FLT3-ITD Expression as a Potential Biomarker for the Assessment of Treatment Response in Patients with Acute Myeloid Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples
2.2. Sample Processing
2.3. FLT3-ITD Mutation Analysis and Quantification
2.4. Measurable Residual Disease and Chimerism Analysis
2.5. Statistical Analysis
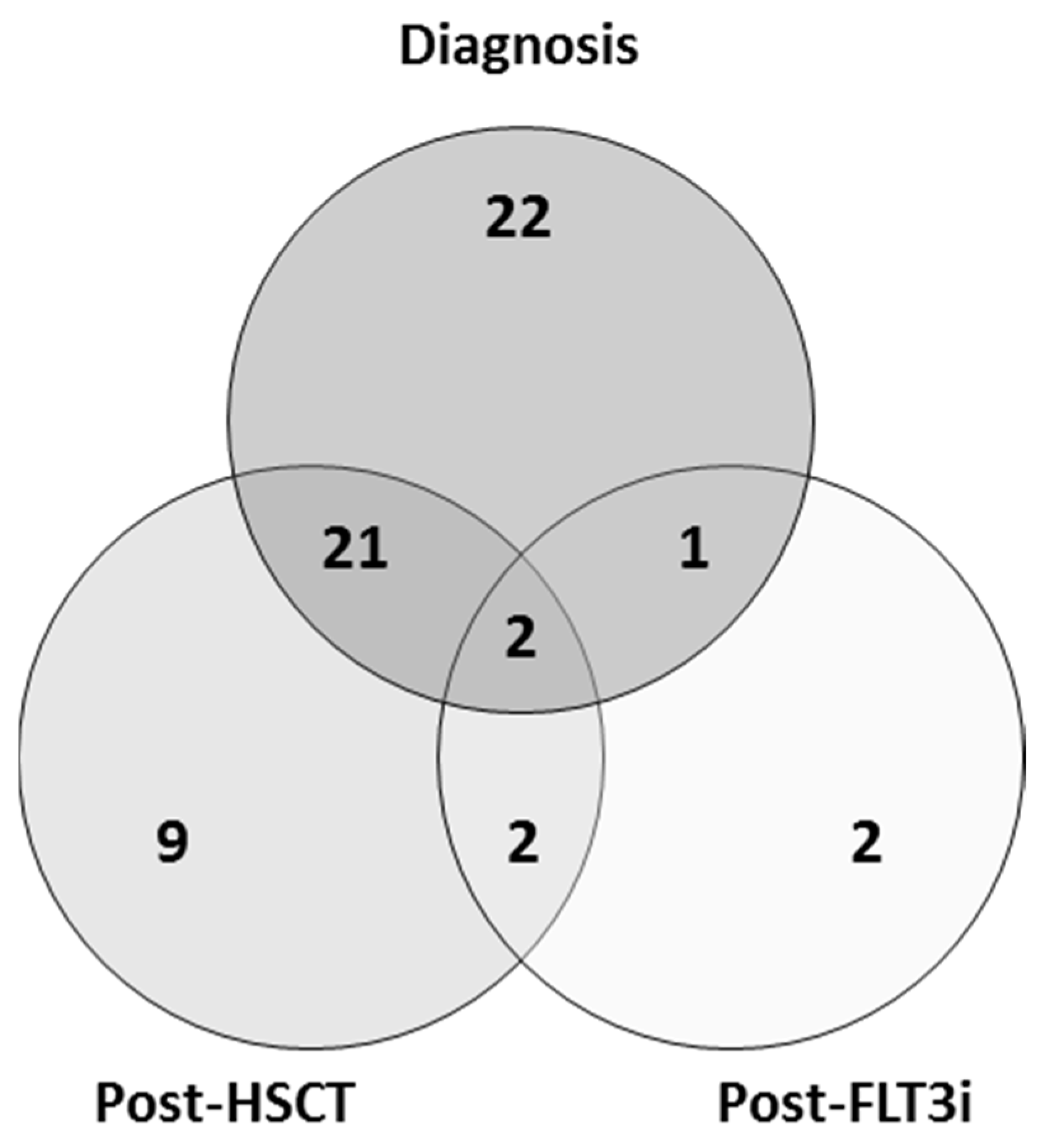
3. Results
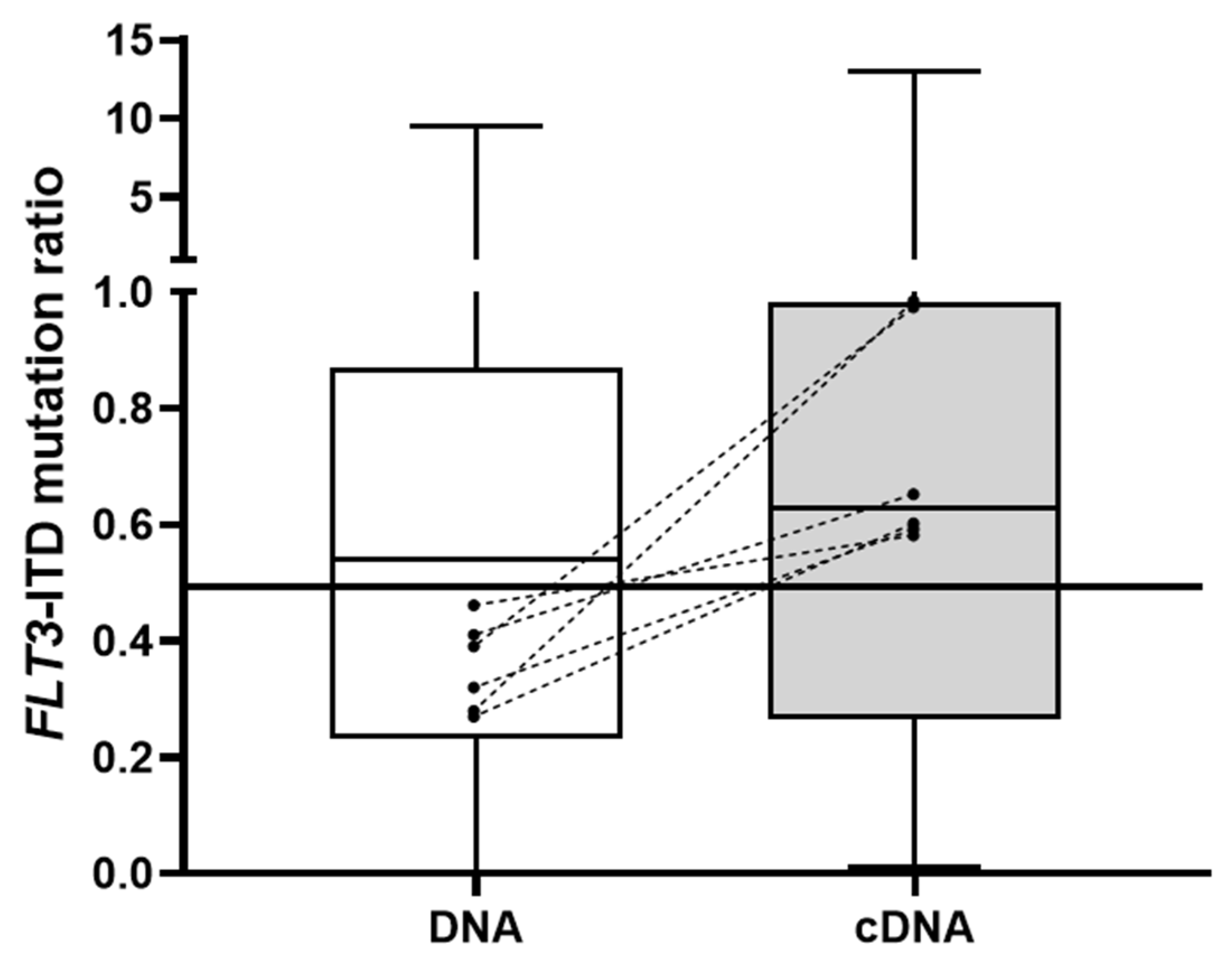
3.1. FLT3-ITD Mutation in DNA and cDNA in Diagnostic Samples (Allelic Ratio)
3.2. FLT3-ITD Mutations in DNA and cDNA for Post-Allo-HSCT Monitoring
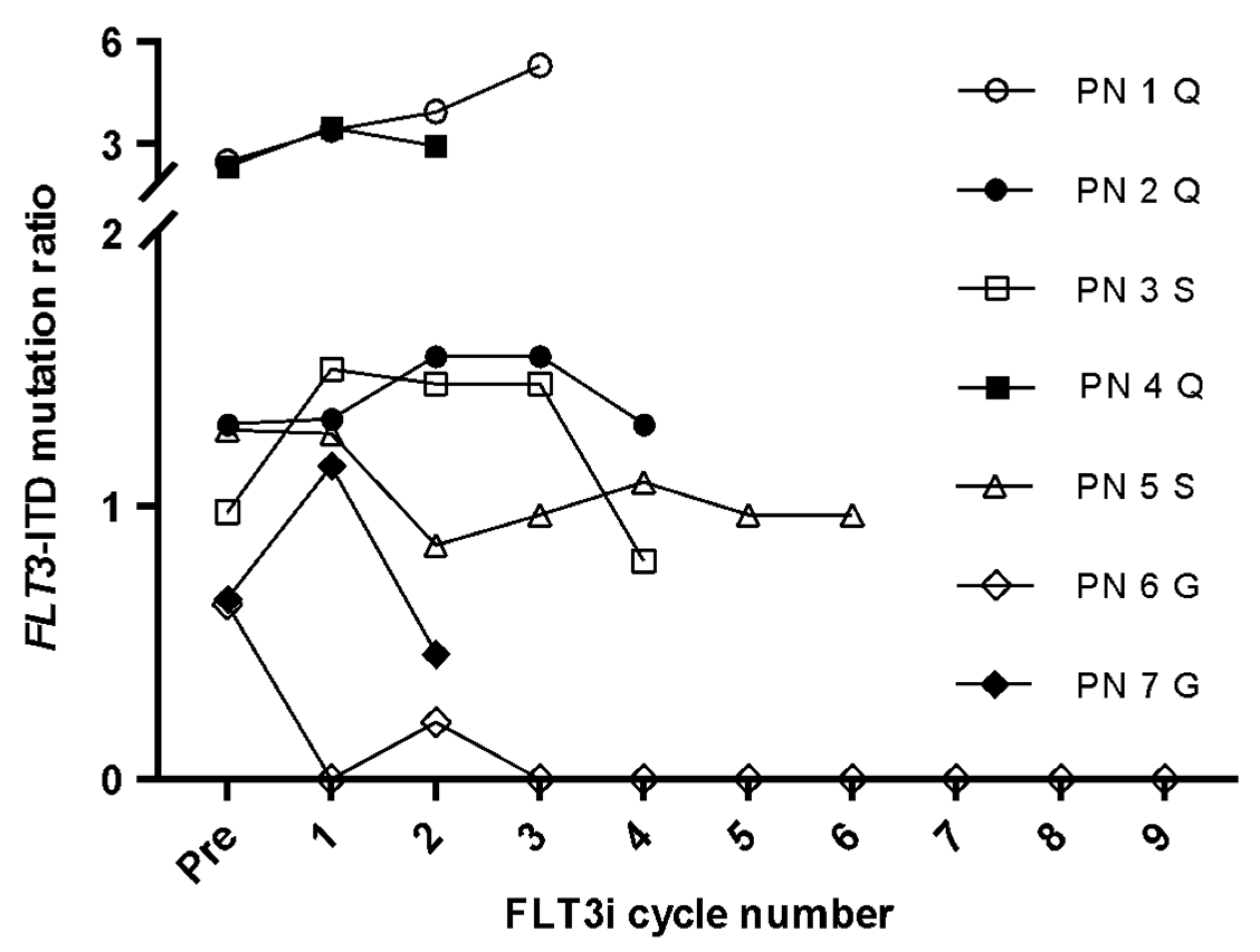
3.3. FLT3-ITD Expression for FLT3i Treatment Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lagunas-Rangel, F.A.; Chávez-Valencia, V. FLT3–ITD and its current role in acute myeloid leukaemia. Med. Oncol. 2017, 34, 114. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Tallman, M.S.; Wang, E.S.; Altman, J.K.; Appelbaum, F.R.; Bhatt, V.R.; Bixby, D.; Coutre, S.E.; De Lima, M.; Fathi, A.T.; Fiorella, M.; et al. Acute Myeloid Leukemia, Version 3.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2019, 17, 721–749. [Google Scholar] [CrossRef]
- Loke, J.; Buka, R.; Craddock, C. Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia: Who, When, and How? Front. Immunol. 2021, 12, 659595. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 mutations in AML: Review of current knowledge and evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Short, N.; Kantarjian, H.; Ravandi, F.; Daver, N. Emerging treatment paradigms with FLT3 inhibitors in acute myeloid leukemia. Ther. Adv. Hematol. 2019, 10, 204062071982731. [Google Scholar] [CrossRef]
- Levis, M. Midostaurin approved for FLT3-mutated AML. Blood 2017, 129, 3403–3406. [Google Scholar] [CrossRef]
- Dhillon, S. Gilteritinib: First Global Approval. Drugs 2019, 79, 331–339. [Google Scholar] [CrossRef]
- Antar, A.; Otrock, Z.K.; El-Cheikh, J.; Kharfan-Dabaja, M.A.; Battipaglia, G.; Mahfouz, R.; Mohty, M.; Bazarbachi, A. Inhibition of FLT3 in AML: A focus on sorafenib. Bone Marrow Transpl. 2016, 52, 344–351. [Google Scholar] [CrossRef][Green Version]
- Levis, M. Quizartinib for the treatment of FLT3/ITD acute myeloid leukemia. Future Oncol. Lond. Engl. 2014, 10, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Kiyoi, H.; Kawashima, N.; Ishikawa, Y. FLT3 mutations in acute myeloid leukemia: Therapeutic paradigm beyond inhibitor development. Cancer Sci. 2020, 111, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.M.; Levis, M.; Hafez, M.J.; Geiger, T.; Cooper, L.C.; Smith, B.; Small, D.; Berg, K.D. Detection of FLT3 Internal Tandem Duplication and D835 Mutations by a Multiplex Polymerase Chain Reaction and Capillary Electrophoresis Assay. J. Mol. Diagn. 2003, 5, 96–102. [Google Scholar] [CrossRef]
- Cloos, J.; Goemans, B.F.; Hess, C.J.; Van Oostveen, J.W.; Waisfisz, Q.; Corthals, S.L.; De Lange, D.; Boeckx, N.; Hählen, K.; Reinhardt, D.; et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia 2006, 20, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Gorello, P.; Cazzaniga, G.; Alberti, F.; Dell’Oro, M.G.; Gottardi, E.; Specchia, G.; Roti, G.; Rosati, R.; Martelli, M.F.; Diverio, D.; et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006, 20, 1103–1108. [Google Scholar] [CrossRef]
- Kwon, M.; Martínez-Laperche, C.; Infante, M.; Carretero, F.; Balsalobre, P.; Serrano, D.; Gayoso, J.; Pérez-Corral, A.; Anguita, J.; Díez-Martín, J.L.; et al. Evaluation of Minimal Residual Disease by Real-Time Quantitative PCR of Wilms’ Tumor 1 Expression in Patients with Acute Myelogenous Leukemia after Allogeneic Stem Cell Transplantation: Correlation with Flow Cytometry and Chimerism. Biol. Blood Marrow Transpl. 2012, 18, 1235–1242. [Google Scholar] [CrossRef]
- Patnaik, M.M. The importance of FLT3 mutational analysis in acute myeloid leukemia. Leuk. Lymphoma 2017, 59, 2273–2286. [Google Scholar] [CrossRef]
- Kondo, M.; Horibe, K.; Takahashi, Y.; Matsumoto, A.; Fukuda, M.; Inaba, J.; Kato, K.; Kojima, S.; Matsuyama, T. Prognostic value of internal tandem duplication of the FLT3 gene in childhood acute myelogenous leukemia. Med. Pediatr. Oncol. 1999, 33, 525–529. [Google Scholar] [CrossRef]
- Cucchi, D.J.G.; Denys, B.; Kaspers, G.J.L.; Janssen, J.J.W.M.; Ossenkoppele, G.J.; De Haas, V.; Zwaan, C.M.; Heuvel-Eibrink, M.M.V.D.; Philippé, J.; Csikós, T.; et al. RNA-based FLT3-ITD allelic ratio is associated with outcome and ex vivo response to FLT3 inhibitors in pediatric AML. Blood 2018, 131, 2485–2489. [Google Scholar] [CrossRef]
- Cucchi, D.G.J.; Vonk, C.M.; Rijken, M.; Kavelaars, F.G.; Merle, P.A.; Verhoef, E.; Venniker-Punt, B.; Kwidama, Z.J.; Gradowska, P.; Löwenberg, B.; et al. DNA vs cDNA FLT3-ITD allelic ratio and length measurements in adult acute myeloid leukemia. Blood Adv. 2021, 5, 4476–4479. [Google Scholar] [CrossRef]
- Whitman, S.P.; Archer, K.; Feng, L.; Baldus, C.; Becknell, B.; Carlson, B.D.; Carroll, A.J.; Mrózek, K.; Vardiman, J.W.; George, S.L.; et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A cancer and leukemia group B study. Cancer Res. 2001, 61, 7233–7239. [Google Scholar] [PubMed]
- Nazha, A.; Cortes, J.; Faderl, S.; Pierce, S.; Daver, N.; Kadia, T.; Borthakur, G.; Luthra, R.; Kantarjian, H.; Ravandi, F. Activating internal tandem duplication mutations of the fms-like tyrosine kinase-3 (FLT3-ITD) at complete response and relapse in patients with acute myeloid leukemia. Haematologica 2012, 97, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Ossenkoppele, G.; Schuurhuis, G.J. MRD in AML: Does it already guide therapy decision-making? Hematology 2016, 2016, 356–365. [Google Scholar] [CrossRef]
- Chou, W.-C.; Hou, H.-A.; Liu, C.-Y.; Chen, C.-Y.; Lin, L.-I.; Huang, Y.-N.; Chao, Y.-C.; Hsu, C.-A.; Huang, C.-F.; Tien, H.-F. Sensitive measurement of quantity dynamics of FLT3 internal tandem duplication at early time points provides prognostic information. Ann. Oncol. 2010, 22, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Schiller, J.; Praulich, I.; Rocha, C.K.; Kreuzer, K.-A. Patient-specific analysis of FLT3 internal tandem duplications for the prognostication and monitoring of acute myeloid leukemia. Eur. J. Haematol. 2012, 89, 53–62. [Google Scholar] [CrossRef]
- Grunwald, M.R.; Tseng, L.-H.; Lin, M.-T.; Pratz, K.W.; Eshleman, J.R.; Levis, M.J.; Gocke, C.D. Improved FLT3 Internal Tandem Duplication PCR Assay Predicts Outcome after Allogeneic Transplant for Acute Myeloid Leukemia. Biol. Blood Marrow Transpl. 2014, 20, 1989–1995. [Google Scholar] [CrossRef]
- Levis, M.J.; Perl, A.E.; Altman, J.K.; Gocke, C.D.; Bahceci, E.; Hill, J.; Liu, C.; Xie, Z.; Carson, A.R.; McClain, V.; et al. A next-generation sequencing–based assay for minimal residual disease assessment in AML patients with FLT3-ITD mutations. Blood Adv. 2018, 2, 825–831. [Google Scholar] [CrossRef]
- Perl, A.E. The role of targeted therapy in the management of patients with AML. Hematology 2017, 2017, 54–65. [Google Scholar] [CrossRef]
- Daver, N.; Venugopal, S.; Ravandi, F. FLT3 mutated acute myeloid leukemia: 2021 treatment algorithm. Blood Cancer J. 2021, 11, 104. [Google Scholar] [CrossRef]
- Altman, J.K.; Perl, A.E.; Hill, J.E.; Rosales, M.; Bahceci, E.; Levis, M.J. The impact of FLT3 mutation clearance and treatment response after gilteritinib therapy on overall survival in patients with FLT3 mutation–positive relapsed/refractory acute myeloid leukemia. Cancer Med. 2021, 10, 797–805. [Google Scholar] [CrossRef]


| Cohort | Diagnosis | Post-HSCT | Post-FLT3i |
|---|---|---|---|
| n | 46 | 34 | 7 |
| Age, median (range), years | 63 (27–91) | 45 (27–65) | 52 (31–65) |
| Female sex, n (%) | 15 (32.6) | 14 (41.2) | 3 (42.9) |
| Induction therapy, n (%) | |||
| IA 3 × 7 | 27 (58.7) | 29 (85.3) | 5 (71.4) |
| IA 3 × 7 + FLT3i | 5 (10.9) | 2 (5.9) | 2 (28.6) |
| Hypomethylating | 3 (6.5) | 0 (0.0) | 0 (0.0) |
| Other | 1 (2.1) | 3 (8.8) | 0 (0.0) |
| Palliative care | 10 (21.8) | 0 (0.0) | 0 (0.0) |
| Allo-HSCT | |||
| HSCT type, n (%) | |||
| Haploidentical | - | 17 (50.0) | - |
| HLA-identical | - | 15 (44.1) | - |
| Haplo-cord | - | 2 (5.9) | - |
| Conditioning regimen, n (%) | |||
| Myeloablative | - | 26 (76.5) | - |
| Reduced intensity | - | 8 (23.5) | - |
| FLT3i in patients with R/R AML | |||
| Quizartinib | - | - | 3 (42.8) |
| Sorafenib | - | - | 2 (28.6) |
| Gilteritinib | - | - | 2 (28.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell, D.; Chicano, M.; Cardero, A.J.; Gómez-Centurión, I.; Bailén, R.; Oarbeascoa, G.; Martínez-Señarís, D.; Franco, C.; Muñiz, P.; Anguita, J.; et al. FLT3-ITD Expression as a Potential Biomarker for the Assessment of Treatment Response in Patients with Acute Myeloid Leukemia. Cancers 2022, 14, 4006. https://doi.org/10.3390/cancers14164006
Carbonell D, Chicano M, Cardero AJ, Gómez-Centurión I, Bailén R, Oarbeascoa G, Martínez-Señarís D, Franco C, Muñiz P, Anguita J, et al. FLT3-ITD Expression as a Potential Biomarker for the Assessment of Treatment Response in Patients with Acute Myeloid Leukemia. Cancers. 2022; 14(16):4006. https://doi.org/10.3390/cancers14164006
Chicago/Turabian StyleCarbonell, Diego, María Chicano, Alfonso J. Cardero, Ignacio Gómez-Centurión, Rebeca Bailén, Gillen Oarbeascoa, Diana Martínez-Señarís, Carolina Franco, Paula Muñiz, Javier Anguita, and et al. 2022. "FLT3-ITD Expression as a Potential Biomarker for the Assessment of Treatment Response in Patients with Acute Myeloid Leukemia" Cancers 14, no. 16: 4006. https://doi.org/10.3390/cancers14164006
APA StyleCarbonell, D., Chicano, M., Cardero, A. J., Gómez-Centurión, I., Bailén, R., Oarbeascoa, G., Martínez-Señarís, D., Franco, C., Muñiz, P., Anguita, J., Kwon, M., Díez-Martín, J. L., Buño, I., & Martínez-Laperche, C. (2022). FLT3-ITD Expression as a Potential Biomarker for the Assessment of Treatment Response in Patients with Acute Myeloid Leukemia. Cancers, 14(16), 4006. https://doi.org/10.3390/cancers14164006






