Early Palliative Care in Acute Myeloid Leukemia
Abstract
:Simple Summary
Abstract
1. Introduction
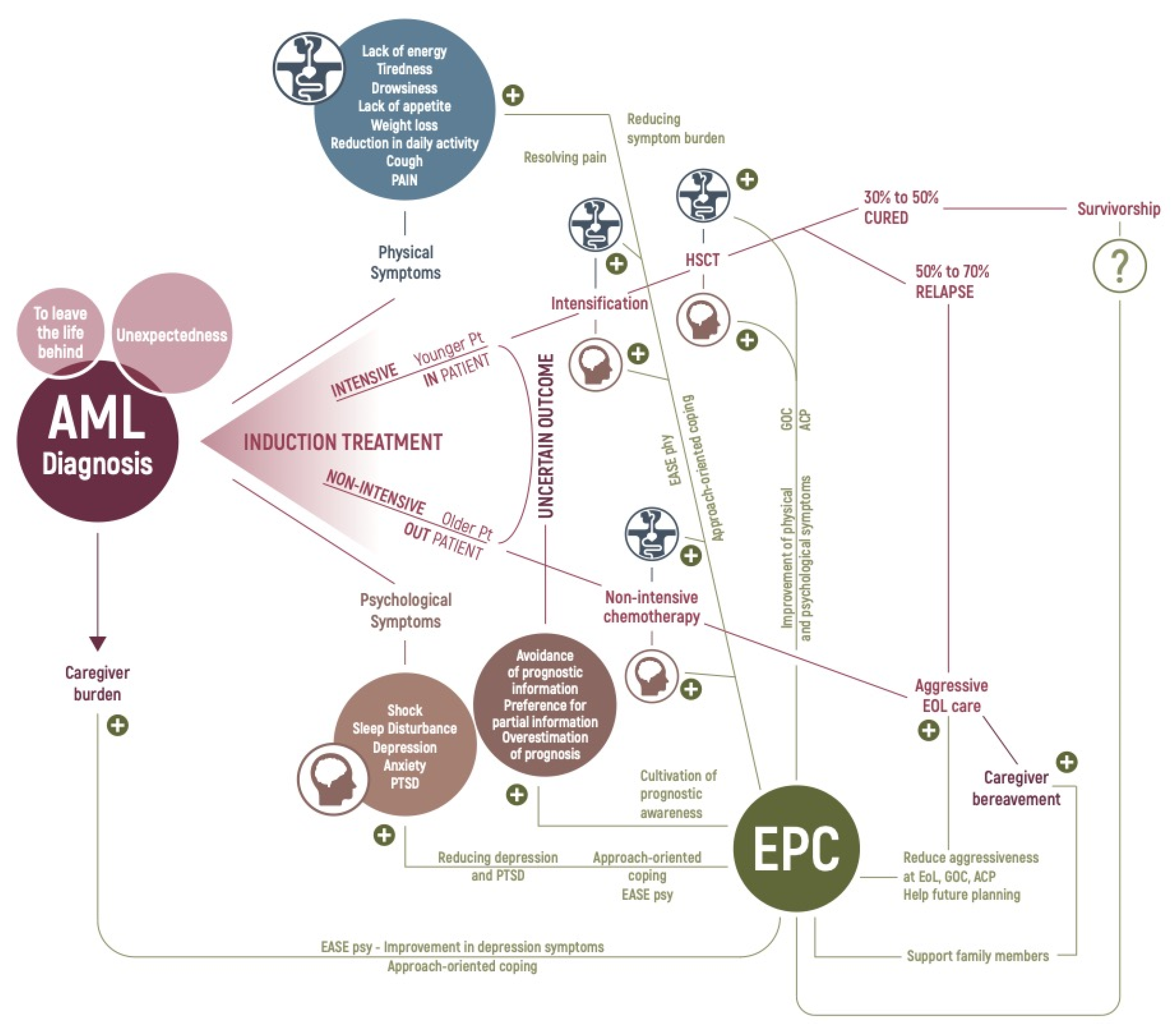
2. AML Patients’ Physical Symptoms
3. AML Patients’ Illness Experience
4. Healthcare Utilization and End-of-Life Care in Patients with AML
5. Early Palliative Care: Lessons from Advanced Solid Cancer Patients
6. What Does the Intervention Consist of?
7. Early Palliative Care in Patients with Hematologic Malignancies
8. Early Palliative Care in AML Patients
9. Future Challenges
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- De Kouchkovsky, I.; Abdul-Hay, M. Acute Myeloid Leukemia: A Comprehensive Review and 2016 Update. Blood Cancer J. 2016, 6, e441. [Google Scholar] [CrossRef]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute Myeloid Leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Wei, A.H. How I Treat Acute Myeloid Leukemia in the Era of New Drugs. Blood 2020, 135, 85–96. [Google Scholar] [CrossRef]
- Megías-Vericat, J.E.; Martínez-Cuadrón, D.; Solana-Altabella, A.; Montesinos, P. Precision Medicine in Acute Myeloid Leukemia: Where Are We Now and What Does the Future Hold? Expert Rev. Hematol. 2020, 13, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Kaasa, S.; Loge, J.H.; Aapro, M.; Albreht, T.; Anderson, R.; Bruera, E.; Brunelli, C.; Caraceni, A.; Cervantes, A.; Currow, D.C.; et al. Integration of Oncology and Palliative Care: A Lancet Oncology Commission. Lancet Oncol. 2018, 19, e588–e653. [Google Scholar] [CrossRef] [Green Version]
- El-Jawahri, A.; Nelson, A.M.; Gray, T.F.; Lee, S.J.; LeBlanc, T.W. Palliative and End-of-Life Care for Patients with Hematologic Malignancies. J. Clin. Oncol. 2020, 38, 944–953. [Google Scholar] [CrossRef]
- Oswald, L.B.; Venditti, A.; Cella, D.; Cottone, F.; Candoni, A.; Melillo, L.; Cairoli, R.; Storti, G.; Salutari, P.; Luppi, M.; et al. Fatigue in Newly Diagnosed Acute Myeloid Leukaemia: General Population Comparison and Predictive Factors. BMJ Support. Palliat Care 2021. [Google Scholar] [CrossRef]
- Manitta, V.; Zordan, R.; Cole-Sinclair, M.; Nandurkar, H.; Philip, J. The Symptom Burden of Patients with Hematological Malignancy: A Cross-Sectional Observational Study. J. Pain Symptom Manag. 2011, 4, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Hochman, M.J.; Yu, Y.; Wolf, S.P.; Samsa, G.P.; Kamal, A.H.; LeBlanc, T.W. Comparing the Palliative Care Needs of Patients with Hematologic and Solid Malignancies. J. Pain Symptom Manag. 2018, 55, 82–88.e1. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, C.; Yuen, D.; Mischitelle, A.; Minden, M.D.; Brandwein, J.M.; Schimmer, A.; Gagliese, L.; Lo, C.; Rydall, A.; Rodin, G. Symptom Burden and Supportive Care in Patients with Acute Leukemia. Leuk. Res. 2013, 37, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Efficace, F.; Gaidano, G.; Breccia, M.; Criscuolo, M.; Cottone, F.; Caocci, G.; Bowen, D.; Lübbert, M.; Angelucci, E.; Stauder, R.; et al. Prevalence, Severity and Correlates of Fatigue in Newly Diagnosed Patients with Myelodysplastic Syndromes. Br. J. Haematol. 2015, 168, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Shaulov, A.; Rodin, G.; Popovic, G.; Caraiscos, V.B.; Le, L.W.; Rydall, A.; Schimmer, A.D.; Zimmermann, C. Pain in Patients with Newly Diagnosed or Relapsed Acute Leukemia. Support. Care Cancer 2019, 27, 2789–2797. [Google Scholar] [CrossRef]
- Morselli, M.; Bandieri, E.; Zanin, R.; Buonaccorso, L.; D’Amico, R.; Forghieri, F.; Pietramaggiori, A.; Potenza, L.; Berti, A.; Cacciapaglia, G.; et al. Pain and Emotional Distress in Leukemia Patients at Diagnosis. Leuk. Res. 2010, 34, e67–e68. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, T.A.; Boyiadzis, M.; Elswick, R.K.; Starkweather, A.; Rosenzweig, M. Symptom Management and Psychosocial Needs of Adults with Acute Myeloid Leukemia During Induction Treatment: A Pilot Study. Cancer Nurs. 2017, 40, E31–E38. [Google Scholar] [CrossRef]
- Rodin, G.; Yuen, D.; Mischitelle, A.; Minden, M.D.; Brandwein, J.; Schimmer, A.; Marmar, C.; Gagliese, L.; Lo, C.; Rydall, A.; et al. Traumatic Stress in Acute Leukemia. Psychooncology 2013, 22, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Batty, G.D.; Russ, T.C.; Stamatakis, E.; Kivimäki, M. Psychological Distress in Relation to Site Specific Cancer Mortality: Pooling of Unpublished Data from 16 Prospective Cohort Studies. BMJ 2017, 356, j108. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, T.W.; Fish, L.J.; Bloom, C.T.; El-Jawahri, A.; Davis, D.M.; Locke, S.C.; Steinhauser, K.E.; Pollak, K.I. Patient Experiences of Acute Myeloid Leukemia: A Qualitative Study about Diagnosis, Illness Understanding, and Treatment Decision-Making. Psychooncology 2017, 26, 2063–2068. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Stone, R.M.; Zahrieh, D.; Neuberg, D.; Morrison, V.; De Angelo, D.J.; Galinsky, I.; Lee, S.J. Decision-Making and Quality of Life in Older Adults with Acute Myeloid Leukemia or Advanced Myelodysplastic Syndrome. Leukemia 2004, 18, 809–816. [Google Scholar] [CrossRef] [Green Version]
- Friis, L.S.; Elverdam, B.; Schmidt, K.G. The Patient’s Perspective: A Qualitative Study of Acute Myeloid Leukaemia Patients’ Need for Information and Their Information-Seeking Behaviour. Support. Care Cancer 2003, 11, 162–170. [Google Scholar] [CrossRef]
- Nissim, R.; Zimmermann, C.; Minden, M.; Rydall, A.; Yuen, D.; Mischitelle, A.; Gagliese, L.; Schimmer, A.; Rodin, G. Abducted by the Illness: A Qualitative Study of Traumatic Stress in Individuals with Acute Leukemia. Leuk. Res. 2013, 37, 496–502. [Google Scholar] [CrossRef] [Green Version]
- Boucher, N.A.; Johnson, K.S.; LeBlanc, T.W. Acute Leukemia Patients’ Needs: Qualitative Findings and Opportunities for Early Palliative Care. J. Pain Symptom Manag. 2018, 55, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Nipp, R.D.; Greer, J.A.; El-Jawahri, A.; Moran, S.M.; Traeger, L.; Jacobs, J.M.; Jacobsen, J.C.; Gallagher, E.R.; Park, E.R.; Ryan, D.P.; et al. Coping and Prognostic Awareness in Patients with Advanced Cancer. J. Clin. Oncol. 2017, 35, 2551–2557. [Google Scholar] [CrossRef] [PubMed]
- Yogaparan, T.; Panju, A.; Minden, M.; Brandwein, J.; Mohamedali, H.Z.; Alibhai, S.M.H. Information Needs of Adult Patients 50 or Older with Newly Diagnosed Acute Myeloid Leukemia. Leuk. Res. 2009, 33, 1288–1290. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Nelson-Lowe, M.; VanDusen, H.; Traeger, L.; Abel, G.A.; Greer, J.A.; Fathi, A.; Steensma, D.P.; LeBlanc, T.W.; Li, Z.; et al. Patient-Clinician Discordance in Perceptions of Treatment Risks and Benefits in Older Patients with Acute Myeloid Leukemia. Oncologist 2019, 24, 247–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Locke, S.C.; Wolf, S.P.; Albrecht, T.A.; Troy, J.D.; Derry, H.; El-Jawahri, A.; LeBlanc, T.W. The Relationship between Emotional Well-Being and Understanding of Prognosis in Patients with Acute Myeloid Leukemia (AML). Support. Care Cancer 2021, 30, 897–906. [Google Scholar] [CrossRef]
- Kassim, A.A.; Savani, B.N. Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia: A Review. Hematol. Oncol. Stem Cell Ther. 2017, 10, 245–251. [Google Scholar] [CrossRef]
- Odejide, O.O.; Salas Coronado, D.Y.; Watts, C.D.; Wright, A.A.; Abel, G.A. End-of-Life Care for Blood Cancers: A Series of Focus Groups with Hematologic Oncologists. J. Oncol. Pr. 2014, 10, e396–e403. [Google Scholar] [CrossRef]
- Hui, D.; Bansal, S.; Park, M.; Reddy, A.; Cortes, J.; Fossella, F.; Bruera, E. Differences in Attitudes and Beliefs toward End-of-Life Care between Hematologic and Solid Tumor Oncology Specialists. Ann. Oncol. 2015, 26, 1440–1446. [Google Scholar] [CrossRef]
- Hui, D.; Park, M.; Liu, D.; Reddy, A.; Dalal, S.; Bruera, E. Attitudes and Beliefs Toward Supportive and Palliative Care Referral Among Hematologic and Solid Tumor Oncology Specialists. Oncologist 2015, 20, 1326–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, D.; Didwaniya, N.; Vidal, M.; Shin, S.H.; Chisholm, G.; Roquemore, J.; Bruera, E. Quality of End-of-Life Care in Patients with Hematologic Malignancies: A Retrospective Cohort Study. Cancer 2014, 120, 1572–1578. [Google Scholar] [CrossRef]
- Odejide, O.O.; Cronin, A.M.; Condron, N.B.; Fletcher, S.A.; Earle, C.C.; Tulsky, J.A.; Abel, G.A. Barriers to Quality End-of-Life Care for Patients with Blood Cancers. J. Clin. Oncol. 2016, 34, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Earle, C.C.; Park, E.R.; Lai, B.; Weeks, J.C.; Ayanian, J.Z.; Block, S. Identifying Potential Indicators of the Quality of End-of-Life Cancer Care from Administrative Data. J. Clin. Oncol. 2003, 21, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zeidan, A.M.; Halene, S.; Xu, X.; Davidoff, A.J.; Huntington, S.F.; Podoltsev, N.A.; Gross, C.P.; Gore, S.D.; Ma, X. Health Care Use by Older Adults with Acute Myeloid Leukemia at the End of Life. J. Clin. Oncol. 2017, 35, 3417–3424. [Google Scholar] [CrossRef] [PubMed]
- El-Jawahri, A.R.; Abel, G.A.; Steensma, D.P.; LeBlanc, T.W.; Fathi, A.T.; Graubert, T.A.; DeAngelo, D.J.; Wadleigh, M.; Ballen, K.K.; Foster, J.E.; et al. Health Care Utilization and End-of-Life Care for Older Patients with Acute Myeloid Leukemia. Cancer 2015, 121, 2840–2848. [Google Scholar] [CrossRef] [Green Version]
- Lowe, J.R.; Yu, Y.; Wolf, S.; Samsa, G.; LeBlanc, T.W. A Cohort Study of Patient-Reported Outcomes and Healthcare Utilization in Acute Myeloid Leukemia Patients Receiving Active Cancer Therapy in the Last Six Months of Life. J. Palliat Med. 2018, 21, 592–597. [Google Scholar] [CrossRef]
- Cheng, H.-W.B.; Li, C.-W.; Chan, K.-Y.; Au, H.-Y.; Chan, P.-F.; Sin, Y.-C.; Szeto, Y.; Sham, M.-K. End-of-Life Characteristics and Palliative Care Provision for Elderly Patients Suffering from Acute Myeloid Leukemia. Support. Care Cancer 2015, 23, 111–116. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Keenan, T.; Abel, G.A.; Steensma, D.P.; LeBlanc, T.W.; Chen, Y.-B.; Hobbs, G.; Traeger, L.; Fathi, A.T.; DeAngelo, D.J.; et al. Potentially Avoidable Hospital Admissions in Older Patients with Acute Myeloid Leukaemia in the USA: A Retrospective Analysis. Lancet Haematol. 2016, 3, e276–e283. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, T.W.; Egan, P.C.; Olszewski, A.J. Transfusion Dependence, Use of Hospice Services, and Quality of End-of-Life Care in Leukemia. Blood 2018, 132, 717–726. [Google Scholar] [CrossRef]
- Lin, R.J.; Elko, T.A.; Perales, M.-A.; Alexander, K.; Jakubowski, A.A.; Devlin, S.M.; Dahi, P.B.; Papadopoulos, E.B.; Klimek, V.M.; Giralt, S.A.; et al. End-of-Life Care for Older AML Patients Relapsing after Allogeneic Stem Cell Transplant at a Dedicated Cancer Center. Bone Marrow Transpl. 2019, 54, 700–706. [Google Scholar] [CrossRef]
- Odejide, O.O.; Uno, H.; Murillo, A.; Tulsky, J.A.; Abel, G.A. Goals of Care Discussions for Patients with Blood Cancers: Association of Person, Place, and Time with End-of-Life Care Utilization. Cancer 2020, 126, 515–522. [Google Scholar] [CrossRef]
- Cheung, M.C.; Croxford, R.; Earle, C.C.; Singh, S. Days Spent at Home in the Last 6 Months of Life: A Quality Indicator of End of Life Care in Patients with Hematologic Malignancies. Leuk. Lymphoma 2020, 61, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Bakitas, M.; Lyons, K.D.; Hegel, M.T.; Balan, S.; Brokaw, F.C.; Seville, J.; Hull, J.G.; Li, Z.; Tosteson, T.D.; Byock, I.R.; et al. Effects of a Palliative Care Intervention on Clinical Outcomes in Patients with Advanced Cancer: The Project ENABLE II Randomized Controlled Trial. JAMA 2009, 302, 741–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmermann, C.; Swami, N.; Krzyzanowska, M.; Hannon, B.; Leighl, N.; Oza, A.; Moore, M.; Rydall, A.; Rodin, G.; Tannock, I.; et al. Early Palliative Care for Patients with Advanced Cancer: A Cluster-Randomised Controlled Trial. Lancet 2014, 383, 1721–1730. [Google Scholar] [CrossRef]
- Bakitas, M.A.; Tosteson, T.D.; Li, Z.; Lyons, K.D.; Hull, J.G.; Li, Z.; Dionne-Odom, J.N.; Frost, J.; Dragnev, K.H.; Hegel, M.T.; et al. Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Grudzen, C.R.; Richardson, L.D.; Johnson, P.N.; Hu, M.; Wang, B.; Ortiz, J.M.; Kistler, E.A.; Chen, A.; Morrison, R.S. Emergency Department-Initiated Palliative Care in Advanced Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016, 2, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temel, J.S.; Greer, J.A.; El-Jawahri, A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; et al. Effects of Early Integrated Palliative Care in Patients with Lung and GI Cancer: A Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 834–841. [Google Scholar] [CrossRef]
- Dionne-Odom, J.N.; Azuero, A.; Lyons, K.D.; Hull, J.G.; Tosteson, T.; Li, Z.; Li, Z.; Frost, J.; Dragnev, K.H.; Akyar, I.; et al. Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients with Advanced Cancer: Outcomes from the ENABLE III Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 1446–1452. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Greer, J.A.; Pirl, W.F.; Park, E.R.; Jackson, V.A.; Back, A.L.; Kamdar, M.; Jacobsen, J.; Chittenden, E.H.; Rinaldi, S.P.; et al. Effects of Early Integrated Palliative Care on Caregivers of Patients with Lung and Gastrointestinal Cancer: A Randomized Clinical Trial. Oncologist 2017, 22, 1528–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, J.; Swami, N.; Hannon, B.; Lo, C.; Pope, A.; Oza, A.; Leighl, N.; Krzyzanowska, M.K.; Rodin, G.; Le, L.W.; et al. Impact of Early Palliative Care on Caregivers of Patients with Advanced Cancer: Cluster Randomised Trial. Ann. Oncol. 2017, 28, 163–168. [Google Scholar] [CrossRef]
- Bandieri, E.; Sichetti, D.; Romero, M.; Fanizza, C.; Belfiglio, M.; Buonaccorso, L.; Artioli, F.; Campione, F.; Tognoni, G.; Luppi, M. Impact of Early Access to a Palliative/Supportive Care Intervention on Pain Management in Patients with Cancer. Ann. Oncol. 2012, 23, 2016–2020. [Google Scholar] [CrossRef] [PubMed]
- Bandieri, E.; Banchelli, F.; Artioli, F.; Mucciarini, C.; Razzini, G.; Cruciani, M.; Potenza, L.; D’Amico, R.; Efficace, F.; Bruera, E.; et al. Early versus Delayed Palliative/Supportive Care in Advanced Cancer: An Observational Study. BMJ Support. Palliat Care 2020, 10, e32. [Google Scholar] [CrossRef]
- Haun, M.W.; Estel, S.; Rücker, G.; Friederich, H.-C.; Villalobos, M.; Thomas, M.; Hartmann, M. Early Palliative Care for Adults with Advanced Cancer. Cochrane Database Syst. Rev. 2017, 6, CD011129. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.J.; LeBlanc, T.W.; Cutson, T.M.; Porter Starr, K.N.; Kamal, A.; Ramos, K.; Freiermuth, C.E.; McDuffie, J.R.; Kosinski, A.; Adam, S.; et al. Integrated Outpatient Palliative Care for Patients with Advanced Cancer: A Systematic Review and Meta-Analysis. Palliat Med. 2019, 33, 123–134. [Google Scholar] [CrossRef]
- Ferrell, B.R.; Temel, J.S.; Temin, S.; Alesi, E.R.; Balboni, T.A.; Basch, E.M.; Firn, J.I.; Paice, J.A.; Peppercorn, J.M.; Phillips, T.; et al. Integration of Palliative Care into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 96–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, T.H.; Jackson, V.A.; Carlson, H.; Rinaldi, S.; Sousa, A.; Hansen, A.; Kamdar, M.; Jacobsen, J.; Park, E.R.; Pirl, W.F.; et al. Communication Differences between Oncologists and Palliative Care Clinicians: A Qualitative Analysis of Early, Integrated Palliative Care in Patients with Advanced Cancer. J. Palliat Med. 2019, 22, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jackson, V.A.; Jacobsen, J.; Greer, J.A.; Pirl, W.F.; Temel, J.S.; Back, A.L. The Cultivation of Prognostic Awareness through the Provision of Early Palliative Care in the Ambulatory Setting: A Communication Guide. J. Palliat Med. 2013, 16, 894–900. [Google Scholar] [CrossRef]
- Zimmermann, C.; Ryan, S.; Hannon, B.; Saltman, A.; Rodin, G.; Mak, E.; Al-Awamer, A.; Lau, J. Team-Based Outpatient Early Palliative Care: A Complex Cancer Intervention. BMJ Support. Palliat Care 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvaggi, K.J.; Vick, J.B.; Jessell, S.A.; Lister, J.; Abrahm, J.L.; Bernacki, R. Bridging the Gap: A Palliative Care Consultation Service in a Hematological Malignancy-Bone Marrow Transplant Unit. J. Community Support. Oncol. 2014, 12, 50–55. [Google Scholar] [CrossRef]
- Loggers, E.T.; LeBlanc, T.W.; El-Jawahri, A.; Fihn, J.; Bumpus, M.; David, J.; Horak, P.; Lee, S.J. Pretransplantation Supportive and Palliative Care Consultation for High-Risk Hematopoietic Cell Transplantation Patients. Biol. Blood Marrow Transpl. 2016, 22, 1299–1305. [Google Scholar] [CrossRef] [Green Version]
- Foxwell, A.M.; Moyer, M.E.; Casarett, D.J.; O’Connor, N.R. Palliative Care Office Hours for Patients with Hematologic Malignancies: An Innovative Model for Symptom Management and Education. J. Palliat Med. 2017, 20, 1148–1151. [Google Scholar] [CrossRef] [PubMed]
- Porta-Sales, J.; Guerrero-Torrelles, M.; Moreno-Alonso, D.; Sarrà-Escarré, J.; Clapés-Puig, V.; Trelis-Navarro, J.; Sureda-Balarí, A.; Fernández De Sevilla-Ribosa, A. Is Early Palliative Care Feasible in Patients with Multiple Myeloma? J. Pain Symptom Manag. 2017, 54, 692–700. [Google Scholar] [CrossRef] [Green Version]
- El-Jawahri, A.; LeBlanc, T.; VanDusen, H.; Traeger, L.; Greer, J.A.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; Spitzer, T.R.; et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA 2016, 316, 2094–2103. [Google Scholar] [CrossRef]
- El-Jawahri, A.; Traeger, L.; Greer, J.A.; VanDusen, H.; Fishman, S.R.; LeBlanc, T.W.; Pirl, W.F.; Jackson, V.A.; Telles, J.; Rhodes, A.; et al. Effect of Inpatient Palliative Care During Hematopoietic Stem-Cell Transplant on Psychological Distress 6 Months After Transplant: Results of a Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- El-Jawahri, A.; LeBlanc, T.W.; Kavanaugh, A.; Webb, J.A.; Jackson, V.A.; Campbell, T.C.; O’Connor, N.; Luger, S.M.; Gafford, E.; Gustin, J.; et al. Effectiveness of Integrated Palliative and Oncology Care for Patients with Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA Oncol. 2021, 7, 238–245. [Google Scholar] [CrossRef]
- Rodin, G.; Malfitano, C.; Rydall, A.; Schimmer, A.; Marmar, C.M.; Mah, K.; Lo, C.; Nissim, R.; Zimmermann, C. Emotion and Symptom-Focused Engagement (EASE): A Randomized Phase II Trial of an Integrated Psychological and Palliative Care Intervention for Patients with Acute Leukemia. Support. Care Cancer 2020, 28, 163–176. [Google Scholar] [CrossRef]
- Potenza, L.; Scaravaglio, M.; Fortuna, D.; Giusti, D.; Colaci, E.; Pioli, V.; Morselli, M.; Forghieri, F.; Bettelli, F.; Messerotti, A.; et al. Early Palliative/Supportive Care in Acute Myeloid Leukaemia Allows Low Aggression End-of-Life Interventions: Observational Outpatient Study. BMJ Support. Palliat Care 2021. [Google Scholar] [CrossRef]
- Nelson, A.M.; Amonoo, H.L.; Kavanaugh, A.R.; Webb, J.A.; Jackson, V.A.; Rice, J.; Lavoie, M.W.; Fathi, A.T.; Brunner, A.M.; Greer, J.A.; et al. Palliative Care and Coping in Patients with Acute Myeloid Leukemia: Mediation Analysis of Data from a Randomized Clinical Trial. Cancer 2021, 127, 4702–4710. [Google Scholar] [CrossRef]
- Amonoo, H.L.; Bodd, M.H.; Reynolds, M.J.; Nelson, A.M.; Newcomb, R.A.; Johnson, P.C.; Dhawale, T.M.; Plotke, R.; Heuer, L.; Gillani, S.; et al. Coping Strategies in Patients with Acute Myeloid Leukemia. Blood Adv. 2021. [Google Scholar] [CrossRef] [PubMed]
- Amonoo, H.L.; LeBlanc, T.W.; Kavanaugh, A.R.; Webb, J.A.; Traeger, L.N.; Jagielo, A.D.; Vaughn, D.M.; Elyze, M.; Longley, R.M.; Fathi, A.T.; et al. Posttraumatic Stress Disorder Symptoms in Patients with Acute Myeloid Leukemia. Cancer 2021, 127, 2500–2506. [Google Scholar] [CrossRef]
- Potenza, L.; Luppi, M.; Efficace, F.; Bruera, E.; Bandieri, E. Early Palliative Care: A Necessary Intervention for Patients Ineligibile to Approved Potentially Life-Saving CAR T-Cell Therapy. Clin. Lymphoma Myeloma Leuk. 2020, 20, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Gritti, G.; Rambaldi, A. Immunotherapy of Acute Lymphoblastic Leukemia and Lymphoma with T Cell-Redirected Bispecific Antibodies. J. Clin. Oncol. 2021, 39, 444–455. [Google Scholar] [CrossRef]
- Chong, E.A.; Ruella, M.; Schuster, S.J.; Lymphoma Program Investigators at the University of Pennsylvania. Five-Year Outcomes for Refractory B-Cell Lymphomas with CAR T-Cell Therapy. N. Engl. J. Med. 2021, 384, 673–674. [Google Scholar] [CrossRef]
- Potenza, L.; Luppi, M.; Borelli, E.; Bigi, S.; Bandieri, E. Education of Early Palliative Care Specialists among Hematologists and Oncologists to Address Patients’ Rather than Physicians’ Rights. Ann. Hematol. 2021, 100, 2857–2858. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Urbauer, D.L.; Bruera, E.; Hui, D. Recommendations for Palliative and Hospice Care in NCCN Guidelines for Treatment of Cancer. Oncologist 2021, 26, 77–83. [Google Scholar] [CrossRef]
- Bramati, P.; Hui, D.; Bruera, E. Integrated Palliative and Oncology Care for Patients with Acute Myeloid Leukemia-Moving from Evidence to Practice. JAMA Oncol. 2021, 7, 943. [Google Scholar] [CrossRef] [PubMed]
| First Author | Study Design | Population | Intervention | Endpoints | Scales and Measures | Results |
|---|---|---|---|---|---|---|
| El-Jawahri A [65] 2020 and Nelson AM [68] 2021 | Multisite, nonblinded, phase III randomized clinical trial | 160 pts: 86 EPC 74 SC | EPC: inpatient PC physician, an AP nurse, or physician assistant. First visit within 72 of randomization. At least 2 visits a week during hospitalization up to 1 year after randomization. No outpatient visits SC: supportive care measures as per their oncology team. PC allowed at patients’ request or at the request of their oncologist. | Primary: QOL at week 2 Secondary: symptom burden, anxiety, depression, PTSD, patient reported EOL discussions, hospitalizations in the last week of life, chemotherapy in the last 30 days of life, and hospice use | FACT-Leuk ESAS PHQ-9 HADS PTSD Checklist–Civilia Brief COPE [68] | Better QOL (EPC:116.45 vs. SC:107.59; p = 0.04). Lower depression (EPC: 5.68 vs. SC: 7.20; p = 0.02; and EPC: 6.34 vs. SC: 8.00; p = 0.04). Lower anxiety (EPC: 4.53 vs. SC: 5.94; p = 0.02). Lower PTSD symptoms (EPC:27.79 vs. SC: 31.69; p = 0.01). Greater use of approach-oriented coping at 2 and 24 weeks (B = 1.85; SE = 0.62; p = 0.004 and B = –0.39; SE = 0.15; p = 0.01) [68]. Lower use of avoidant coping at week 2 (B = –0.70; SE = 0.29; p = 0.02) [68]. Better QOL and lower anxiety, depression, and PTSD symptoms were maintained longitudinally. Higher frequency of discussion about EOL care preferences (EPC: 21 of 28 [75.0%] vs. SC: 12 of 30 [40.0%]; p = 0.01) and lower frequency of chemotherapy in the last 30 days of life (EPC: 15 of 43 [34.9%] vs. SC: 27 of 41 [65.9%]; p = 0.01). No differences in symptom burden, PHQ-9 scores, or changes in the use of avoidant coping strategies [68], longitudinally. No differences in hospice use, hospice length of stay, or hospitalization in the last week of life. |
| Rodin G [66] 2020 | Single-center phase II trial evaluating feasibility and tolerability, calculation of sample size, and timing of the primary endpoint | 31 pts: 17 EPC 14 SC | EPC: mainly inpatient 8–12 psychotherapeutic sessions, over 8 weeks by a trained mental health clinician (EASE-psy), and systematic screening of physical symptoms (EASE-phys) with triggered referral to PC. PC team: a physician and nurse. First visit within 1 month of inpatient admission. Rare outpatient evaluation SC: PC allowed at request | Primary: severity of traumatic stress symptoms Secondary: physical symptom burden, pain, QOL, depressive symptoms and patients’ satisfaction with care | ESAS-AL SASRQ MSAS BPI FACIT- Sp BDI-II FAMCARE-P16 | Feasibility outcome met Reduced traumatic stress symptoms at 4 and 12 weeks: EASE group: M (SE) = 24.26 (5.63), vs. SC group, M (SE) = 40.13 (5.50), p = 0.048; M (SE) = 21.03 (5.71), vs. SC group, M (SE) = 38.27 (5.46), p = 0.033 Lower pain intensity and pain interference with daily activities at 12 weeks, EASE group: M (SE) = 2.23 (2.66) vs. SC: M (SE) = 9.66 (2.09), p = 0.032. EASE group: M (SE) = 4.68 (6.27) vs. SC: M (SE) = 27.73 (4.88), p = 0.006. Lower rates of pts with ASD or threshold ASD at 12 weeks: EASE group: 7.7% (1/13) vs. SC: 42.1% (8/19), p = 0.05. No differences in physical symptom severity, symptom-related distress, depressive symptoms, satisfaction with care, and overall quality of life. |
| Potenza L [67] 2021 | Single-center observational retrospective | 215 pts: 131 EPC 84 late referrals to PC | EPC: exclusively outpatient One trained physician and one fellow First visit at a median of 5 weeks after the diagnosis. Monthly visits or frequency driven by disease trajectory. At least three visits Late PC: patients with only 1 or 2 visits of PC | Primary: presence of quality indicators of PC and EOL care | 5 indicators of quality for PC [30]: psychological support, assessing and managing pain, GOC and prognosis, ACP, accessing home-care service 14 indicators of quality of EOL care [27] | Higher rates of Assessment and management of pain (EPC 100% vs. LatePC 46%; p = 0.00001) GOC (EPC 71.8% vs. LatePC 43%; p = 0.00001) ACP (EPC 57.3% vs. LatePC 2.3%; p = 0.00001) Home care service (EPC 43.5% vs. LatePC 14.2%; p = 0.00001) Lower rate of Chemotherapy in the last 14 days of life (EPC 2.7% vs. LatePC 13.9%; p = 0.0228) ICU admission and intubation in the last month of life (EPC 0% and 0% vs. LatePC 14.7% and 6.1%; p = 0.0007 and 0.0314) Access to ED ≥2 within 30 days of death (EPC 4% vs. LatePC 23.5%; p = 0.001) Death in acute facilities (EPC 5.3% vs. LatePC 31.4%; p = 0.002) RC transfusion in the last week of life (EPC 49.3% vs. LatePC 28.12%; p = 0.0315). No differences in Hospitalization ≥2 within 30 days of death, hospice length of stay > 7 days, platelet transfusion in the last week of life |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potenza, L.; Borelli, E.; Bigi, S.; Giusti, D.; Longo, G.; Odejide, O.; Porro, C.A.; Zimmermann, C.; Efficace, F.; Bruera, E.; et al. Early Palliative Care in Acute Myeloid Leukemia. Cancers 2022, 14, 478. https://doi.org/10.3390/cancers14030478
Potenza L, Borelli E, Bigi S, Giusti D, Longo G, Odejide O, Porro CA, Zimmermann C, Efficace F, Bruera E, et al. Early Palliative Care in Acute Myeloid Leukemia. Cancers. 2022; 14(3):478. https://doi.org/10.3390/cancers14030478
Chicago/Turabian StylePotenza, Leonardo, Eleonora Borelli, Sarah Bigi, Davide Giusti, Giuseppe Longo, Oreofe Odejide, Carlo Adolfo Porro, Camilla Zimmermann, Fabio Efficace, Eduardo Bruera, and et al. 2022. "Early Palliative Care in Acute Myeloid Leukemia" Cancers 14, no. 3: 478. https://doi.org/10.3390/cancers14030478
APA StylePotenza, L., Borelli, E., Bigi, S., Giusti, D., Longo, G., Odejide, O., Porro, C. A., Zimmermann, C., Efficace, F., Bruera, E., Luppi, M., & Bandieri, E. (2022). Early Palliative Care in Acute Myeloid Leukemia. Cancers, 14(3), 478. https://doi.org/10.3390/cancers14030478








