Health-Related Quality of Life as Assessed by the EQ-5D-5L Predicts Outcomes of Patients Treated with Azacitidine—A Prospective Cohort Study by the AGMT
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analyses
3. Results
3.1. Myeloid Patient Characteristics
3.2. Patients Treated with Azacitidine Reveal Profound Impairments in HRQoL
3.3. Comparison of HRQoL with a Reference Population Matched by Age, Sex and Number of Comorbidities
3.4. IPSS and R-IPSS Prognosticate OS and TTNT
3.5. EQ-5D-5L Composite Scores at Azacitidine Start Provide Added Value to the (R)-IPSS
3.6. EQ-5D-5L Composite Scores at Azacitidine Start Impact Time-to-Event Endpoints
3.7. EQ-5D-5L Composite Scores at Azacitidine Start Prognosticate the Likelihood of Response to Azacitidine
3.8. Longitudinal Assessment of EQ-5D-5L Responses and Clinical Parameters
3.9. Minimally Clinically Important Differences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Demakos, E.P.; Peterson, B.L.; Kornblith, A.B.; Holland, J.C.; Odchimar-Reissig, R.; Stone, R.M.; Nelson, D.; Powell, B.L.; DeCastro, C.M.; et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J. Clin. Oncol. 2002, 20, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- Pleyer, L.; Leisch, M.; Kourakli, A.; Padron, E.; Maciejewski, J.P.; Xicoy Cirici, B.; Kaivers, J.; Ungerstedt, J.; Heibl, S.; Patiou, P.; et al. Outcomes of patients with chronic myelomonocytic leukaemia treated with non-curative therapies: A retrospective cohort study. Lancet Haematol. 2021, 8, e135–e148. [Google Scholar] [CrossRef] [PubMed]
- FDA. Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009; pp. 1–39. Available online: https://www.fda.gov/media/77832/download (accessed on 1 June 2022).
- FDA. Core Patient-Reported Outcomes in Cancer Clinical Trials Guidance for Industry—Draft Guidance. 2021; pp. 1–7. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/core-patient-reported-outcomes-cancer-clinical-trials (accessed on 1 June 2022).
- EMA. Reflection Paper on the Regulatory Guidance for the use of Health-related Quality of Life (HRQoL) Measures in the Evaluation of Medical Products. 27 July 2005. pp. 1–5. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-regulatory-guidance-use-healthrelated-quality-life-hrql-measures-evaluation_en.pdf (accessed on 1 June 2022).
- EMA. Integrating Patients’ Views in Clinical Studies of Anticancer Medicines. 22 April 2016. Available online: https://www.ema.europa.eu/en/news/integrating-patients-views-clinical-studies-anticancer-medicines (accessed on 1 June 2022).
- Malcovati, L.; Hellstrom-Lindberg, E.; Bowen, D.; Ades, L.; Cermak, J.; Del Canizo, C.; Della Porta, M.G.; Fenaux, P.; Gattermann, N.; Germing, U.; et al. Diagnosis and treatment of primary myelodysplastic syndromes in adults: Recommendations from the European LeukemiaNet. Blood 2013, 122, 2943–2964. [Google Scholar] [CrossRef] [PubMed]
- De Swart, L.; Crouch, S.; Hoeks, M.; Smith, A.; Langemeijer, S.; Fenaux, P.; Symeonidis, A.; Cermak, J.; Hellstrom-Lindberg, E.; Stauder, R.; et al. Impact of red blood cell transfusion dose density on progression-free survival in patients with lower-risk myelodysplastic syndromes. Haematologica 2020, 105, 632–639. [Google Scholar] [CrossRef]
- Stauder, R.; Yu, G.; Koinig, K.A.; Bagguley, T.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Cermak, J.; Mittelman, M.; Hellstrom-Lindberg, E.; et al. Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: A European LeukemiaNet study. Leukemia 2018, 32, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Szende, A.; Schaefer, C.; Goss, T.F.; Heptinstall, K.; Knight, R.; Lubbert, M.; Deschler, B.; Fenaux, P.; Mufti, G.J.; Killick, S.; et al. Valuation of transfusion-free living in MDS: Results of health utility interviews with patients. Health Qual. Life Outcomes 2009, 7, 81. [Google Scholar] [CrossRef]
- Oliva, E.N.; Finelli, C.; Santini, V.; Poloni, A.; Liso, V.; Cilloni, D.; Impera, S.; Terenzi, A.; Levis, A.; Cortelezzi, A.; et al. Quality of life and physicians’ perception in myelodysplastic syndromes. Am. J. Blood Res. 2012, 2, 136–147. [Google Scholar]
- Uyl-de Groot, C.A.; Lowenberg, B.; Vellenga, E.; Suciu, S.; Willemze, R.; Rutten, F.F. Cost-effectiveness and quality-of-life assessment of GM-CSF as an adjunct to intensive remission induction chemotherapy in elderly patients with acute myeloid leukemia. Br. J. Haematol. 1998, 100, 629–636. [Google Scholar] [CrossRef]
- Slovacek, L.; Slovackova, B.; Jebavy, L.; Macingova, Z. Psychosocial, health and demographic characteristics of quality of life among patients with acute myeloid leukemia and malignant lymphoma who underwent autologous hematopoietic stem cell transplantation. Sao Paulo Med. J. 2007, 125, 359–361. [Google Scholar] [CrossRef]
- Leunis, A.; Redekop, W.K.; Uyl-de Groot, C.A.; Lowenberg, B. Impaired health-related quality of life in acute myeloid leukemia survivors: A single-center study. Eur. J. Haematol. 2014, 93, 198–206. [Google Scholar] [CrossRef]
- Kurosawa, S.; Yamaguchi, T.; Mori, T.; Kanamori, H.; Onishi, Y.; Emi, N.; Fujisawa, S.; Kohno, A.; Nakaseko, C.; Saito, B.; et al. Patient-reported quality of life after allogeneic hematopoietic cell transplantation or chemotherapy for acute leukemia. Bone Marrow Transplant 2015, 50, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen-Leunis, A.; Redekop, W.K.; Uyl-de Groot, C.A. Which Questionnaire Should Be Used to Measure Quality-of-Life Utilities in Patients with Acute Leukemia? An Evaluation of the Validity and Interpretability of the EQ-5D-5L and Preference-Based Questionnaires Derived from the EORTC QLQ-C30. Value Health 2016, 19, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Mamolo, C.M.; Cappelleri, J.C.; Hoang, C.J.; Kim, R.; Hadfield, A.; Middleton, C.; Rider, A.; Walter, R.B. A real-world, cross-sectional, community survey of symptoms and health-related quality of life of adults with acute myeloid leukemia. Future Oncol. 2019, 15, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Horvath Walsh, L.E.; Rider, A.; Piercy, J.; Pike, J.; Wilson, S.; Pandya, B.J.; Medeiros, B.C. Real-World Impact of Physician and Patient Discordance on Health-Related Quality of Life in US Patients with Acute Myeloid Leukemia. Oncol Ther. 2019, 7, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zeng, X.; Sui, M.; Liu, R.; Tan, R.L.; Yang, J.; Huang, W.; Luo, N. A head-to-head comparison of measurement properties of the EQ-5D-3L and EQ-5D-5L in acute myeloid leukemia patients. Qual. Life Res. 2020, 30, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Peipert, J.D.; Yount, S.E.; Efficace, F.; Loefgren, C.; Pierson, R.; He, J.; Cella, D. Validation of the Functional Assessment of Cancer Therapy-Leukemia instrument in patients with acute myeloid leukemia who are not candidates for intensive therapy. Cancer 2020, 126, 3542–3551. [Google Scholar] [CrossRef]
- Pratz, K.W.; Panayiotidis, P.; Recher, C.; Wei, X.; Jonas, B.A.; Montesinos, P.; Ivanov, V.; Schuh, A.C.; DiNardo, C.D.; Novak, J.; et al. Venetoclax combinations delay the time to deterioration of HRQoL in unfit patients with acute myeloid leukemia. Blood Cancer J. 2022, 12, 71. [Google Scholar] [CrossRef]
- Pleyer, L.; Dohner, H.; Dombret, H.; Seymour, J.F.; Schuh, A.C.; Beach, C.L.; Swern, A.S.; Burgstaller, S.; Stauder, R.; Girschikofsky, M.; et al. Azacitidine for Front-Line Therapy of Patients with AML: Reproducible Efficacy Established by Direct Comparison of International Phase 3 Trial Data with Registry Data from the Austrian Azacitidine Registry of the AGMT Study Group. Int. J. Mol. Sci. 2017, 18, 415. [Google Scholar] [CrossRef]
- Leisch, M.; Pfeilstocker, M.; Stauder, R.; Heibl, S.; Sill, H.; Girschikofsky, M.; Stampfl-Mattersberger, M.; Tinchon, C.; Hartmann, B.; Petzer, A.; et al. Adverse Events in 1406 Patients Receiving 13,780 Cycles of Azacitidine within the Austrian Registry of Hypomethylating Agents-A Prospective Cohort Study of the AGMT Study-Group. Cancers 2022, 14, 2459. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; Le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Sole, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Grochtdreis, T.; Dams, J.; Konig, H.H.; Konnopka, A. Health-related quality of life measured with the EQ-5D-5L: Estimation of normative index values based on a representative German population sample and value set. Eur. J. Health. Econ. 2019, 20, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.; Graf von der Schulenburg, J.M.; Greiner, W. German Value Set for the EQ-5D-5L. Pharmacoeconomics 2018, 36, 663–674. [Google Scholar] [CrossRef]
- Efficace, F.; Gaidano, G.; Breccia, M.; Voso, M.T.; Cottone, F.; Angelucci, E.; Caocci, G.; Stauder, R.; Selleslag, D.; Sprangers, M.; et al. Prognostic value of self-reported fatigue on overall survival in patients with myelodysplastic syndromes: A multicentre, prospective, observational, cohort study. Lancet Oncol. 2015, 16, 1506–1514. [Google Scholar] [CrossRef] [PubMed]
- Starkman, R.; Alibhai, S.; Wells, R.A.; Geddes, M.; Zhu, N.; Keating, M.M.; Leber, B.; Chodirker, L.; Sabloff, M.; Christou, G.; et al. An MDS-specific frailty index based on cumulative deficits adds independent prognostic information to clinical prognostic scoring. Leukemia 2020, 34, 1394–1406. [Google Scholar] [CrossRef]
- Efficace, F.; Cottone, F.; Abel, G.; Niscola, P.; Gaidano, G.; Bonnetain, F.; Anota, A.; Caocci, G.; Cronin, A.; Fianchi, L.; et al. Patient-reported outcomes enhance the survival prediction of traditional disease risk classifications: An international study in patients with myelodysplastic syndromes. Cancer 2018, 124, 1251–1259. [Google Scholar] [CrossRef]
- Efficace, F.; Santini, V.; La Nasa, G.; Cottone, F.; Finelli, C.; Borin, L.; Quaresmini, G.; Di Tucci, A.A.; Volpe, A.; Cilloni, D.; et al. Health-related quality of life in transfusion-dependent patients with myelodysplastic syndromes: A prospective study to assess the impact of iron chelation therapy. BMJ Support Palliat. Care 2016, 6, 80–88. [Google Scholar] [CrossRef]
- Madry, K.; Lis, K.; Tukiendorf, A.; Szwedyk, P.; Kapelko-Slowik, K.; Subocz, E.; Golos, A.; Makowska, W.; Masternak, A.; Kopinska, A.; et al. Low serum albumin level deteriorates prognosis in azacitidine-treated myelodysplastic syndromes patients—Results of the PALG study ‘PolAZA’. Hematology 2021, 26, 556–564. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Corrales-Yepez, M.; Kharfan-Dabaja, M.A.; Al Ali, N.H.; Padron, E.; Rollison, D.E.; Pinilla-Ibarz, J.; Zhang, L.; Epling-Burnette, P.K.; Lancet, J.E.; et al. Hypoalbuminemia is an independent prognostic factor for overall survival in myelodysplastic syndromes. Am. J. Hematol. 2012, 87, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Laribi, K.; Bolle, D.; Alani, M.; Ghnaya, H.; Le Bourdelles, S.; Besancon, A.; Farhi, J.; Denizon, N.; Baugier de Materre, A. Prognostic impact of elevated pretreatment serum ferritin in patients with high-risk MDS treated with azacitidine. Exp. Hematol. 2018, 65, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, P.T.; Kotsianidis, I.; Symeonidis, A.; Pappa, V.; Galanopoulos, A.; Gogos, D.; Karakatsanis, S.; Papadaki, H.; Palla, A.; Hatzimichael, E.; et al. Chronic myelomonocytic leukemia treated with 5-azacytidine—Results from the Hellenic 5-Azacytidine Registry: Proposal of a new risk stratification system. Leuk Lymphoma 2019, 60, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Silveira, D.R.A.; Quek, L.; Santos, I.S.; Corby, A.; Coelho-Silva, J.L.; Pereira-Martins, D.A.; Vallance, G.; Brown, B.; Nardinelli, L.; Silva, W.F.; et al. Integrating clinical features with genetic factors enhances survival prediction for adults with acute myeloid leukemia. Blood Adv. 2020, 4, 2339–2350. [Google Scholar] [CrossRef]
- Palmieri, R.; Othus, M.; Halpern, A.B.; Percival, M.M.; Godwin, C.D.; Becker, P.S.; Walter, R.B. Accuracy of SIE/SIES/GITMO Consensus Criteria for Unfitness to Predict Early Mortality After Intensive Chemotherapy in Adults With AML or Other High-Grade Myeloid Neoplasm. J. Clin. Oncol. 2020, 38, 4163–4174. [Google Scholar] [CrossRef]
- Roussel, M.; Moreau, P.; Hebraud, B.; Laribi, K.; Jaccard, A.; Dib, M.; Slama, B.; Dorvaux, V.; Royer, B.; Frenzel, L.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab for transplantation-eligible patients with newly diagnosed multiple myeloma (CASSIOPEIA): Health-related quality of life outcomes of a randomised, open-label, phase 3 trial. Lancet Haematol. 2020, 7, e874–e883. [Google Scholar] [CrossRef]
- Fizazi, K.; Kramer, G.; Eymard, J.C.; Sternberg, C.N.; de Bono, J.; Castellano, D.; Tombal, B.; Wulfing, C.; Liontos, M.; Carles, J.; et al. Quality of life in patients with metastatic prostate cancer following treatment with cabazitaxel versus abiraterone or enzalutamide (CARD): An analysis of a randomised, multicentre, open-label, phase 4 study. Lancet Oncol. 2020, 21, 1513–1525. [Google Scholar] [CrossRef]
- Devlin, N.; Parkin, D.; Jenssen, B. Economic evaluation compares the costs and consequences of alternative courses of action. In Methods for Analysing and Reporting EQ-5D Data; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- EuroQol (Ed.) Measuring Self-Reported Population Health: An International Perspective Based on Eq-5d; Spring Med Publishing: Gosford, Australia, 2004. [Google Scholar]
- EuroQol. Choosing a Value Set. Available online: https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/valuation/choosing-a-value-set/ (accessed on 14 December 2020).
- Feng, Y.; Devlin, N.; Bateman, A.; Zamora, B.; Parkin, D. The distribution of the EQ-5D-5L index in patients populations. Value Health 2016, 19, A84. [Google Scholar] [CrossRef]
- EuroQol. EQ-5D-3L User Guide, Basic Information on How to Use the EQ-5D-3L Instrument, Version 6.0. December 2018. Available online: https://euroqol.org/publications/user-guides/ (accessed on 14 December 2020).
- EuroQol. EQ-5D-5L User Guide, Basic Information on How to Use the EQ-5D-5L Instrument, Version 3.0. Sept 2019. Available online: https://euroqol.org/publications/user-guides/ (accessed on 14 December 2020).
- Gerlinger, C.; Bamber, L.; Leverkus, F.; Schwenke, C.; Haberland, C.; Schmidt, G.; Endrikat, J. Comparing the EQ-5D-5L utility index based on value sets of different countries: Impact on the interpretation of clinical study results. BMC Res. Notes 2019, 12, 18. [Google Scholar] [CrossRef]
- Zrubka, Z.; Beretzky, Z.; Hermann, Z.; Brodszky, V.; Gulácsi, L.; Rencz, F.; Baji, P.; Golicki, D.; Prevolnik-Rupel, V.; Péntek, M. A comparison of European, Polish, Slovenian and British EQ-5D-3L value sets using a Hungarian sample of 18 chronic diseases. Eur. J. Health Econ. 2019, 20, 119–132. [Google Scholar] [CrossRef]
- Greiner, W.; Claes, C.; Busschbach, J.; Von Der Schulenburg, J.-M.G. Validating the EQ-5D with time trade off for the German population. Eur. J. Health Econ. 2005, 6, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Janssen, M.F.; Birnie, E.; Haagsma, J.A.; Bonsel, G.J. Comparing the Standard EQ-5D Three-Level System with a Five-Level Version. Value Health 2008, 11, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Pickard, A.S.; Kohlmann, T.; Janssen, M.F.; Bonsel, G.; Rosenbloom, S.; Cella, D. Evaluating equivalency between response systems: Application of the Rasch model to a 3-level and 5-level EQ-5D. Med. Care 2007, 45, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Devlin, N.J.; Krabbe, P.F. The development of new research methods for the valuation of EQ-5D-5L. Eur J Health Econ 2013, 14 (Suppl. 1), S1–S3. [Google Scholar] [CrossRef]
- Oppe, M.; Devlin, N.J.; van Hout, B.; Krabbe, P.; de Charro, F. A Program of Methodological Research to Arrive at the New International EQ-5D-5L Valuation Protocol. Value Health 2014, 17, 445–453. [Google Scholar] [CrossRef]
- NICE. Position Statement on Use of the EQ-5D-5L Value Set for England (Updated October 2019). Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l#:~:text=We%20do%20not%20recommend%20using,set%20for%20reference%2Dcase%20analyses (accessed on 22 December 2020).
- Huber, M.B.; Felix, J.; Vogelmann, M.; Leidl, R. Health-Related Quality of Life of the General German Population in 2015: Results from the EQ-5D-5L. Int. J. Environ. Res. Public Health 2017, 14, 426. [Google Scholar] [CrossRef] [PubMed]
- Konig, H.H.; Bernert, S.; Angermeyer, M.C. Health Status of the German population: Results of a representative survey using the EuroQol questionnaire. Gesundheitswesen 2005, 67, 173–182. [Google Scholar]
- Hinz, A.; Klaiberg, A.; Brahler, E.; Konig, H.H. The Quality of Life Questionnaire EQ-5D: Modelling and norm values for the general population. Psychother. Psychosom. Med. Psychol. 2006, 56, 42–48. [Google Scholar] [CrossRef]
- Hinz, A.; Kohlmann, T.; Stöbel-Richter, Y.; Zenger, M.; Brähler, E. The quality of life questionnaire EQ-5D-5L: Psychometric properties and normative values for the general German population. Qual. Life Res. 2014, 23, 443–447. [Google Scholar] [CrossRef]
- van Hout, B.; Janssen, M.F.; Feng, Y.S.; Kohlmann, T.; Busschbach, J.; Golicki, D.; Lloyd, A.; Scalone, L.; Kind, P.; Pickard, A.S. Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 2012, 15, 708–715. [Google Scholar] [CrossRef]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Henry, E.B.; Barry, L.E.; Hobbins, A.P.; McClure, N.S.; O’Neill, C. Estimation of an Instrument-Defined Minimally Important Difference in EQ-5D-5L Index Scores Based on Scoring Algorithms Derived Using the EQ-VT Version 2 Valuation Protocols. Value Health 2020, 23, 936–944. [Google Scholar] [CrossRef]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]


| First Author | Year Published | Patients, n | Disease | EQ-5D, Type | EQ-VAS, Mean (SD) | Index Value, Mean (SD) | Index Value, Median (IQR) | Impact on Time-to-Event Endpoint |
|---|---|---|---|---|---|---|---|---|
| MDS | ||||||||
| Szende A. [11] | 2009 | 47 | MDS | 3L | NR | 0.78 (NR) | NR | NR |
| Oliva E. [12] | 2012 | 148 | MDS | 3L | 60 (20) | NR | 0.74 (0.62–0.85) | NR |
| Stauder R. [10] | 2018 | 1683 | Lower-risk MDS | 3L | 69.6 (20.1) | 0.74 (0.23) | NR | NR |
| de Swart L. [9] | 2020 | NR | Lower-risk MDS | 3L | 70.5 (19.7) | NR | NR | EQ-5D-3L index was significantly associated with progression-free survival in univariate analysis |
| Pleyer L. (this article) | 2023 | 162 | MDS/CMML | 5L | 64.4 (21.2) | 0.79 (0.3) | 0.88 (0.73–0.95) | EQ-5D-5L index, LSS and EQ-VAS were significantly associated with overall survival and the likelihood to respond to azacitidine in univariate analysis; EQ-5D-5L index was significantly associated with overall survival, time with clinical benefit and time to next treatment in multivariate-adjusted analyses. LSS was significantly associated with the likelihood to respond to azacitidine in multivariate analysis. |
| AML | ||||||||
| Uyl-de Groot C.A. [13] | 1998 | NR NR | AML | 3L | 70.6 (NR) 64.8 (NR) | NR NR | NR NR | NR |
| Slovacek L. [14] | 2007 | NR | AML | 3L | 67.5 (NR) | NR | NR | NR |
| Leunis A. [15] | 2014 | 88 | AML | 3L | 74.6 (17.4) | 0.82 (17.4) | NR | NR |
| Kurosowa S. [16] | 2015 | 392 | AML | 3L | NR | NR | NR | NR |
| van Dongen-Leunis, A. [17] | 2016 | 111 | AML | 5L | NR | 0.81 (0.22) | 0.87 (NR-NR) | NR |
| Mamolo C. [18] | 2019 | NR | AML | 3L | 61.2 (NR) | 0.74 (NR) | NR | NR |
| Horvath Walsh L. [19] | 2019 | 75 | AML | 3L | 61.2 (NR) | 0.74 | NR | NR |
| Yu H. [20] | 2020 | NR/168 NR/168 | AML | 3L 5L | 76.9 (15.1) | 0.829 (0.16) 0.786 (0.25) | NR NR | NR |
| Peipert J. [21] | 2020 | 307 | AML | 5L | 61.9 (20.1) | 0.67 (0.26) | NR | NR |
| Pratz K.W. [22] | 2022 | 642 | AML | 5L | NR | NR | NR | NR |
| Pleyer L. (this article) | 2023 | 110 | AML | 5L | 64.7 (21.7) | 0.83 (0.2) | 0.89 (0.76–0.98) | EQ-5D-5L index, LSS and EQ-VAS were significantly associated with overall survival and the likelihood to respond to azacitidine in univariate analysis; EQ-5D-5L index was significantly associated with overall survival, time with clinical benefit and time to next treatment in multivariate-adjusted analyses. LSS was significantly associated with the likelihood to respond to azacitidine in multivariate analysis. |
| Mobility Problem 2 | Selfcare Problem 2 | Usual Activities Problem 2 | Pain/Discomfort Problem 2 | Anxiety/Depression Problem 2 | Level Sum Score 3 | Index Value 4 | EQ-VAS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/n (%) | p 5 | n/n (%) | p 5 | n/n (%) | p 5 | n/n (%) | p 5 | n/n (%) | p 5 | n | Mean (SD) | p 5 | n | Mean (SD) | p 6 | n | Mean (SD) | p 6 | |
| Total cohort | |||||||||||||||||||
| 1st available EQ-5D | 136/272 (50.0) | NA | 68/72 (25.0) | NA | 150/272 (55.1) | NA | 138/272 (50.7) | NA | 125/272 (46.0) | NA | 266 | 9.1 (3.9) | NA | 266 | 0.807 (0.232) | NA | 263 | 63.9 (21.6) | NA |
| EQ-5D in cycle 1 or 2 | 104/205 (50.7) | NA | 46/205 (22.4) | NA | 120/205 (58.5) | NA | 102/205 (49.8) | NA | 100/205 (48.8) | NA | 200 | 9.2 (3.9) | NA | 198 | 0.810 (0.229) | NA | 200 | 64.5 (21.4) | NA |
| Disease-related parameters 1 | |||||||||||||||||||
| Azacitidine ≥2nd line: No Yes | 75/145 (52.1) 29/59 (49.2) | 0.7045 | 35/143 (24.5) 11/59 (18.6) | 0.3688 | 86/141 (61.0) 34/59 (557.6) | 0.6577 | 76/143 (53.1) 26/59 (44.1) | 0.2406 | 72/143 (50.3) 28/59 (47.5) | 0.7085 | 141 59 | 9.3 (4.0) 8.7 (3.5) | 0.3288 | 141 59 | 0.800 (0.243) 0.831 (0.192) | 0.4282 | 141 57 | 63.3 (22.0) 67.5 (19.7) | 0.2136 |
| Diagnosis: MDS or CMML AML | 59/112 (52.7) 45/91 (49.5) | 0.6472 | 28/111 (25.2) 18/91 (19.8) | 0.3585 | 66/109 (60.6) 54/91 (59.3) | 0.8619 | 66/111 (59.5) 36/91 (39.6) | 0.0049 | 53/111 (47.4) 47/91 (51.6) | 0.5812 | 109 91 | 9.4 (4.0) 8.8 (3.6) | 0.2921 | 109 91 | 0.788 (0.256) 0.835 (0.192) | 0.2160 | 110 88 | 64.4 (21.2) 64.7 (21.7) | 0.9440 |
| Treatment-related disease: No Yes | 89/175 (50.9) 12/24 (50.0) | 0.9372 | 39/174 (22.4) 6/24 (25.0) | 0.7769 | 102/172 (59.3) 14/24 (58.3) | 0.9279 | 84/174 (48.3) 15/24 (62.5) | 0.1914 | 79/174 (45.4) 17/24 (70.8) | 0.0194 | 172 24 | 9.1 (3.9) 9.5 (3.7) | 0.4741 | 172 24 | 0.810 (0.238) 0.809 (0.182) | 0.4869 | 170 24 | 64.7 (21.8) 64.4 (19.9) | 0.7998 |
| IPSS: Low or intermediate-1 Intermediate-2 or high | 39/72 (54.2) 62/125 (49.6) | 0.5369 | 17/71 (23.9) 27/125 (21.6) | 0.7055 | 40/69 (58.0) 75/125 (60.0) | 0.7830 | 39/71 (54.9) 58/125 (46.4) | 0.2510 | 32/71 (45.1) 64/125 (51.2) | 0.4093 | 69 125 | 9.3 (4.2) 8.9 (3.5) | 0.7663 | 69 125 | 0.789 (0.274) 0.836 (0.169) | 0.7950 | 70 122 | 65.6 (20.7) 64.6 (21.8) | 0.6783 |
| R-IPSS: Very low or low Intermediate, poor, very poor | 11/26 (42.3) 90/169 (53.3) | 0.2984 | 6/26 (23.1) 39/168 (23.2) | 0.9877 | 13/26 (50.0) 102/166 (61.4) | 0.2682 | 15/26 (57.7) 82/168 (48.8) | 0.3992 | 12/26 (46.2) 84/168 (50.0) | 0.7151 | 26 166 | 9.3 (5.0) 9.1 (3.6) | 0.5927 | 26 166 | 0.758 (0.369) 0.821 (0.188) | 0.7551 | 25 165 | 64.6 (21.8) 64.7 (21.1) | 0.9609 |
| IPSS cytogenetic risk: good Intermediate or poor | 60/125 (48.0) 34/56 (60.7) | 0.1135 | 29/124 (23.4) 14/56 (25.0) | 0.8143 | 67/123 (54.5) 35/55 (63.6) | 0.2534 | 62/124 (50.0) 29/56 (51.8) | 0.8244 | 64/124 (50.0) 27/56 (48.2) | 0.6729 | 123 55 | 9.0 (3.9) 9.5 (3.8) | 0.2706 | 123 55 | 0.814 (0.228) 0.806 (0.216) | 0.3255 | 122 54 | 65.7 (21.4) 64.1 (21.1) | 0.6006 |
| Peripheral blood blasts: <10% ≥10% | 78/156 (50.0) 26/47 (55.3) | 0.5225 | 34/155 (21.9) 12/47 (25.5) | 0.6065 | 94/153 (61.4) 26/47 (55.3) | 0.4539 | 83/155 (53.5) 19/47 (40.4) | 0.1150 | 77/155 (49.7) 23/47 (48.9) | 0.9291 | 153 47 | 9.3 (4.0) 8.8 (3.3) | 0.7245 | 153 47 | 0.798 (0.246) 0.847 (0.162) | 0.4270 | 153 45 | 64.8 (20.9) 63.8 (23.1) | 0.7879 |
| Monocytes: <10% ≥10% | 56/121 (46.3) 44/75 (58.7) | 0.0918 | 23/121 (19.0) 22/74 (29.7) | 0.0846 | 61/119 (51.3) 52/74 (70.3) | 0.0091 | 60/121 (49.6) 41/74 (55.4) | 0.4301 | 52/121 (43.0) 43/74 (58.1) | 0.0402 | 119 74 | 8.5 (3.5) 10.1 (4.3) | 0.0053 | 119 74 | 0.850 (0.193) 0.752 (0.267) | 0.0052 | 118 73 | 67.7 (19.8) 61.5 (22.5) | 0.0626 |
| Haemoglobin: <10.0 g/dL ≥10.0 g/dL | 81/142 (57.0) 23/61 (37.7) | 0.0115 | 37/141 (26.2) 9/61 (14.8) | 0.0739 | 89/139 (64.0) 31/61 (50.8) | 0.0792 | 73/141 (51.8) 29/61 (47.5) | 0.5807 | 71/141 (50.4) 29/61 (47.5) | 0.7135 | 139 61 | 9.5 (4.0) 8.3 (3.5) | 0.0295 | 139 61 | 0.790 (0.242) 0.855 (0.191) | 0.0429 | 137 61 | 62.8 (21.0) 68.5 (21.8) | 0.0545 |
| Red blood cell transfusions: ≤3 >3 | 62/138 (44.9) 22/26 (84.6) | 0.0002 | 31/137 (22.6) 8/26 (30.8) | 0.3724 | 75/135 (55.6) 20/26 (76.9) | 0.0425 | 73/137 (53.3) 16/26 (61.5) | 0.4384 | 64/137 (46.7) 15/26 (57.7) | 0.3045 | 135 26 | 9.0 (4.0) 9.9 (3.2) | 0.0723 | 135 26 | 0.809 (0.247) 0.864 (0.163) | 0.1412 | 134 25 | 65.6 (21.7) 55.2 (17.6) | 0.0147 |
| Platelet count: <100 G/L ≥100 G/L | 36/65 (55.4) 68/138 (49.3) | 0.4165 | 17/65 (26.2) 29/137 (21.2) | 0.4299 | 39/63 (61.9) 81/137 (59.1) | 0.7092 | 34/65 (52.3) 68/137 (49.6) | 0.7226 | 30/65 (46.2) 70/137 (51.1) | 0.5117 | 63 137 | 9.4 (4.0) 9.0 (3.8) | 0.4665 | 61 137 | 0.797 (0.254) 0.815 (0.218) | 0.4980 | 64 134 | 65.8 (19.9) 63.9 (22.1) | 0.6100 |
| Patient-related parameters 1 | |||||||||||||||||||
| Sex male: No Yes | 45/81 (55.6) 59/122 (48.4) | 0.3152 | 21/81 (25.9) 25/121 (20.7) | 0.3819 | 49/79 (62.0) 71/121 (58.7) | 0.6366 | 44/81 (54.3) 58/121 (47.9) | 0.3735 | 47/81 (58.0) 53/121 (43.8) | 0.0475 | 79 121 | 9.6 (4.0) 8.9 (3.7) | 0.1644 | 79 121 | 0.786 (0.261) 0.825 (0.206) | 0.2445 | 77 121 | 66.3 (21.9) 63.4 (21.1) | 0.2408 |
| Age ≥75 yrs: No Yes | 47/105 (44.8) 57/98 (58.2) | 0.0563 | 19/104 (18.3) 27/89 (27.6) | 0.1159 | 64/103 (62.1) 56/97 (57.7) | 0.5252 | 44/104 (42.3) 59/98 (59.2) | 0.0165 | 51/104 (49.0) 49/98 (50.0) | 0.8913 | 103 97 | 8.7 (3.4) 9.6 (4.2) | 0.2478 | 103 97 | 0.832 (0.191) 0.785 (0.263) | 0.2429 | 103 95 | 66.9 (21.0) 60.0 (21.6) | 0.1083 |
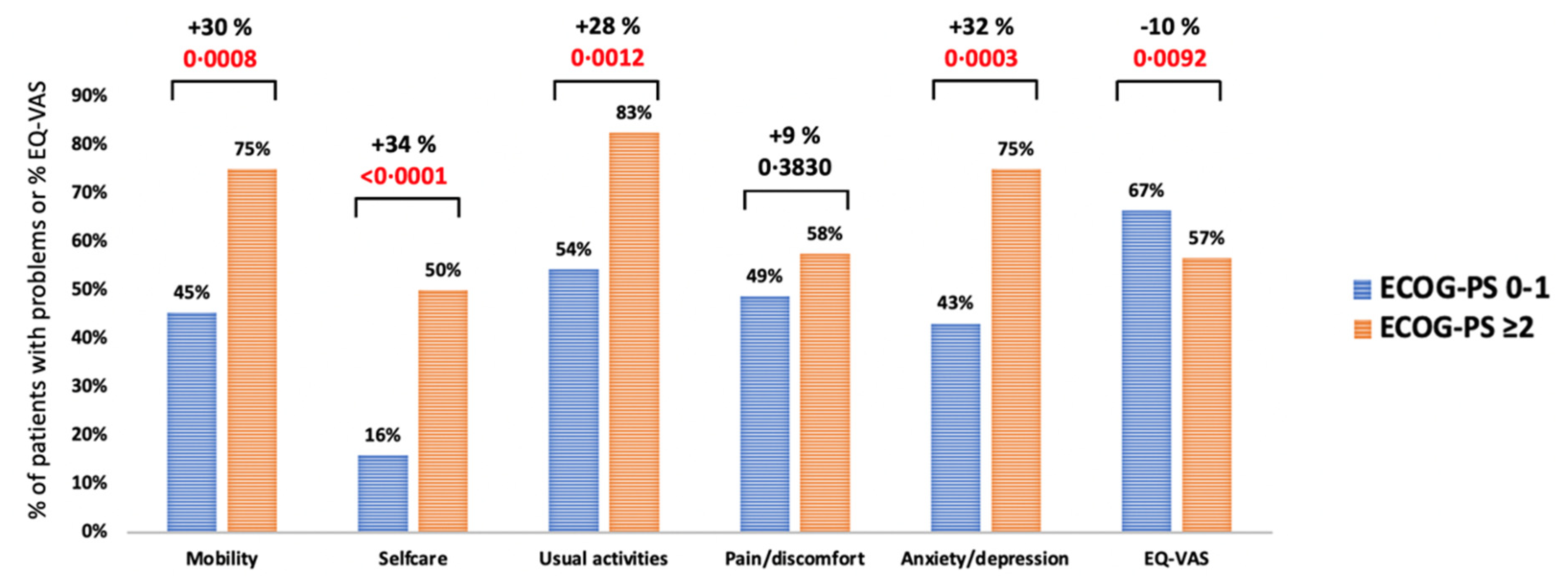
| ECOG-PS: 0–1 ≥2 | 74/163 (45.4) 30/40 (75.0) | 0.0008 | 26/162 (16.0) 20/40 (50.0) | <0.0001 | 87/160 (54.4) 33/40 (82.5) | 0.0012 | 79/162 (48.8) 23/40 (57.5) | 0.3224 | 70/162 (43.2) 30/40 (75.0) | 0.0003 | 160 40 | 8.4 (3.4) 12.0 (4.3) | <0.0001 | 160 40 | 0.847 (0.185) 0.659 (0.315) | <0.0001 | 159 39 | 66.5 (20.8) 56.6 (22.3) | 0.0092 |
| HCT-CI: Low risk Intermediate risk High risk | 31/77 (40.3) 33/65 (50.8) 40/61 (65.6) | 0.0127 | 13/77 (16.9) 12/65 (18.5) 21/60 (35.0) | 0.0259 | 40/75 (53.3) 36/65 (55.4) 44/60 (73.3) | 0.0406 | 38/77 (49.4) 26/65 (40.0) 38/60 (63.3) | 0.0324 | 36/77 (46.8) 30/65 (46.2) 34/60 (56.7) | 0.4155 | 75 65 60 | 8.3 (3.3) 8.9 (3.7) 10.4 (4.4) | 0.0133 | 75 65 60 | 0.849 (0.186) 0.822 (0.224) 0.748 (0.271) | 0.0189 | 75 64 59 | 67.8 (20.1) 65.4 (21.0) 59.5 (22.7) | 0.0750 |
| No. of comorbidities: 0–1 ≥2 | 52/116 (44.8) 52/87 (59.8) | 0.0350 | 23/116 (19.8) 23/86 (26.7) | 0.2464 | 66/114 (57.9) 54/86 (62.8) | 0.4841 | 57/116 (49.1) 45/86 (52.3) | 0.6541 | 52/116 (44.8) 48/86 (55.8) | 0.1225 | 114 86 | 8.7 (3.5) 9.8 (4.3) | 0.0689 | 114 86 | 0.839 (0.183) 0.770 (0.276) | 0.0703 | 113 85 | 66.8 (21.0) 61.6 (21.6) | 0.0829 |
| Mobility Problem 3 | Selfcare Problem 3 | Usual Activities Problem 3 | Pain/Discomfort Problem 3 | Anxiety/Depression Problem 3 | Index Value | EQ-VAS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/n (%) | p4 | n/n (%) | p4 | n/n (%) | p4 | n/n (%) | p4 | n/n (%) | p4 | n | Mean (SD) | p5 | n | Mean (SD) | p5 | |
| Total cohort Austrian Registry German Norm | 136/269 (50.6) 1772/5001 (35.4) | <0.0001 | 68/268 (25.4) 360/5001 (7.2) | <0.0001 | 150/266 (56.4) 1417/5001 (28.3) | <0.0001 | 138/268 (51.5) 2847/5001 (56.9) | 0.0802 | 125/269 (46.5) 1256/5001 (25.1) | <0.0001 | 266 5001 | 0.81 (0.23) 0.88 (0.18) | <0.0001 | 260 4997 | 63.9 (21.6) 71.6 (21.4) | <0.0001 |
| ≥75 years Austrian Registry German Norm | 74/130 (56.9) 399/593 (67.3) | 0.0245 | 39/130 (30.0) 111/593 (18.7) | 0.0041 | 71/129 (55.0) 281/593 (47.4) | 0.1151 | 75/130 (57.7) 418/593 (70.5) | 0.0046 | 59/131 (45.0) 160/593 (27.0) | <0.0001 | 129 593 | 0.79 (0.25) 0.80 (0.28) | 0.7547 | 127 590 | 61.7 (22.4) 60.9 (26.2) | 0.7662 |
| 65 < 75 years Austrian Registry German Norm | 50/105 (47.6) 324/654 (46.1) | 0.7146 | 22/105 (21.0) 69/654 (10.6) | 0.0023 | 60/104 (57.7) 198/654 (30.3) | <0.0001 | 49/105 (46.7) 411/654 (62.8) | 0.0016 | 49/105 (46.7) 158/654 (24.2) | <0.0001 | 104 654 | 0.84 (0.19) 0.85 (0.240 | 0.5650 | 102 654 | 66.8 (19.3) 66.1 (25.5) | 0.7777 |
| <65 years Austrian Registry German Norm | 12/34 (35.3) 1049/3754 (27.9) | 0.3420 | 7/33 (21.2) 180/3754 (4.8) | <0.0001 | 19/33 (57.6) 938/3754 (25.0) | <0.0001 | 14/33 (42.4) 2017/3754 (53.7) | 0.1948 | 17/33 (51.5) 938/3754 (25.0) | 0.0005 | 33 3754 | 0.77 (0.26) 0.90 (0.15) | <0.0001 | 34 3753 | 63.5 (24.0) 74.2 (19.1) | 0.0011 |
| Females Austrian Registry German Norm | 59/103 (57.3) 980/2584 (37.9) | <0.0001 | 30/103 (29.1) 203/2584 (7.9) | <0.0001 | 63/101 (62.4) 789/2584 (30.5) | <0.0001 | 60/103 (58.3) 1497/2584 (57.9) | 0.9487 | 56/103 (54.5) 734/2584 (28.4) | <0.0001 | 101 2584 | 0.78 (0.26) 0.86 (0.20) | <0.0001 | 98 2581 | 64.6 (21.8) 71.1 (22.2) | 0.0048 |
| Males Austrian Registry German Norm | 77/166 (46.4) 791/2417 (32.7) | 0.0003 | 38/165 (23.0) 157/2417 (6.5) | <0.0001 | 86/165 (52.7) 628/2417 (26.0) | <0.0001 | 78/165 (47.3) 1350/2417 (55.9) | 0.0319 | 69/166 (41.6) 522/2417 (21.6) | <0.0001 | 165 2417 | 0.83 (0.21) 0.90 (0.16) | <0.0001 | 165 2416 | 63.5 (21.5) 72.1 (20.5) | <0.0001 |
| One comorbidity Austrian Registry German Norm | 24/66 (36.4) 455/1432 (31.8) | 0.4344 | 9/66 (13.6) 74/1432 (5.2) | 0.0033 | 31/64 (48.4) 361/1433 (25.2) | <0.0001 | 32/66 (48.5) 813/1432 (56.8) | 0.1843 | 31/67 (46.3) 317/1432 (22.1) | <0.0001 | 64 1432 | 0.87 (0.17) 0.90 (0.15) | 0.0861 | 64 1432 | 66.3 (22.8) 73.0 (19.2) | 0.0067 |
| Two comorbidities Austrian Registry German Norm | 42/85 (50.6) 378/820 (46.1) | 0.4295 | 23/85 (27.1) 74/821 (9.0) | <0.0001 | 49/85 (57.7) 294/821 (35.8) | <0.0001 | 43/85 (50.6) 570/821 (69.4) | 0.0004 | 31/85 (37.7) 245/821 (29.8) | 0.1370 | 85 821 | 0.82 (0.21) 0.85 (0.18) | 0.1154 | 83 821 | 65.7 (20.6) 65.1 (21.9) | 0.7841 |
| ≥Three comorbidities Austrian Registry German Norm | 69/118 (58.5) 627/870 (72.1) | 0.0024 | 36/117 (30.8) 179/871 (20.6) | 0.0119 | 70/117 (59.8) 536/870 (61.6) | 0.7104 | 63/117 (53.9) 748/871 (85.9) | <0.0001 | 62/117 (53.0) 374/871 (42.9) | 0.0398 | 117 871 | 0.77 (0.27) 0.72 (0.28) | 0.0944 | 116 871 | 61.3 (21.4) 55.2 (24.0) | 0.0093 |
| (R)-IPSS | (R)-IPSS + LSS | (R)-IPSS + EQ-VAS | (R)-IPSS + Index | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Months [95% CI] 1 | LHR | p 6 | LHR | p 6 | LHR | p 6 | LHR | p 6 | |
| Overall survival | |||||||||
|
IPSS: Lower-risk 2 Higher-risk 3 | 21.0 [14.6–30.3] 12.8 [10.2–16.9] | 7.3195 | 0.0068 | 10.6911 | 0.0048 | 11.5552 | 0.0031 | 13.0219 | 0.0015 |
|
R-IPSS: Lower-risk 4 Higher-risk 5 | 30.3 [11.2–39.3] 14.6 [11.9–17.8] | 5.3691 | 0.0205 | 9.0542 | 0.0108 | 10.2840 | 0.0058 | 13.4753 | 0.0012 |
| Time with clinical benefit | |||||||||
|
IPSS: Lower-risk 2 Higher-risk 3 | 8.9 [5.6–13.1] 7.9 [5.2–9.6] | 1.0693 | 0.3011 | 3.6196 | 0.1637 | 1.9171 | 0.3835 | 3.6196 | 0.1637 |
|
R-IPSS: Lower-risk 4 Higher-risk 5 | 7.8 [3.4–14.9] 8.0 [6.4–9.6] | 0.0757 | 0.7832 | 4.0208 | 0.1339 | 1.5603 | 0.4583 | 4.0208 | 0.1339 |
| Time to next treatment | |||||||||
|
IPSS: Lower-risk 2 Higher-risk 3 | 14.6 [9.5–19.3] 11.3 [8.9–12.6] | 3.5998 | 0.0578 | 5.7236 | 0.0572 | 4.7933 | 0.0910 | 6.3834 | 0.0411 |
|
R-IPSS: Lower-risk 4 Higher-risk 5 | 17.6 [6.9–37.7] 10.8 [9.3–12.6] | 4.3114 | 0.0379 | 7.7372 | 0.0209 | 6.8408 | 0.0327 | 6.5489 | 0.0378 |
| Univariate (n = 205) | Multivariate 4 (n = 205) | |||||
|---|---|---|---|---|---|---|
| Months [95% CI] | p | HR [95% CI] | Months [95% CI] | p | HR [95% CI] | |
| Overall Survival | ||||||
|
Level Sum Score: <median 1 ≥median | 19.3 [14.6–21.5] 12.4 [8.7–15.0] | 0.0407 | 1.408 [1.013–1.956] | 16.9 [12.9–37.4] 14.2 [11.7–17.8] | 0.2286 | 1.234 [0.876–1.737] |
|
EQ-VAS (health today): ≥median 2 <median | 17.9 [13.8–21.3] 12.8 [8.7–16.8] | 0.0141 | 1.511 [1.084–2.106] | 16.9 [12.9–30.6] 14.0 [11.4–24.7] | 0.2293 | 1.242 [0.872–1.769] |
|
EQ-5D-5L index: ≥median 3 <median | 18.5 [15.0–21.0] 11.9 [8.5–14.9] | 0.0093 | 1.536 [1.109–2.127] | 17.9 [14.0–21.0] 12.9 [10.3–16.8] | 0.0143 | 1.523 [1.088–2.131] |
| Time with Clinical Benefit | ||||||
|
Level Sum Score: <median 1 ≥median | 10.2 [6.6–13.2] 6.1 [4.3–8.2] | 0.0573 | 1.340 [0.989–1.815] | 8.7 [6.5–11.8] 6.8 [5.2–8.8] | 0.2174 | 1.221 [0.889–1.677] |
|
EQ-VAS (health today): ≥median 2 <median | 9.6 [6.6–12.1] 6.7 [4.6–8.5] | 0.1841 | 1.227 [0.906–1.662] | 8.4 [6.4–11.4] 7.7 [5.6–9.6] | 0.5233 | 1.111 [0.998–1.012] |
|
EQ-5D-5L index: ≥median 3 <median | 10.2 [7.2–12.8] 6.1 [4.0–8.2] | 0.0134 | 1.456 [1.078–1.966] | 9.6 [6.8–12.1] 6.6 [4.9–8.5] | 0.0258 | 1.425 [1.044–1.945] |
| Time to Next Treatment | ||||||
|
Level Sum Score: <median 1 ≥median | 13.5 [9.8–17.6] 9.4 [7.6–11.9] | 0.0633 | 1.347 [0.982–1.846] | 12.6 [10.2–16.5] 10.8 [8.9–12.6] | 0.1144 | 1.302 [0.938–1.806] |
|
EQ-VAS (health today): ≥median 2 <median | 12.6 [9.4–16.8] 11.1 [8.5–12.8] | 0.1034 | 1.305 [0.946–1.801] | 11.9 [9.7–14.6] 11.1 [9.0–20.2] | 0.4197 | 1.150 [0.819–1.614] |
|
EQ-5D-5L index: ≥median 3 <median | 13.1 [10.8–17.4] 9.2 [6.7–11.9] | 0.0414 | 1.383 [1.011–1.890] | 12.8 [10.5–20.2] 9.8 [8.5–11.9] | 0.0332 | 1.420 [1.028–1.962] |
| Univariate p | Multivariate 4 p | Multivariate 4 OR [95% CI] | |
|---|---|---|---|
| Level Sum Score: ≥ vs. < median 1 | 0.0009 | 0.0160 | 0.451 [0.235–0.852] |
| EQ-VAS: < vs. ≥ median 2 | 0.0237 | 0.1065 | 0.590 [0.321–1.116] |
| EQ-5D-5L index: < vs. ≥ median 3 | 0.0110 | 0.0627 | 0.522 [0.296–1.032] |
| Mobility | Selfcare | Usual Activities | Pain/Discomfort | Anxiety/Depression | Level Sum Score 2 | EQ-VAS | EQ-5D-5L Index | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
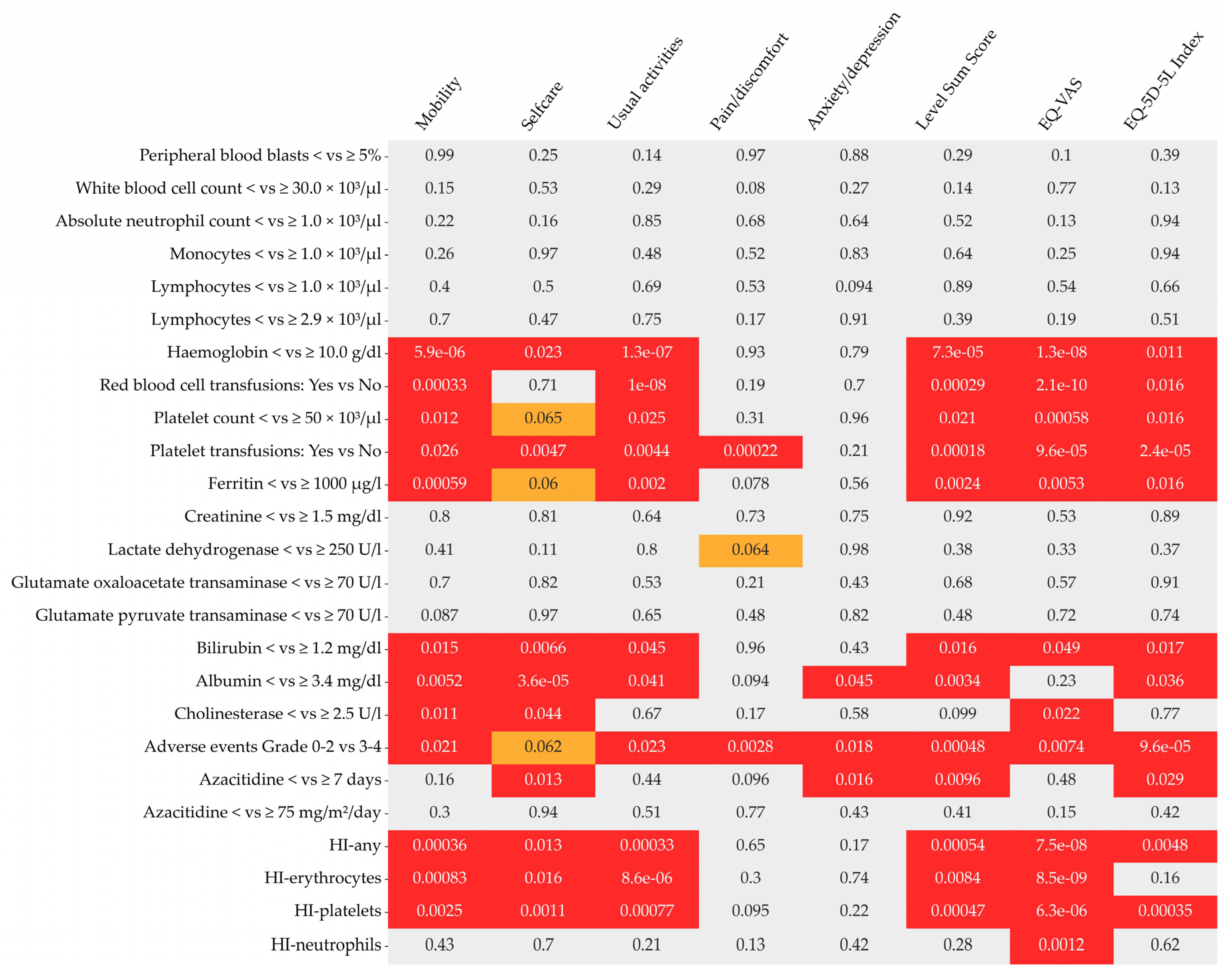
| Differential blood count | n 3 | p | n | p | n | p | n | p | n | p | n | p | n | p | n | p |
| Peripheral blood blasts< vs. ≥5% | 1425 | 0.9897 | 1417 | 0.2548 | 1417 | 0.1447 | 1421 | 0.9703 | 1416 | 0.8775 | 1395 | 0.2930 | 1365 | 0.0996 | 1395 | 0.3916 |
| White blood cell count< vs. ≥30.0 G/L | 1429 | 0.1502 | 1421 | 0.5278 | 1421 | 0.2869 | 1425 | 0.0801 | 1420 | 0.2674 | 1399 | 0.1371 | 1368 | 0.7712 | 1399 | 0.1272 |
| Absolute neutrophil count< vs. ≥1.0 G/L | 1415 | 0.2206 | 1407 | 0.1586 | 1407 | 0.8529 | 1411 | 0.6784 | 1406 | 0.6362 | 1385 | 0.5171 | 1355 | 0.1329 | 1385 | 0.9389 |
| Monocytes< vs. ≥1.0 G/L | 1417 | 0.2559 | 1409 | 0.9738 | 1409 | 0.4770 | 1413 | 0.5203 | 1408 | 0.8287 | 1387 | 0.6366 | 1357 | 0.2476 | 1387 | 0.9439 |
| Lymphocytes< vs. ≥1.0 G/L | 1402 | 0.4021 | 1394 | 0.5043 | 1394 | 0.6879 | 1398 | 0.5349 | 1393 | 0.0941 | 1372 | 0.8871 | 1343 | 0.5429 | 1372 | 0.6557 |
| Haemoglobin< vs. ≥10.0 g/dL | 1429 | <0.0001 | 1421 | 0.0227 | 1421 | <0.0001 | 1425 | 0.9289 | 1420 | 0.7871 | 1399 | <0.0001 | 1368 | <0.0001 | 1399 | 0.0110 |
| Red blood cell transfusions: Yes vs. No | 1429 | 0.0003 | 1421 | 0.7072 | 1421 | <0.0001 | 1425 | 0.1935 | 1420 | 0.6996 | 1399 | 0.0003 | 1368 | <0.0001 | 1399 | 0.0161 |
| Platelet count< vs. ≥50 G/L | 1429 | 0.0122 | 1421 | 0.0647 | 1421 | 0.0248 | 1425 | 0.3142 | 1420 | 0.9574 | 1399 | 0.0212 | 1368 | 0.0006 | 1399 | 0.0156 |
| Platelet transfusions: Yes vs. No | 1429 | 0.0257 | 1421 | 0.0047 | 1421 | 0.0044 | 1425 | 0.0002 | 1420 | 0.2067 | 1399 | 0.0002 | 1368 | <0.0001 | 1399 | <0.0001 |
| Comorbidity/toxicity | ||||||||||||||||
| Ferritin< vs. ≥1000 µg/L | 723 | 0.0006 | 720 | 0.0598 | 720 | 0.0020 | 722 | 0.0785 | 718 | 0.5635 | 709 | 0.0024 | 703 | 0.0053 | 709 | 0.0163 |
| Creatinine< vs. ≥1.5 mg/dL | 1417 | 0.7976 | 1409 | 0.8133 | 1409 | 0.6386 | 1413 | 0.7286 | 1408 | 0.7550 | 1387 | 0.9162 | 1356 | 0.5338 | 1387 | 0.8874 |
| Lactate dehydrogenase, U/L | 1399 | 0.4066 | 1391 | 0.1095 | 1392 | 0.7977 | 1395 | 0.0642 | 1390 | 0.9778 | 1370 | 0.3834 | 1337 | 0.3343 | 1370 | 0.3673 |
| Glutamate oxaloacetate transaminase, U/L | 1406 | 0.7039 | 1398 | 0.8181 | 1399 | 0.5276 | 1402 | 0.2078 | 1397 | 0.4316 | 1377 | 0.6822 | 1345 | 0.5734 | 1377 | 0.9119 |
| Glutamate pyruvate transaminase, U/L | 1348 | 0.0867 | 1340 | 0.9662 | 1340 | 0.6501 | 1344 | 0.4822 | 1339 | 0.8201 | 1318 | 0.4770 | 1288 | 0.7212 | 1318 | 0.7369 |
| Bilirubin< vs. ≥1.2 mg/dL | 1407 | 0.0149 | 1399 | 0.0066 | 1399 | 0.0451 | 1403 | 0.9600 | 1398 | 0.4338 | 1377 | 0.0158 | 1346 | 0.0494 | 1377 | 0.0170 |
| Albumin< vs. ≥3.4 mg/dL | 583 | 0.0052 | 579 | <0.0001 | 578 | 0.0412 | 580 | 0.0942 | 576 | 0.0454 | 567 | 0.0034 | 565 | 0.2309 | 567 | 0.0355 |
| Cholinesterase< vs. ≥3.7 U/L | 584 | 0.0108 | 581 | 0.0437 | 580 | 0.6728 | 582 | 0.1706 | 580 | 0.5751 | 567 | 0.0992 | 567 | 0.0216 | 567 | 0.7691 |
| Adverse events 4 Grade 0–2 vs. 3–4 | 1429 | 0.0208 | 1421 | 0.0616 | 1421 | 0.0229 | 1425 | 0.0028 | 1420 | 0.0179 | 1399 | 0.0005 | 1368 | 0.0074 | 1399 | <0.0001 |
| Azacitidine dose/regimen | ||||||||||||||||
| Azacitidine< vs. ≥7 days | 1429 | 0.1648 | 1421 | 0.0129 | 1421 | 0.4369 | 1425 | 0.0964 | 1420 | 0.0158 | 1399 | 0.0096 | 1368 | 0.4788 | 1399 | 0.0288 |
| Azacitidine< vs. ≥75 mg/m2/day | 1426 | 0.1485 | 1418 | 0.1155 | 1418 | 0.0249 | 1422 | 0.0168 | 1417 | 0.0001 | 1396 | 0.0003 | 1365 | 0.0040 | 1396 | 0.0013 |
| Haematologic improvement (HI) | ||||||||||||||||
| HI-any 5: Yes vs. No | 1275 | 0.0004 | 1268 | 0.0130 | 1270 | 0.0003 | 1272 | 0.6473 | 1266 | 0.1747 | 1248 | 0.0005 | 1221 | <0.0001 | 1248 | 0.0048 |
| HI-Erythrocytes: Yes vs. No | 1296 | 0.0008 | 1289 | 0.0163 | 1291 | <0.0001 | 1293 | 0.2981 | 1287 | 0.7419 | 1269 | 0.0084 | 1239 | <0.0001 | 1269 | 0.1645 |
| HI-Platelets: Yes vs. No | 1317 | 0.0025 | 1310 | 0.0011 | 1311 | 0.0008 | 1315 | 0.0951 | 1310 | 0.2232 | 1288 | 0.0005 | 1262 | <0.0001 | 1288 | 0.0003 |
| HI-Neutrophils: Yes vs. No | 1362 | 0.4299 | 1355 | 0.7016 | 1354 | 0.2083 | 1358 | 0.1326 | 1353 | 0.4239 | 1333 | 0.2837 | 1303 | 0.0012 | 1333 | 0.6162 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleyer, L.; Heibl, S.; Tinchon, C.; Vallet, S.; Schreder, M.; Melchardt, T.; Stute, N.; Föhrenbach Quiroz, K.T.; Leisch, M.; Egle, A.; et al. Health-Related Quality of Life as Assessed by the EQ-5D-5L Predicts Outcomes of Patients Treated with Azacitidine—A Prospective Cohort Study by the AGMT. Cancers 2023, 15, 1388. https://doi.org/10.3390/cancers15051388
Pleyer L, Heibl S, Tinchon C, Vallet S, Schreder M, Melchardt T, Stute N, Föhrenbach Quiroz KT, Leisch M, Egle A, et al. Health-Related Quality of Life as Assessed by the EQ-5D-5L Predicts Outcomes of Patients Treated with Azacitidine—A Prospective Cohort Study by the AGMT. Cancers. 2023; 15(5):1388. https://doi.org/10.3390/cancers15051388
Chicago/Turabian StylePleyer, Lisa, Sonja Heibl, Christoph Tinchon, Sonia Vallet, Martin Schreder, Thomas Melchardt, Norbert Stute, Kim Tamara Föhrenbach Quiroz, Michael Leisch, Alexander Egle, and et al. 2023. "Health-Related Quality of Life as Assessed by the EQ-5D-5L Predicts Outcomes of Patients Treated with Azacitidine—A Prospective Cohort Study by the AGMT" Cancers 15, no. 5: 1388. https://doi.org/10.3390/cancers15051388
APA StylePleyer, L., Heibl, S., Tinchon, C., Vallet, S., Schreder, M., Melchardt, T., Stute, N., Föhrenbach Quiroz, K. T., Leisch, M., Egle, A., Scagnetti, L., Wolf, D., Beswick, R., Drost, M., Larcher-Senn, J., Grochtdreis, T., Vaisband, M., Hasenauer, J., Zaborsky, N., ... Stauder, R. (2023). Health-Related Quality of Life as Assessed by the EQ-5D-5L Predicts Outcomes of Patients Treated with Azacitidine—A Prospective Cohort Study by the AGMT. Cancers, 15(5), 1388. https://doi.org/10.3390/cancers15051388







