-engaging BiAb designed to redirect CTLs in a major histocompatibility complex (MHC)-independent manner to CD123-expressing AML cells [26,27]. Dissimilar to previously described bispecific antibody fragments, APVO436 with its ADAPTIR format binds bivalently to both CD123 and CD3, yet does not cross-link and activate T-cells without a target present [26,27]. APVO436 also incorporates a modified antibody Fc region that improves serum stability, but does not cross-link T-cells or target cells through Fc gamma receptors such as CD16 or CD64 [26]. Preclinical data comparing APVO436 with a CD3xCD123 dual-affinity re-targeting (DART) molecule, MGD006, evaluating T-cell activation, proliferation, cytotoxicity and cytokine secretion, showed that APVO436 and MDG006 are both effective at stimulating a tumor-directed immune response by inducing a comparable T-cell activation, proliferation and cytotoxicity [27]. However, in these preclinical studies, APVO436 induced lower levels of several T-cell cytokines, including interferon gamma (IFNγ), interleukin-2 (IL-2), interleukin-(IL-6), tumor necrosis factor alpha (TNFα) and several additional cytokines, suggesting a potential safety advantage with APVO436 [27]. This is reminiscent of the published data on the first generation ADAPTIR candidate, APVO414, showing a reduced cytokine release upon T-cell engagement compared to another bispecific format [28].
In a Phase 1B dose-finding study in relapsed/refractory AML and MDS patients (ClinicalTrials.gov identifier: NCT03647800), this CD3-engaging bispecific antibody exhibited promising single-agent activity (29). While the safety profile was overall favorable and the maximum tolerated dose (MTD) was >60 mcg/week, some patients experienced CRS as a potentially life-threatening treatment-emergent complication [29]. Within the confines of a small patient and heterogeneous patient population, the CRS rate of 21.7% in the Phase 1B study of APVO436 appeared to compare favorably with the reported CRS rates for the anti-AML bispecific antibodies: CD33xCD3 bispecific antibody AMG330 (67%—Clinicaltrial.gov; identifier: NCT#02520427), CD33xCD3 bispecific antibody AMG673 (63%—Clinicaltrial.gov; identifier: NCT03224819), CD3xCD123 bispecific, DART antibody Flotetuzumab (96%) [11] and CD3xCD123 bispecific antibody Vibecotamab (XmAb14045) (58%) [30]. If these preliminary results pertaining to the tolerability of APVO436 and low CRS rate are confirmed in an additional clinical evaluation of APVO436, APVO436 may emerge as a clinically meaningful adjunct to existing AML drugs. Nevertheless, CRS was the second most common APVO436-related AE causing an interruption of infusions, dose delays, dose reductions as well as the discontinuations of protocol therapy [29].
In order to mitigate the risk of CRS in APVO436-receiving AML/MDS patients, a better understanding of the mechanism and kinetics of CRS after APVO436 administration, its predictive clinical and laboratory biomarkers as well as the effectiveness of available CRS-treatment algorithms in preventing and/or managing APVO436-asociated CRS will be of paramount importance. Here, we extend our recently published observations regarding the tolerability of APVO436 and APVO436-associated CRS [29] and report, for the first time, the kinetics of cytokine responses in APVO436-treated patients who developed moderate–severe CRS, an effective CRS management algorithm, the clinical significance as well as risk factors for CRS.
2. Materials and Methods
2.1. Investigational Medicinal Product
APVO436 is a humanized BiAb that binds to both CD123 and CD3 [26]. It is a homodimeric antibody comprised of two sets of binding domains linked to a human immunoglobulin (Ig) G1 fragment crystallizable (Fc) domain [29]. The CD123 binding domain is a fully human single-chain variable fragment (scFv) directed against human CD123. The CD3 binding domain is a humanized scFv derived from a murine antibody that binds human CD3. APVO436 drug substance (DS) was produced in accordance with current Good Manufacturing Practices (GMP) by the contract manufacturer KBI Biopharma, Inc (Durham, NC, USA) using a master cell bank transfected with an expression plasmid encoding APVO436.
2.2. Study Design and Patients
The clinical trial was registered in the clinical trial database ClinicalTrials.gov with the identifier number NCT03647800. In total, 58 patients were screened and 46 R/R AML/MDS patients who met the study eligibility criteria were enrolled. The details of the study design and patient eligibility criteria were recently reported [29].
2.3. Study Conduct
The open-label Phase 1 study was performed at the following 10 centers in the US as an open-label study sponsored by Aptevo Therapeutics [29]. The starting dose in Cohort 1 was 0.3 mcg (~0005 mcg/kg for a 60 kg patient), which was the Minimum Anticipated Biological Effect Level (MABEL) [31]. The assigned weekly target dose levels for Cohorts 2–10 ranged from 1 mcg to 60 mcg according to a 3 + 3 dose escalation scheme [29].
2.4. Ethics Statement and Study Approval
The study protocol was approved by the WCG-Central Institutional Review Board (IRB) (OHRP/FDA registration number: IRB00000533) and the local IRB at participating centers [29]. The Central IRB-approved study/protocol number was 20181730. The study was performed in compliance with the International Conference on Harmonization (ICH) guidelines for Good Clinical Practice (ICHE6/GCP). Each patient provided a written informed consent (ICF) prior to enrollment.
2.5. Grading and Management of Cytokine Release Syndrome (CRS)
CRS was defined and graded and managed, as detailed in Tables S1 and S2, respectively [21]. We also applied the 2019 American Society for Transplantation and Cellular Therapy (ASTCT) Consensus Grading criteria [32].
2.6. Measurement of Serum Cytokine Levels and Flow Cytometry
The MESO Scale Discovery (MSD) U-PLEX assay platform and an MSD MESO QuickPlex SQ 120 Reader Instrument (MESO Scale Diagnostics, Rockville, MD, USA) were used in a Central Laboratory setup for measurement of serum levels of the proinflammatory cytokines interleukin-5 (IL-5), IL-6, interleukin-10 (IL-10), interleukin-17A (IL-17A), IFN-γ, monocyte chemoattractant protein 1 (MCP-1) and TNF-α by electrochemiluminescence in duplicate serum samples from 3 patients with CRS (1 with Grade 2 CRS, 1 with Grade 3 CRS and 1 with Grade 4 CRS) [29]. In one additional case with Grade 3 CRS, serum IL-6 levels were determined by the local laboratory. The longitudinal changes in serum cytokine levels were evaluated in patients with CRS by comparing the mean concentrations for each time point to baseline concentrations. Immunophenotyping was performed on cryopreserved peripheral blood mononuclear cells from patients by standard flow cytometry using a BD LSR II flow cytometer (BD Biosciences, San Jose, CA, USA) and FACSDiva Software Version 8.0.2 fluorochrome-labeled monoclonal antibodies reactive with CD5 (anti-human CD5, clone REA782 (PE-Vio770), CD45 (anti-human CD45, Clone H130, V500, BD Biosciences #560777), CD34 (anti-human CD34, Clone REA1164, Vio Bright 515, Miltenyi Biotec #130-120-517, Auburn, CA, USA), CD38 (anti-human CD38, clone HIT-2, BV605, Biolegend #303532, San Diego, CA, USA), and CD123 (anti-human CD123, Clone 9F5, AF647, BD Biosciences #563599) antigens.
2.7. Statistical Analyses
Standard statistical methods were applied for the analysis of the clinical data. Survival data were analyzed by the Kaplan–Meier method using the GraphPad Prism 9 statistical program (GraphPad Software, LLC, San Diego, CA, USA). Log-rank statistics were used to compare the differences between patient subgroups.
3. Results
3.1. Cytokine Release Syndrome and Its Predictors
APVO436 exhibited an overall favorable safety profile with an acceptable tolerability and manageable treatment-emergent adverse events (AEs) in 46 patients with relapsed/refractory AML/MDS who were treated on the Phase 1B study 5001 [29]. The MTD was not reached at a weekly dose of 60 mcg which was tolerated by all four patients enrolled without any dose-limiting toxicities (DLTs) or Grade 3–4 treatment-emergent AEs. The single dose recommended Phase 2 dose (RP2D) level has been identified as an 18 mcg flat dose (Cohort 6; ~0.2 mcg/kg based on the body weights of the patients enrolled), which was 30% of the Cohort 10 dose level [29].
Grade 3–4 CRS was the 6th most common Grade ≥3 AE, following febrile neutropenia, anemia, hyperglycemia, decreased platelet count and sepsis by the Medical Dictionary for Regulatory Activities (MedDRA) preferred term (PT) occurring in patients treated with APVO436 in Study 5001 regardless of any relationship with the study drug APVO436, and it was encountered in four patients (8.7%) [29]. CRS was reported as a serious adverse event (SAE) in 7 (70%) of the 10 patients who developed CRS (Table 1). Table S3 shows the listing of all AE leading to dose modifications of APCO436. CRS led to dose interruptions in four patients, a dose reduction in one patient and permanent discontinuation of the study drug in one patient (Tables S4 and S5). Only 2 of the 46 patients experienced DLT and it was related to CRS in both patients (Tables S4 and S5).

Table 1.
Summary tabulation of CRS data (all grades) from study 5001 dose-escalation phase using 2019 ASTCT criteria.
Premedication with steroids (dexamethasone) did not eliminate the risk of CRS. Of four patients who developed Grade ≥3 CRS, two had received steroid prophylaxis (Table 1). Notably, CRS did not show an apparent dose relationship. The average dose levels were 0.28 ± 0.21 (median: 0.19) µg/kg for those patients who developed CRS and 0.28 ± 0.27 (Median: 0.20) µg/kg for those who did not develop CRS (p = 0.97). A total of 6 of 18 patients treated in cohorts 4–6 and 3 of 17 patients treated in cohorts 7–10 developed CRS (Table 1). In total, 2 of 18 patients from cohorts 4 to 6 and 1 of 17 patients from cohorts 7 to 10 developed ≥Grade 3 CRS (Table 1). There was a borderline significant age difference between patients who did versus patients who did not develop CRS (72.9 ± 1.6 years (Median 73.5 years) vs. 63.5 ± 2.3 years (Median: 65.0 years) (p = 0.04). APVO436 dose, gender or race did not affect the incidence of CRS. Importantly, the percentage of T-cells in peripheral blood did not predict CRS (Table 2). There was a statistically insignificant (p = 0.1) trend towards a higher absolute lymphocyte count for patients who experienced CRS. Patients with a higher leukemia burden as determined by a higher total WBC, higher percentage of blasts in bone marrow, or higher percentage of blasts in peripheral blood (by hematopathology or immunophenotyping) did not have a higher incidence of CRS (Table 2).

Table 2.
Predictors of CRS and its impact on survival.
Obesity is considered a significant contributor to inflammatory cytokine production [31,32], and it was reported as a risk factor for both CRS and neurotoxicity in patients treated with IL-2 [33]. We, therefore, sought to determine if obesity was a risk factor for APVO436-related CRS. BMI values were available for 45 of the 46 patients in the safety population. While 9 of 32 non-obese patients (28.1%) developed CRS, only 1 of 13 obese patients (7.7%) developed CRS, consistent with a trend towards a lower risk of CRS for obese patients that was not statistically significant (p = 0.14). Only one of the 10 patients who developed CRS was obese (212-0005), and he had Grade 1 CRS with a total duration of 2 days without any use of tocilizumab (Table 1). The BMI of the 10 patients who developed CRS was not higher than the average BMI of patients who did not experience CRS (Table 2). Hence, our results did not indicate that obesity is a significant contributing factor to treatment-emergent CRS in APVO436-receiving patients.
3.2. Serum Cytokine Profiles of Patients Who Developed CRS
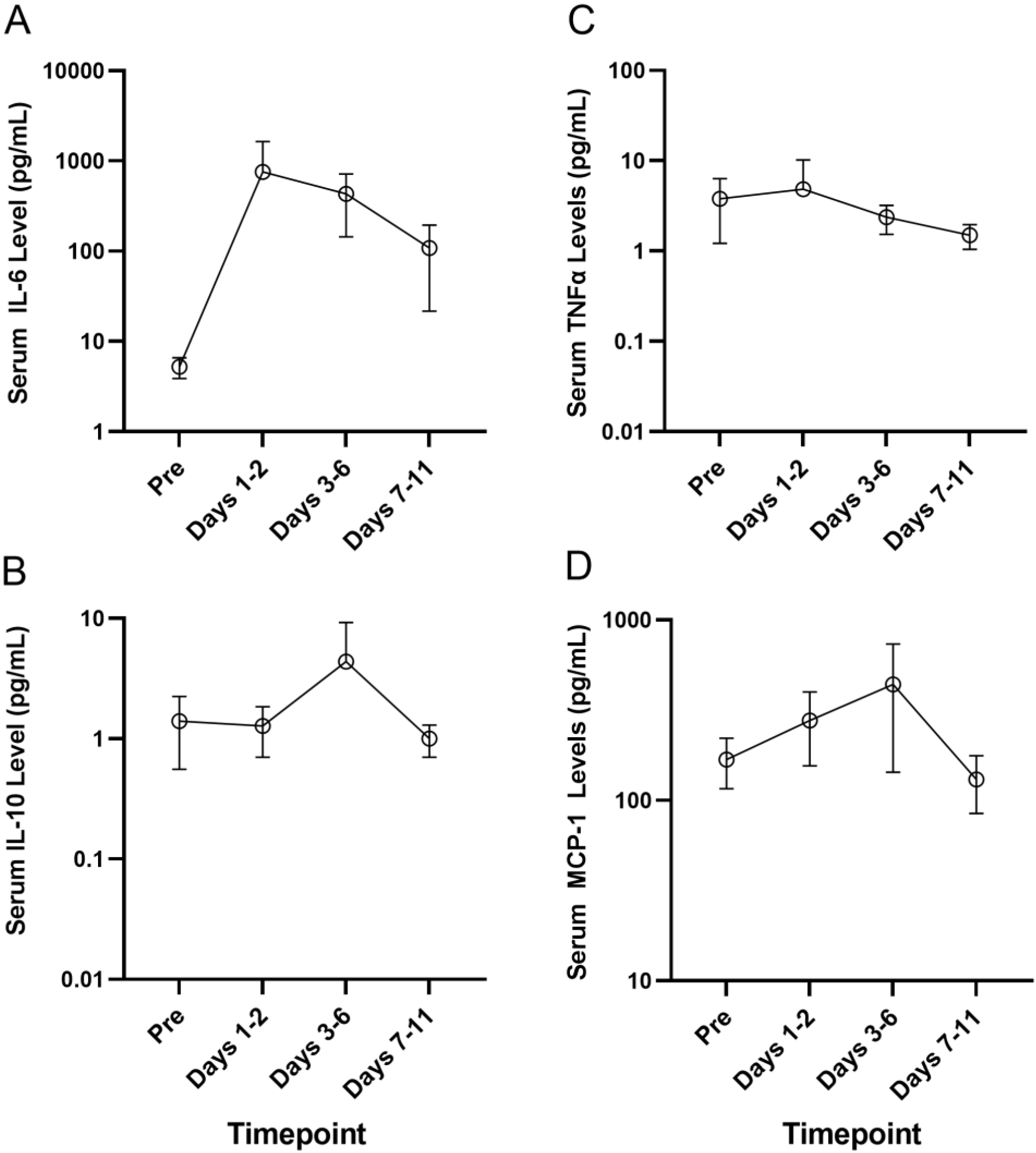
The MSD U-PLEX assay platform was used for the measurement of serum levels of the proinflammatory cytokines by electrochemiluminescence in serum samples from a select group of four primary AML patients who developed Grade 2–4 CRS, including 214-0002 in Cohort 1 who developed Grade 3 CRS, 217-0002 in Cohort 4 who developed Grade 4 CRS, 203-0004 in Cohort 6A who developed Grade 3 CRS and 214-0011 in Cohort 7 who developed Grade 2 CRS. Serum samples obtained pretreatment and at multiple timepoints after the initiation of APVO436 treatment were used to monitor the longitudinal changes in serum levels of inflammatory cytokines. There was a marked and sustained increase in serum IL-6 levels detected between days 1 and 6 post exposure to APVO436 (Table S6, Figure 1). Within 1–2 days, following the first dose of APVO436, the mean serum IL-6 concentration was elevated 145-fold over the baseline (755 vs. 5.2) and at the end of one week it was still elevated 83-fold over the baseline. By comparison, the surge in the serum levels of IL-5, IL-10, MCP-1 and TNF-α were transient with a mild–moderate increase over baseline levels (Table S6, Figure 1). Levels of IL-17A and IFN-γ did not show a significant or consistent elevation (Table S6, Figure 1). These results indicated that the APVO436-related CRS in AML patients was a largely IL-6-dominated systemic inflammatory process. Six patients received tocilizumab as part of their standard of care CRS management, three patients had dose reductions and/or dose delays, two patients had a temporary interruption of their APVO436 therapy plan and in three patients (214-0002, 203-0003, 217-0002) with Grade 2–4 CRS whose CRS was reported as an SAE, APVO436 was permanently discontinued. No changes to dose or schedule were determined in 219-0003 who developed a Grade 1 CRS that resolved within a day (Table 1).
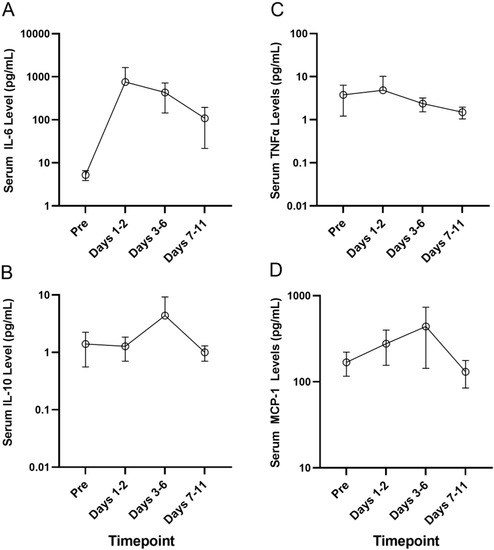
Figure 1.
Serum cytokine levels of patients who developed CRS after APVO436. The MSD U-PLEX assay platform was used for measurement of serum levels of the proinflammatory cytokines IL-5, IL-6, IL-10 and TNF-α by electrochemiluminescence in serum samples from a select group of 4 primary AML patients who experienced Grade 2–4 CRS. Serum samples obtained pretreatment and at multiple timepoints after initiation of APVO436 treatment were used to understand the longitudinal changes in serum cytokine levels. The results are also presented in Table S6. See text for detailed discussion of the results. (A) Serum IL-6 levels (B) Serum IL-10 levels (C) Serum TNFα levels (D) Serum MCP-1 levels.
3.3. Clinical Responses to APVO436 in Patients Who Developed CRS
Of the 39 R/R AML patients, 34 were evaluated for a response. Twelve patients (35.3%) had progressive disease (PD) and died of leukemia between 29 and 70 days (median: 43 days). A total of 22 of these 34 patients (64.7%) had stable disease (SD) as their best overall response [30]. In 8 of these 22 patients, SD was achieved between 31 and 75 days after study entry and lasted ~3 months or longer [29].
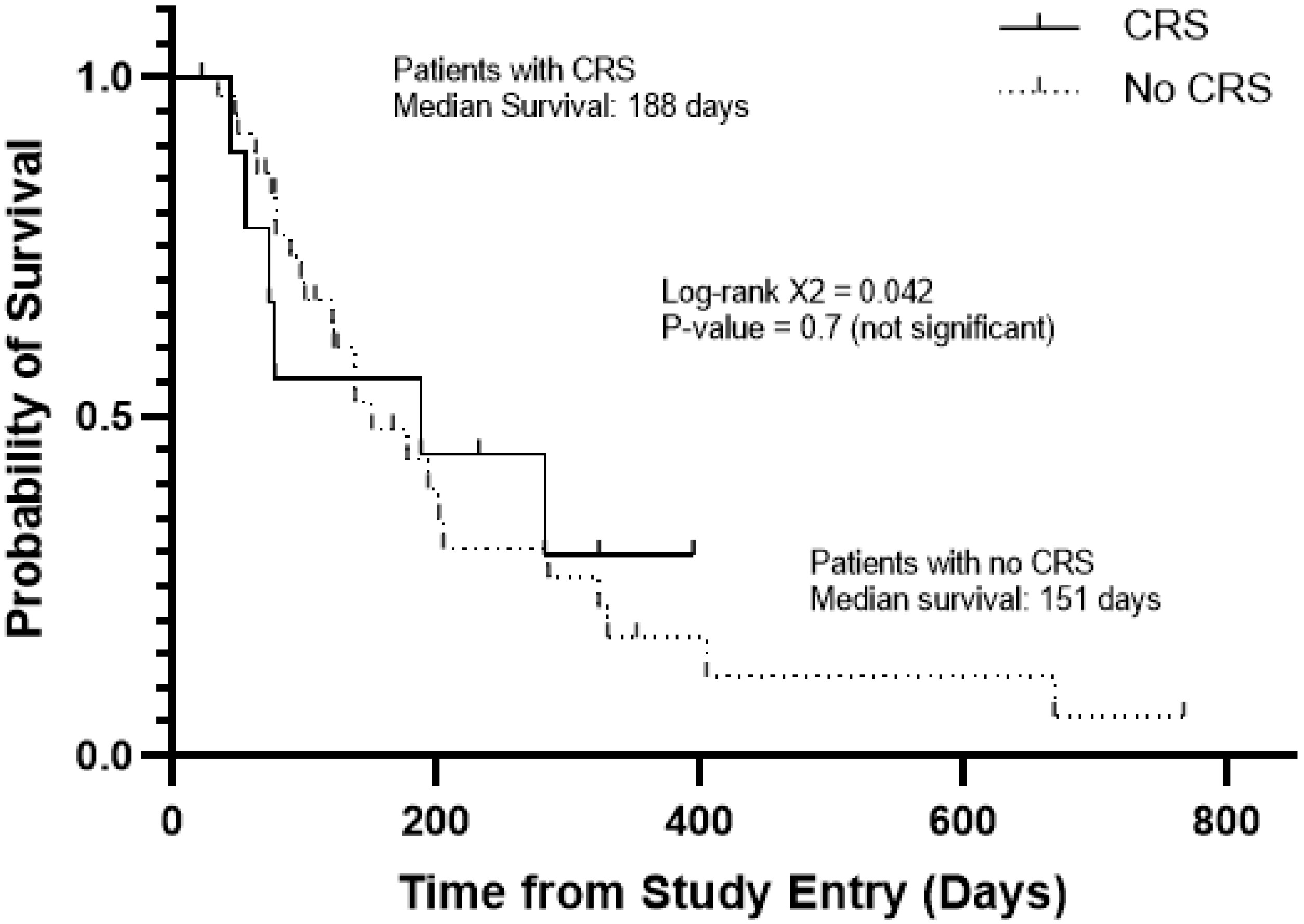
Among the patients with favorable responses, four experienced CRS and four did not. APVO436-related CRS was not required for clinically meaningful responses in R/R AML patients (Table 3), and it did not affect the survival outcome (Figure 2). The median survival was 188 days for patients with CRS and 151 days for those without CRS (Log-rang X2 = 0.042, p = 0.7) (Figure 2). Prolonged stabilization of disease, partial remissions and complete remissions were achieved in both patients who experienced CRS as well as patients who did not experience CRS after APVO436 infusions.

Table 3.
CRS history of APVO436-treated R/R AML patients with favorable responses.
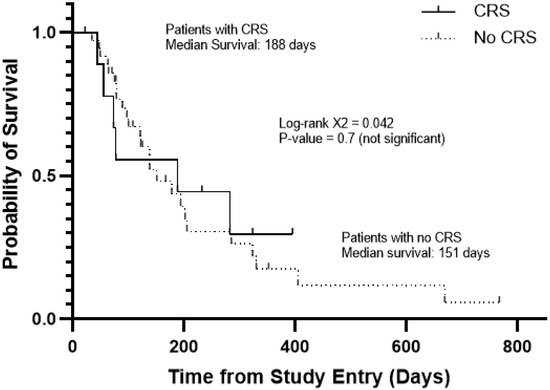
Figure 2.
Survival outcome of AML/MDS patients according to development of CRS in the course of their APVO436 therapy. Depicted are the overall survival curves of the 10 patients who developed CRS and 36 patients who did not. See also Table 2.
4. Discussion
A common complication of bispecific antibody treatments is CRS [17,18,19,20,21]. For example, the human bispecific antibody AMG330 binds CD33 antigen on AML cells and CD3Ԑ on T-cells. In an open-label Phase 1 study (Clinicaltrial.gov, identifier: NCT02520427), AMG330 was given at doses ranging from 0.5 to 720 μg/d in the manner of continuous IV infusion among 55 patients with R/R AML. AMG 330-related AEs included CRS (67%; Grade ≥ 3 in 13%) as the most frequent AEs. Similarly, CRS was observed in 63% of AML patients treated with AMG673, a new version of AMG330 (Grade ≥ 3 in 18%; Clinicaltrial.gov, identifier: NCT03224819) [34]. Flotetuzumab (MGD006) is a bispecific DART antibody reactive with both a CD3 antigen on T-cells and a CD123 antigen on AML cells. This CD3-engaging BiAb exhibited promising single-agent activity in therapy-refractory AML patients with primary induction failure as well as patients with an early first relapse. CRS was observed in all AML patients treated with Flotetuzumab [11] and 58% of AML patients treated with Vibecotamab (XmAb14045), another CD3xCD123 BiAb [35]. By comparison, only 10 of 46 patients (21.7%) treated with APVO436 developed CRS.
IL-6 is one of the driving pro-inflammatory cytokines that contributes to CRS and its pulmonary, cardiovascular, renal and neurologic complications [17,18,19,20,21,36,37]. Cytokine profiling in patients who developed CRS after APVO436 infusion indicated that the predominant cytokine in this inflammatory cytokine response was IL-6, which was in agreement with our current knowledge regarding CRS that occurs in the context of BiAb therapy [14,15,16,38,39,40]. Within 1–2 days following the first dose of APVO436, the mean serum IL-6 concentration was elevated 145-fold over the baseline (755 vs. 5.2) and at the end of one week it was still elevated 83-fold over the baseline. In most cases, CRS events were transient and medically manageable with standard of care, including the use of dexamethasone and anti-IL-6:IL-rR antibody tocilizumab or anti-IL-6 antibody siltuximab (antibody against IL-6). One patient who developed Grade 2 CRS died due to complications from acute renal failure. Notably, patients who developed CRS after APVO436 therapy were not more or less likely to have a favorable response. As patients received dexamethasone as a premedication and for the treatment of CRS, these results suggest that dexamethasone does not prevent favorable responses to APVO436 at the applied dose level and schedule.
5. Conclusions
APVO436-related CRS was encountered in 10 of 46 patients (21.7%) treated with APVO436 with a cumulative Grade 3/4 CRS incidence of 8.7%. Cytokine profiling in patients who developed CRS after APVO436 infusion indicated that the predominant cytokine in this inflammatory cytokine response was IL-6. APVO436-associated CRS was generally manageable with standard of care and resolved rapidly with the administration of tocilizumab at standard doses combined with dexamethasone. APVO436-related CRS was not required for clinically meaningful responses in R/R AML patients, and it did not affect their survival outcome. The prolonged stabilization of disease, partial remissions and complete remissions were achieved in both patients who experienced CRS as well as patients who did not experience CRS after APVO436 infusions.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13215287/s1, Table S1: severity grades of CRS, Table S2: protocol 5001 management guidelines for cytokine release syndrome (CRS), Table S3: listing of all adverse events leading to dose modifications of the study drug APVO436 in study 5001, Table S4: summary tabulation by MedDRA PT of all adverse events leading to dose interruption, dose reduction or drug withdrawal occurring in patients treated with APVO436 in study 5001, Table S5: listing of dose-limiting toxicities (DLTs) in study 5001, Table S6: CRS-associated changes in serum cytokine levels of APVO436-treated R/R AML/MDS patients.
Author Contributions
All authors equally contributed to this manuscript. T.L.L., A.S.M., P.P., P.J.S., E.C., C.R.C., E.W. and J.W. assisted in the study design, served as site investigators, screened and recruited participants, administered treatments, assessed adverse events and disease responses, collected safety and efficacy data, met regularly to review study data during the study and reviewed the manuscript; F.M.U. designed the evaluations reported in this paper, directed the data compilation and analysis and prepared the initial draft of the manuscript; F.M.U. and C.L. analyzed and validated data and performed statistical analyses; each author reviewed and revised the manuscript and provided the final approval for the submission of the final version. No medical writer was involved. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Aptevo Research and Development LLC, a wholly owned subsidiary of Aptevo Therapeutics Inc. (OTCQB:APVO). The sponsor did not participate in the safety/efficacy assessments of the investigators. Among the authors, F.M.U. and C.L., who participated in the analysis and decision to submit the manuscript for publication, are affiliated with the sponsor.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the WCG-Central Institutional Review Board (IRB) (OHRP/FDA registration number: IRB00000533) and the local IRB at participating centers [29]. The Central IRB-approved study/protocol number was 20181730.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Each patient provided written informed consent (ICF) prior to enrollment.
Data Availability Statement
Will individual participant data be available (including data dictionaries)? Yes. What data in particular will be shared? Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices). What other documents will be available? Study protocol. When will data be available (start and end dates)? Beginning 3 months and ending 5 years following article publication. With whom? Researchers who provide a methodologically sound proposal. For what types of analyses? To achieve aims in the approved proposal.
Acknowledgments
The authors thank the patients who participated in this trial and their families, the coinvestigators, nurses and study coordinators at each of the sites. This study was sponsored by Aptevo Therapeutics, which provided APVO436 and worked with investigators to design the study, as well as collect, analyze and interpret the data. We thank the Aptevo Lab Personnel for centralized laboratory services for immunophenotyping by flow cytometry. We thank the research coordinators from the participating clinical sites for their assistance with study coordination and data management.
Conflicts of Interest
T.L.L., A.S.M., P.P., P.J.S., E.C., C.R.C., E.W., and J.W. and their institutions received research funding in the form of investigative site awards from Aptevo Therapeutics for conducting the study. F.M.U. and C.L. received compensation from Aptevo Therapeutics as consultants. No other conflicts are reported.
References
- Mims, A.S.; Blum, W. Progress in the problem of relapsed or refractory acute myeloid leukemia. Curr. Opin. Hematol. 2019, 26, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Muller-Tidow, C.; Benner, A.; Kieser, M. Relapsed/refractory acute myeloid leukemia: Any progress? Curr. Opin. Oncol. 2017, 29, 467–473. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, C.; Doucette, K.; Norsworthy, K. Recent drug approvals for acute myeloid leukemia. J. Hematol. Oncol. 2019, 12, 100. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Lessi, F.; Vitagliano, O.; Birkenghi, E.; Rossi, G. Current Therapeutic Results and Treatment Options for Older Patients with Relapsed Acute Myeloid Leukemia. Cancers 2019, 11, 224. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Wei, A.H. How I treat acute myeloid leukemia in the era of new drugs. Blood 2020, 135, 85–96. [Google Scholar] [CrossRef]
- Blum, W.G.; Mims, A.S. Treating acute myeloid leukemia in the modern era: A primer. Cancer 2020, 126, 4668–4677. [Google Scholar] [CrossRef]
- Thol, F.; Heuser, M. Treatment for Relapsed/Refractory Acute Myeloid Leukemia. Hemasphere 2021, 5, e572. [Google Scholar] [CrossRef]
- Short, N.J.; Konopleva, M.; Kadia, T.M.; Borthakur, G.; Ravandi, F.; DiNardo, C.D.; Daver, N. Advances in the Treatment of Acute Myeloid Leukemia: New Drugs and New Challenges. Cancer Discov. 2020, 10, 506–525. [Google Scholar] [CrossRef] [Green Version]
- Daver, N.; Wei, A.H.; Pollyea, D.A.; Fathi, A.T.; Vyas, P.; DiNardo, C.D. New Directions for Emerging Therapies in Acute Myeloid Leukemia: The Next Chapter. Blood Cancer J. 2020, 10, 107. [Google Scholar] [CrossRef]
- Uy, G.L.; Aldoss, I.; Foster, M.C.; Sayre, P.H.; Wieduwilt, M.J.; Advani, A.S.; Godwin, J.E.; Arellano, M.L.; Sweet, K.L.; Emadi, A.; et al. Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 2021, 137, 751–762. [Google Scholar] [CrossRef]
- Daver, N.; Alotaibi, A.S.; Bücklein, V.; Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: Current concepts and future developments. Leukemia 2021, 35, 1843–1863. [Google Scholar] [CrossRef] [PubMed]
- Kovtun, Y.; Jones, G.E.; Adams, S.; Harvey, L.; Audette, C.A.; Wilhelm, A.; Bai, C.; Rui, L.; Laleau, R.; Liu, F.; et al. A CD123-targeting antibody-drug conjugate, IMGN632, designed to eradicate AML while sparing normal bone marrow cells. Blood Adv. 2018, 2, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Einsele, H.; Borghaei, H.; Orlowski, R.Z.; Subklewe, M.; Roboz, G.J.; Zugmaier, G.; Kufer, P.; Iskander, K.; Kantarjian, H.M. The BiTE (Bispecific T-cell Engager) Platform: Development and Future Potential of a Targeted Immuno-Oncology Therapy across Tumor Types. Cancer 2020, 126, 3192–3201. [Google Scholar] [CrossRef]
- Isidori, A.; Cerchione, C.; Daver, N.; DiNardo, C.; Garcia-Manero, G.; Konopleva, M.; Jabbour, E.; Ravandi, F.; Kadia, T.; Burguera, A.F.; et al. Immunotherapy in Acute Myeloid Leukemia: Where We Stand. Front. Oncol. 2021, 11, 656218. [Google Scholar] [CrossRef] [PubMed]
- Huehls, A.M.; Coupet, T.A.; Sentman, C.L. Bispecific T-cell Engagers for Cancer Immunotherapy. Immunol. Cell Biol. 2015, 93, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldoss, I.; Khaled, S.K.; Budde, E.; Stein, A.S. Cytokine Release Syndrome with the Novel Treatments of Acute Lymphoblastic Leukemia: Pathophysiology, Prevention, and Treatment. Curr. Oncol. Rep. 2019, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Lee, D.W.; Gardner, R.; Porter, D.L.; Louis, C.U.; Ahmed, N.; Jensen, M.; Grupp, S.A.; Mackall, C.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014, 124, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, U.; Riccioni, R.; Coccia, E.; Stellacci, E.; Samoggia, P.; Latagliata, R.; Latagliata, R.; Mariani, G.; Rossini, A.; Battistini, A.; et al. Elevated expression of IL-3Rα in acute myelogenous leukemia is associated with enhanced blast proliferation, increased cellularity and poor prognosis. Blood 2002, 100, 2980–2988. [Google Scholar] [CrossRef]
- Hwang, K.; Park, C.J.; Jang, S.; Chi, H.S.; Kim, D.Y.; Lee, J.H.; Im, H.J.; Seo, J.J. Flow cytometric quantification and immunophenotyping of leukemic stem cells in acute myeloid leukemia. Ann. Hematol. 2012, 91, 1541–1546. [Google Scholar] [CrossRef]
- Jin, L.; Lee, E.M.; Ramshaw, H.S.; Busfiled, S.J.; Peoppl, A.G.; Wilkinson, L.; Wilkinson, L.; Guthridge, M.A.; Thomas, D.; Barry, E.F.; et al. Monoclonal-antibody mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemia stem cells. Cell Stem Cell 2009, 5, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Jordan, C.T.; Upchurch, D.; Szilvassy, S.J.; Guzman, M.L.; Howard, D.S.; Pettigrew, A.L.; Meyerrose, T.; Rossi, R.; Grimes, B.; Rizzieri, D.A.; et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 2000, 14, 1777–1784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comeau, M.R.; Gottschalk, R.; Daugherty, M.; Sewell, T.; Sewell, T.; Misher, L.; Bannink, J.; Johnson, S.; Parr, L.; Kumer, J.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity with limited cytokine release, is well tolerated in repeat dose toxicology studies in cynomolgus macaques. In Proceedings of the American Association for Cancer Research Annual Meeting 2019, Atlanta, GA, USA, 29 March–3 April 2019; AACR: Philadelphia, PA, USA. Cancer Res. 2019, 79 (Suppl. 13). Abstract nr LB-199. [Google Scholar]
- Comeau, M.R.; Miller, R.E.; Bader, R.; Gottschalk, R.; Daughterty, M.; Sewell, T.; Misher, L.; Parr, L.; DeFrancesco, M.; Bienvenue, D.; et al. APVO436, a bispecific anti-CD123 x anti-CD3 ADAPTIR™ molecule for redirected T-cell cytotoxicity, induces potent T-cell activation, proliferation and cytotoxicity with limited cytokine release. In Proceedings of the American Association for Cancer Research Annual Meeting 2018, Chicago, IL, USA, 14–18 April 2018; AACR: Philadelphia, PA, USA. Cancer Res. 2018, 78 (Suppl. 13). Abstract nr 1786. [Google Scholar]
- Hernandez-Hoyos, G.; Sewell, T.; Bader, R.; Bannink, J.; Chenault, R.A.; Daugherty, M.; Dasovich, M.; Fang, H.; Gottschalk, R.; Kumer, J.; et al. MOR209/ES414, a Novel Bispecific Antibody Targeting PSMA for the Treatment of Metastatic Castration-Resistant Prostate Cancer. Mol. Cancer Ther. 2016, 15, 2155–2165. [Google Scholar] [CrossRef] [Green Version]
- Uckun, F.M.; Lin, T.L.; Mims, A.S.; Patel, P.; Lee, C.; Shahidzadeh, A.; Shami, P.J.; Cull, E.; Cogle, C.R.; Watts, J. A Clinical Phase 1B Study of the CD3xCD123 Bispecific Antibody APVO436 in Patients with Relapsed/Refractory Acute Myeloid Leukemia or Myelodysplastic Syndrome. Cancers 2021, 13, 4113. [Google Scholar] [CrossRef]
- Ravandi, F.; Bashey, A.; Stock, W.; Foran, J.M.; Mawad, R.; Egan, D.; Blum, W.; Yang, A.; Pastore, A.; Johnson, C.; et al. Complete Responses in Relapsed/Refractory Acute Myeloid Leukemia (AML) Patients on a Weekly Dosing Schedule of Vibecotamab (XmAb14045), a CD123 X CD3 T Cell-Engaging Bispecific Antibody; Initial Results of a Phase 1 Study. Blood 2020, 136, 4–5. [Google Scholar] [CrossRef]
- Muller, P.Y.; Milton, M.; Lloyd, P.; Sims, J.; Brennan, F.R. The minimum anticipated biological effect level (MABEL) for selection of first human dose in clinical trials with monoclonal antibodies. Curr. Opin. Biotechnol. 2009, 20, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breen, E.C.; Reynolds, S.M.; Cox, C.; Jacobson, L.P.; Magpantay, L.; Mulder, C.B.; Dibben, O.; Margolick, J.B.; Bream, J.H.; Sambrano, E.; et al. Multisite comparison of high-sensitivity multiplex cytokine assays. Clin. Vaccine Immunol. 2011, 18, 1229–1242. [Google Scholar] [CrossRef] [Green Version]
- Canter, R.J.; Le, C.T.; Beerthuijzen, J.; Murphy, W.J. Obesity as an immune-modifying factor in cancer immunotherapy. J. Leukoc. Biol. 2018, 104, 487–497. [Google Scholar] [CrossRef]
- Kim, J.; Nam, J.H. Insight into the relationship between obesity-induced low-level chronic inflammation and COVID-19 infection. Int. J. Obes. 2020, 44, 1541–1542. [Google Scholar] [CrossRef]
- Schwartz, R.N.; Stover, L.; Dutcher, J.P. Managing toxicities of high-dose interleukin-2. Oncology 2002, 16 (Suppl. 13), 11–20. [Google Scholar] [PubMed]
- Subklewe, M.; Stein, A.; Walter, R.B.; Bhatia, R.; Wei, A.H.; Ritchie, D.; Bücklein, V.; Vachhani, P.; Dai, T.; Hindoyan, A.; et al. Updated Results from a Phase 1 First-in-Human Dose Escalation Study of AMG 673, a Novel Anti-CD33/CD3 BiTE® (Bispecific T-Cell Engager). In Patients with Relapsed/Refractory Acute Myeloid Leukemia; Abstract: EP548; European Hematology Association: Brussels, Belgium, 2020. [Google Scholar]
- García Roche, A.; Díaz Lagares, C.; Élez, E.; Ferrer Roca, R. Cytokine release syndrome. Reviewing a new entity in the intensive care unit. Med. Intensiva 2019, 43, 480–488. [Google Scholar] [CrossRef]
- Simbaqueba, C.C.; Aponte, M.P.; Kim, P.; Deswal, A.; Palaskas, N.L.; Iliescu, C.; Jahangir, E.; Yang, E.H.; Steiner, R.E.; Lopez-Mattei, J. Cardiovascular complications of chimeric antigen receptor therapy: Cytokine release syndrome and associated arrhytmias. J. Immunother. Precis. Oncol. 2020, 3, 113–120. [Google Scholar] [CrossRef]
- Chen, L.Y.C.; Biggs, C.M.; Jamal, S.; Stukas, S.; Wellington, C.L.; Sekhon, M.S. Soluble Interleukin-6 Receptor in the COVID-19 Cytokine Storm Syndrome. Cell Rep. Med. 2021, 2, 100269. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).