Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Efficacy of Venetoclax as Compared to Chemotherapy in AML Patients
3. Toxicity of Venetoclax-Based Therapy as Compared to Chemotherapy in AML Patients
4. Sensitivity to Venetoclax-Based Therapies in Molecular Defined Subgroups of AML
5. NPM1-, IDH1/2-, TET2- and Relapsed or Refractory RUNX1-Mutated AML Patients Showed Enhanced Sensitivity to Venetoclax
6. AML Patients with Mutations in FLT3, TP53 and RAS Showed Reduced Sensitivity to Venetoclax
| Total Response | Mutated Response | |||||
|---|---|---|---|---|---|---|
| Mutation | Study | Stage | Combination Therapy | Incidence (%) | CR/CRi (%) | CR/CRi (%) |
| NPM1 | DiNardo (2019) [31] | ND | HMA | 16 | 67 | 91 |
| DiNardo (2020) [37] | ND | HMA/LDAC | 20 | 64 | 72 | |
| DiNardo (2020) [38] | ND | HMA | 17 | 66 | 67 | |
| Morsia (2020) [39] | ND | HMA | 14 | 50 | 60 | |
| Pollyea (2020) [40] | ND | HMA | 17 | 71 | 79 | |
| Wei (2019) [41] | ND | LDAC | 13 | 54 | 89 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 8 | 23 | 67 | |
| IDH1/2 | DiNardo (2019) [31] | ND | HMA | 24 | 67 | 71 |
| DiNardo (2020) [37] | ND | HMA/LDAC | 28 | 64 | 89 | |
| DiNardo (2020) [38] | ND | HMA | 25 | 66 | 75 | |
| Morsia (2020) [39] | ND | HMA | 21 | 50 | 56 | |
| Pollyea (2020) [40] | ND | HMA | 26 | 71 | 86 | |
| Pollyea (2022) [74] | ND | HMA | 26 | 63 | 79 | |
| Wei (2019) [41] | ND | LDAC | 25 | 54 | 72 | |
| Aldoss (2019) [48] | R/R | HMA | 17 | 46 | 60 | |
| DiNardo (2018) [50] | R/R | HMA/LDAC | 26 | 12 | 27 | |
| Konopleva (2016) [52] | R/R | MONO | 12 | 19 | 33 | |
| Morsia (2020) [39] | R/R | HMA | 12 | 33 | 60 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 11 | 23 | 25 | |
| TET2 | DiNardo (2020) [37] | ND | HMA/LDAC | 23 | 64 | 58 |
| Aldoss (2019) [48] | R/R | HMA | 8 | 46 | 86 | |
| RUNX1 | DiNardo (2020) [37] | ND | HMA | 23 | 64 | 50 |
| Aldoss (2019) [48] | R/R | HMA | 22 | 46 | 35 | |
| DiNardo (2018) [50] | R/R | HMA/LDAC | 19 | 12 | 50 | |
| Morsia (2020) [39] | R/R | HMA | 10 | 33 | 75 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 28 | 23 | 36 | |
| FLT3 | DiNardo (2019) [31] | ND | HMA | 12 | 67 | 72 |
| DiNardo (2020) [37] | ND | HMA/LDAC | 7 | 64 | 33 | |
| DiNardo (2020) [38] | ND | HMA | 14 | 66 | 72 | |
| Morsia (2020) [39] | ND | HMA | 23 | 50 | 40 | |
| Pollyea (2020) [40] | ND | HMA | 14 | 71 | 58 | |
| Wei (2019) [41] | ND | LDAC | 23 | 54 | 44 | |
| Aldoss (2019) [48] | R/R | HMA | 27 | 46 | 42 | |
| Aldoss (2020) [88] | ND/R/R | HMA | 100 | 60 | 60 | |
| Morsia (2020) [39] | R/R | HMA | 10 | 33 | 50 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 8 | 23 | 33 | |
| TP53 | DiNardo (2019) [31] | ND | HMA | 25 | 67 | 47 |
| DiNardo (2020) [37] | ND | HMA/LDAC | 23 | 64 | 44 | |
| DiNardo (2020) [38] | ND | HMA | 23 | 66 | 55 | |
| Morsia (2020) [39] | ND | HMA | 21 | 50 | 44 | |
| Pollyea (2020) [40] | ND | HMA | 20 | 71 | 53 | |
| Wei (2019) [41] | ND | LDAC | 14 | 54 | 30 | |
| Aldoss (2019) [91] | R/R | HMA | 14 | 46 | 46 | |
| Morsia (2020) [39] | R/R | HMA | 29 | 33 | 40 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 8 | 23 | 0 | |
| K/NRAS | DiNardo (2020) [37] | ND | HMA | 19 | 64 | 33 |
| Aldoss (2019) [48] | R/R | HMA | 16 | 46 | 36 | |
| Wang (2020) [54] | R/R | HMA/LDAC | 8 | 23 | 0 | |
| Other | ||||||
| Prior HMA | DiNardo (2020) [37] | ND | HMA | 6 | 64 | 20 |
| Wei (2019) [41] | ND | LDAC | 29 | 54 | 33 | |
| Winters (2019) [43] | ND | HMA | 12 | 58 | 0 | |
| Goldberg (2017) [92] | R/R | HMA/LDAC | 76 | 24 | 25 | |
| Morsia (2020) [39] | R/R | HMA | 36 | 33 | 40 |
7. Venetoclax Sensitivity Is Decreased in More Mature AML Cells
8. Prior Treatment with Hypomethylating Agents Decreased Effectiveness of Venetoclax
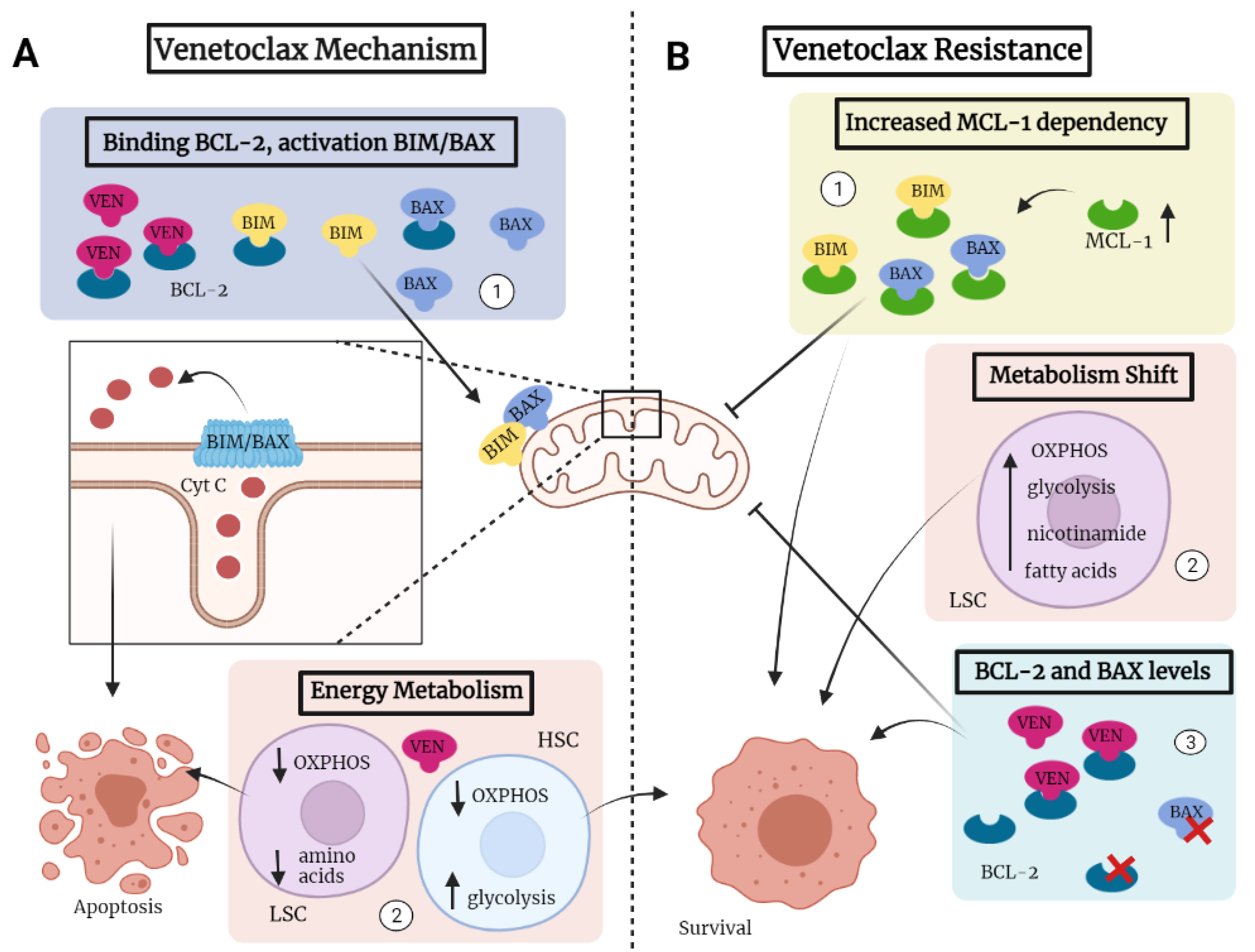
9. Mechanisms Determining Sensitivity of AML to Venetoclax
10. Prediction of Active Venetoclax-Based Combination Therapies for AML
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of Acute Myeloid Leukemia: Recent Progress and Enduring Challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef] [PubMed]
- van Gils, N.; Denkers, F.; Smit, L. Escape from Treatment; the Different Faces of Leukemic Stem Cells and Therapy Resistance in Acute Myeloid Leukemia. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.-T.; Flandrin, G.; Galton, D.A.G.; Gralnick, H.R.; Sultan, C. Proposals for the Classification of the Acute Leukaemias French-American-British (FAB) Co-operative Group. Br. J. Haematol. 1976, 33, 451–458. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Thomas, B.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations from an International Expert Panel. J. Am. Soc. Hematol. 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantarjian, H.; O’Brisn, S.; Cortes, J.; Giles, F.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; Wierda, W.; Pierce, S.; Shan, J.; et al. Results of Intensive Chemotherapy in 998 Patients Age 65 Years or Older with Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome: Predictive Prognostic Models for Outcome. Cancer 2006, 106, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Weisdorf, D.J.; Bloomfield, C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1136–1152. [Google Scholar] [CrossRef] [Green Version]
- Döhner, H.; Estey, E.H.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Burnett, A.K.; Dombret, H.; Fenaux, P.; Grimwade, D.; Larson, R.A.; et al. Diagnosis and Management of Acute Myeloid Leukemia in Adults: Recommendations from an International Expert Panel, on Behalf of the European LeukemiaNet. Blood 2010, 115, 453–474. [Google Scholar] [CrossRef]
- Dombret, H.; Gardin, C. An Update of Current Treatments for Adult Acute Myeloid Leukemia. Blood 2016, 127, 53–61. [Google Scholar] [CrossRef]
- Shah, A.; Andersson, T.M.L.; Rachet, B.; Björkholm, M.; Lambert, P.C. Survival and Cure of Acute Myeloid Leukaemia in England, 1971–2006: A Population-Based Study. Br. J. Haematol. 2013, 162, 509–516. [Google Scholar] [CrossRef]
- Terwijn, M.; Kelder, A.; Huijgens, P.C.; Dräger, A.M.; Oussoren, Y.J.M.; Scholten, W.J.; Snel, A.N.; Ossenkoppele, G.J.; Schuurhuis, G.J.; Biemond, B.J.; et al. High Prognostic Impact of Flow Cytometric Minimal Residual Disease Detection in Acute Myeloid Leukemia: Data from the HOVON/SAKK AML 42A Study. J. Clin. Oncol. 2013, 31, 3889–3897. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Grob, T.; Hanekamp, D.; Kavelaars, F.G.; al Hinai, A.; Zeilemaker, A.; Erpelinck-Verschueren, C.A.J.; Gradowska, P.L.; Meijer, R.; Cloos, J.; et al. Molecular Minimal Residual Disease in Acute Myeloid Leukemia. N. Engl. J. Med. 2018, 378, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Krivtsov, A.V.; Twomey, D.; Feng, Z.; Stubbs, M.C.; Wang, Y.; Faber, J.; Levine, J.E.; Wang, J.; Hahn, W.C.; Gilliland, D.G.; et al. Transformation from Committed Progenitor to Leukaemia Stem Cell Initiated by MLL-AF9. Nature 2006, 442, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Burnett, A.K.; Milligan, D.; Prentice, A.G.; Goldstone, A.H.; McMullin, M.F.; Hills, R.K.; Wheatley, K. A Comparison of Low-Dose Cytarabine and Hydroxyurea with or without All-Trans Retinoic Acid for Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome in Patients Not Considered Fit for Intensive Treatment. Cancer 2007, 109, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Dombret, H.; Seymour, J.F.; Butrym, A.; Wierzbowska, A.; Selleslag, D.; Jang, J.H.; Kumar, R.; Cavenagh, J.; Schuh, A.C.; Candoni, A.; et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients with Newly Diagnosed AML with >30% Blasts. Blood 2015, 126, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra, V.A.; DiNardo, C.; Konopleva, M. Venetoclax-Based Therapies for Acute Myeloid Leukemia. Best Pract. Res. Clin. Haematol. 2019, 32, 145–153. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a Potent and Selective BCL-2 Inhibitor, Achieves Antitumor Activity While Sparing Platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of Cytochrome c Release from Mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [Green Version]
- Westphal, D.; Kluck, R.M.; Dewson, G. Building Blocks of the Apoptotic Pore: How Bax and Bak Are Activated and Oligomerize during Apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Karakas, T.; Maurer, U.; Weidmann, E.; Miething, C.C.; Hoelzer, D.; Bergmann, L. High Expression of Bcl-2 MRNA as a Determinant of Poor Prognosis in Acute Myeloid Leukemia. Ann. Oncol. 1998, 9, 159–165. [Google Scholar] [CrossRef]
- Liu, Y.; He, P.; Liu, F.; Shi, L.; Zhu, H.; Cheng, X.; Zhao, J.; Wang, Y.; Zhang, M. Prognostic Significance of B-Cell Lymphoma 2 Expression in Acute Leukemia: A Systematic Review and Meta-Analysis. Mol. Clin. Oncol. 2014, 2, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Majeti, R. Biology and Relevance of Human Acute Myeloid Leukemia Stem Cells. Blood 2017, 129, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Gerhard, B.; Hogge, D.E. Detection, Isolation, and Stimulation of Quiescent Primitive Leukemic Progenitor Cells from Patients with Acute Myeloid Leukemia (AML). Blood 2003, 101, 3142–3149. [Google Scholar] [CrossRef]
- Costello, R.T.; Mallet, F.; Chambost, H.; Sainty, D.; Arnoulet, C.; Gastaut, J.A.; Olive, D. The Immunophenotype of Minimally Differentiated Acute Myeloid Leukemia (AML-MO): Reduced Immunogenicity and High Frequency of CD34+/CD38- Leukemic Progenitors. Leukemia 1999, 13, 1513–1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagadinou, E.D.; Sach, A.; Callahan, K.; Rossi, R.M.; Neering, S.J.; Minhajuddin, M.; Ashton, J.M.; Pei, S.; Grose, V.; O’Dwyer, K.M.; et al. BCL-2 Inhibition Targets Oxidative Phosphorylation and Selectively Eradicates Quiescent Human Leukemia Stem Cells. Cell Stem Cell 2013, 12, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Abraham, M.; Klein, S.; Bulvik, B.; Wald, H.; Weiss, I.D.; Olam, D.; Weiss, L.; Beider, K.; Eizenberg, O.; Wald, O.; et al. The CXCR4 Inhibitor BL-8040 Induces the Apoptosis of AML Blasts by Downregulating ERK, BCL-2, MCL-1 and Cyclin-D1 via Altered MiR-15a/16-1 Expression. Leukemia 2017, 31, 2336–2346. [Google Scholar] [CrossRef]
- Zhou, J.D.; Zhang, T.J.; Xu, Z.J.; Gu, Y.; Ma, J.C.; Li, X.X.; Guo, H.; Wen, X.M.; Zhang, W.; Yang, L.; et al. BCL2 Overexpression: Clinical Implication and Biological Insights in Acute Myeloid Leukemia. Diagn. Pathol. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Pervaiz, S. Bcl-2 Induces pro-Oxidant State by Engaging Mitochondrial Respiration in Tumor Cells. Cell Death Differ. 2007, 14, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollyea, D.A.; Stevens, B.M.; Jones, C.L.; Winters, A.; Pei, S.; Minhajuddin, M.; D’Alessandro, A.; Culp-Hill, R.; Riemondy, K.A.; Gillen, A.E.; et al. Venetoclax with Azacitidine Disrupts Energy Metabolism and Targets Leukemia Stem Cells in Patients with Acute Myeloid Leukemia. Nat. Med. 2018, 24, 1859–1866. [Google Scholar] [CrossRef]
- Jones, C.L.; Stevens, B.M.; D’Alessandro, A.; Reisz, J.A.; Culp-Hill, R.; Nemkov, T.; Pei, S.; Khan, N.; Adane, B.; Ye, H.; et al. Inhibition of Amino Acid Metabolism Selectively Targets Human Leukemia Stem Cells. Cancer Cell 2018, 34, 724–740.e4. [Google Scholar] [CrossRef] [Green Version]
- DiNardo, C.D.; Pratz, K.; Pullarkat, V.; Jonas, B.A.; Arellano, M.; Becker, P.S.; Frankfurt, O.; Konopleva, M.; Wei, A.H.; Kantarjian, H.M.; et al. Venetoclax Combined with Decitabine or Azacitidine in Treatment-Naive, Elderly Patients with Acute Myeloid Leukemia. Blood 2019, 133, 7–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiti, A.; Rausch, C.R.; Cortes, J.E.; Pemmaraju, N.; Daver, N.G.; Ravandi, F.; Garcia-Manero, G.; Borthakur, G.; Naqvi, K.; Ohanian, M.; et al. Outcomes of Relapsed or Refractory Acute Myeloid Leukemia after Front-Line Hypomethylating Agent and Venetoclax Regimens. Haematologica 2021, 106, 894–898. [Google Scholar] [CrossRef]
- Cui, W.; Zhang, D.; Cunningham, M.T.; Tilzer, L. Leukemia-Associated Aberrant Immunophenotype in Patients with Acute Myeloid Leukemia: Changes at Refractory Disease or First Relapse and Clinicopathological Findings. Int. J. Lab. Hematol. 2014, 36, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Feller, N.; Van Der Velden, V.H.J.; Brooimans, R.A.; Boeckx, N.; Preijers, F.; Kelder, A.; De Greef, I.; Westra, G.; Te Marvelde, J.G.; Aerts, P.; et al. Defining Consensus Leukemia-Associated Immunophenotypes for Detection of Minimal Residual Disease in Acute Myeloid Leukemia in a Multicenter Setting. Blood Cancer J. 2013, 3, e129-8. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.Y. Mechanisms for Resistance in AML Insights into Molecular Pathways Mediating Resistance to Venetoclax. Best Pract. Res. Clin. Haematol. 2021, 34, 101251. [Google Scholar] [CrossRef] [PubMed]
- Asghari, H.; Lee, D.; Deutsch, Y.E.; Chan, O.; Al Ali, N.; Boisclair, S.; Brahim, A.; Padron, E.; Kuykendall, A.T.; List, A.F.; et al. Outcomes of Patients with Relapsed or Refractory Acute Myeloid Leukemia Receiving Hypomethylating Agent and Venetoclax. Blood 2019, 134, 1357. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Tiong, I.S.; Quaglieri, A.; MacRaild, S.; Loghavi, S.; Brown, F.C.; Thijssen, R.; Pomilio, G.; Ivey, A.; Salmon, J.M.; et al. Molecular Patterns of Response and Treatment Failure after Frontline Venetoclax Combinations in Older Patients with AML. Blood 2020, 135, 791–803. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Morsia, E.; McCullough, K.; Joshi, M.; Cook, J.; Alkhateeb, H.B.; Al-Kali, A.; Begna, K.; Elliott, M.; Hogan, W.; Litzow, M.; et al. Venetoclax and Hypomethylating Agents in Acute Myeloid Leukemia: Mayo Clinic Series on 86 Patients. Am. J. Hematol. 2020, 95, 1511–1521. [Google Scholar] [CrossRef]
- Pollyea, D.A.; Pratz, K.; Letai, A.; Jonas, B.A.; Wei, A.H.; Pullarkat, V.; Konopleva, M.; Thirman, M.J.; Arellano, M.; Becker, P.S.; et al. Venetoclax with Azacitidine or Decitabine in Patients with Newly Diagnosed Acute Myeloid Leukemia: Long Term Follow-up from a Phase 1b Study. Am. J. Hematol. 2020, 96, 208–217. [Google Scholar] [CrossRef]
- Wei, A.H.; Strickland, S.A.; Hou, J.; Fiedler, W.; Lin, T.L.; Walter, R.B.; Enjeti, A.; Tiong, I.S.; Savona, M.; Lee, S.; et al. Venetoclax Combined With Low-Dose Cytarabine for Previously Untreated Patients With Acute Myeloid Leukemia: Results From a Phase Ib/II Study Abstract. J. Clin. Oncol. 2019, 37, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Wei, A.H.; Montesinos, P.; Ivanov, V.; DiNardo, C.D.; Novak, J.; Laribi, K.; Kim, I.; Stevens, D.A.; Fiedler, W.; Pagoni, M.; et al. Venetoclax plus LDAC for Newly Diagnosed AML Ineligible for Intensive Chemotherapy: A Phase 3 Randomized Placebo-Controlled Trial. Blood 2020, 135, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Winters, A.C.; Gutman, J.A.; Purev, E.; Nakic, M.; Tobin, J.; Chase, S.; Kaiser, J.; Lyle, L.; Boggs, C.; Halsema, K.; et al. Real-World Experience of Venetoclax with Azacitidine for Untreated Patients with Acute Myeloid Leukemia. Blood Adv. 2019, 3, 2911–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiNardo, C.D.; Lachowiez, C.A.; Takahashi, K.; Loghavi, S.; Xiao, L.; Kadia, T.; Daver, N.; Adeoti, M.; Short, N.J.; Sasaki, K.; et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 2768–2778. [Google Scholar] [CrossRef]
- Liu, B.; Guo, Y.; Deng, L.; Qiao, Y.; Jian, J. The Efficacy and Adverse Events of Venetoclax in Combination with Hypomethylating Agents Treatment for Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Systematic Review and Meta-Analysis. Hematology 2020, 25, 414–423. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Reville, P.K.; Kantarjian, H.; Jabbour, E.; Borthakur, G.; Daver, N.; Loghavi, S.; Furudate, K.; Xiao, L.; Pierce, S.; et al. Venetoclax Combined with Induction Chemotherapy in Patients with Newly Diagnosed Acute Myeloid Leukaemia: A Post-Hoc, Propensity Score-Matched, Cohort Study. Lancet Haematol. 2022, 9, e350–e360. [Google Scholar] [CrossRef]
- Aldoss, I.; Yang, D.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Mei, M.; Salhotra, A.; Khaled, S.; Nakamura, R.; et al. Efficacy of the Combination of Venetoclax and Hypomethylating Agents in Relapsed/Refractory Acute Myeloid Leukemia Venetoclax. Haematologica 2018, 15, 404–407. [Google Scholar] [CrossRef]
- Aldoss, I.; Yang, D.; Pillai, R.; Sanchez, J.F.; Mei, M.; Aribi, A.; Ali, H.; Sandhu, K.; Al Malki, M.M.; Salhotra, A.; et al. Association of Leukemia Genetics with Response to Venetoclax and Hypomethylating Agents in Relapsed/Refractory Acute Myeloid Leukemia. Am. J. Hematol. 2019, 94, E253–E255. [Google Scholar] [CrossRef] [Green Version]
- Byrne, M.; Danielson, N.; Sengsayadeth, S.; Rasche, A.; Culos, K.; Gatwood, K.; Wyatt, H.; Chinratanalab, W.; Dholaria, B.; Ferrell, P.B.; et al. The Use of Venetoclax-Based Salvage Therapy for Post-Hematopoietic Cell Transplantation Relapse of Acute Myeloid Leukemia. Am. J. Hematol. 2020, 95, 1006–1014. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Rausch, C.R.; Benton, C.; Kadia, T.; Jain, N.; Pemmaraju, N.; Daver, N.; Covert, W.; Marx, K.R.; Mace, M.; et al. Clinical Experience with the BCL2-Inhibitor Venetoclax in Combination Therapy for Relapsed and Refractory Acute Myeloid Leukemia and Related Myeloid Malignancies. Am. J. Hematol. 2018, 93, 401–407. [Google Scholar] [CrossRef] [Green Version]
- Gaut, D.; Burkenroad, A.; Duong, T.; Feammelli, J.; Sasine, J.; Schiller, G. Venetoclax Combination Therapy in Relapsed/Refractory Acute Myeloid Leukemia: A Single Institution Experience. Leuk. Res. 2020, 90, 106314. [Google Scholar] [CrossRef] [PubMed]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ram, R.; Amit, O.; Zuckerman, T.; Gurion, R.; Raanani, P.; Bar-On, Y.; Avivi, I.; Wolach, O. Venetoclax in Patients with Acute Myeloid Leukemia Refractory to Hypomethylating Agents—A Multicenter Historical Prospective Study. Ann. Hematol. 2019, 98, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Tsai, C.H.; Lin, C.C.; Tien, F.M.; Chen, Y.W.; Lin, H.Y.; Yao, M.; Lin, Y.C.; Lin, C.T.; Cheng, C.L.; et al. Cytogenetics and Mutations Could Predict Outcome in Relapsed and Refractory Acute Myeloid Leukemia Patients Receiving BCL-2 Inhibitor Venetoclax. Ann. Hematol. 2020, 99, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Bewersdorf, J.P.; Giri, S.; Wang, R.; Williams, R.T.; Tallman, M.S.; Zeidan, A.M.; Stahl, M. Venetoclax as Monotherapy and in Combination with Hypomethylating Agents or Low Dose Cytarabine in Relapsed and Treatment Refractory Acute Myeloid Leukemia: A Systematic Review and Meta-Analysis. Haematologica 2020, 105, 2659–2663. [Google Scholar] [CrossRef] [Green Version]
- Lachowiez, C.A.; Loghavi, S.; Kadia, T.M.; Daver, N.; Borthakur, G.; Pemmaraju, N.; Naqvi, K.; Alvarado, Y.; Yilmaz, M.; Short, N.; et al. Outcomes of Older Patients with NPM1-Mutated AML: Current Treatments and the Promise of Venetoclax-Based Regimens. Blood Adv. 2020, 4, 1311–1320. [Google Scholar] [CrossRef]
- Federici, L.; Falini, B. Nucleophosmin Mutations in Acute Myeloid Leukemia: A Tale of Protein Unfolding and Mislocalization. Protein Sci. 2013, 22, 545–556. [Google Scholar] [CrossRef] [Green Version]
- Murano, K.; Okuwaki, M.; Hisaoka, M.; Nagata, K. Transcription Regulation of the RRNA Gene by a Multifunctional Nucleolar Protein, B23/Nucleophosmin, through Its Histone Chaperone Activity. Mol. Cell. Biol. 2008, 28, 3114–3126. [Google Scholar] [CrossRef] [Green Version]
- Colombo, E.; Alcalay, M.; Pelicci, P.G. Nucleophosmin and Its Complex Network: A Possible Therapeutic Target in Hematological Diseases. Oncogene 2011, 30, 2595–2609. [Google Scholar] [CrossRef] [Green Version]
- Heath, E.M.; Chan, S.M.; Minden, M.D.; Murphy, T.; Shlush, L.I.; Schimmer, A.D. Biological and Clinical Consequences of NPM1 Mutations in AML. Leukemia 2017, 31, 798–807. [Google Scholar] [CrossRef]
- Leong, S.M.; Tan, B.X.; Ahmad, B.B.; Yan, T.; Chee, L.Y.; Ang, S.T.; Tay, K.G.; Koh, L.P.; Yeoh, A.E.J.; Koay, E.S.C.; et al. Mutant Nucleophosmin Deregulates Cell Death and Myeloid Differentiation through Excessive Caspase-6 and -8 Inhibition. Blood 2010, 116, 3286–3296. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; Gundry, M.; Sorcini, D.; Guzman, A.G.; Huang, Y.; Ramabadran, R.; Gionfriddo, I.; Mezzasoma, F.; Milano, F.; Nabet, B.; et al. Mutant NPM1 Maintains the Leukemic State through HOX Expression. Cancer Cell 2018, 34, 499–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, D.H.; Young, M.A.; Lamprecht, T.L.; Helton, N.M.; Fulton, R.; O’Laughlin, M.; Fronick, C.; Magrini, V.; Demeter, R.T.; Miller, C.A.; et al. Epigenomic Analysis of the HOX Gene Loci Reveals Mechanisms That May Control Canonical Expression Patterns in AML and Normal Hematopoietic Cells. Leukemia 2015, 29, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halasi, M.; Gartel, A.L. FOX(M1) News—It Is Cancer. Mol. Cancer Ther. 2013, 12, 245–254. [Google Scholar] [CrossRef] [Green Version]
- Kontro, M.; Kumar, A.; Majumder, M.M.; Eldfors, S.; Parsons, A.; Pemovska, T.; Saarela, J.; Yadav, B.; Malani, D.; Fløisand, Y.; et al. HOX Gene Expression Predicts Response to BCL-2 Inhibition in Acute Myeloid Leukemia. Leukemia 2017, 31, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.J.; Deshpande, A.; Sinha, A.U.; Chen, L.; Chang, J.; Cihan, A.; Fazio, M.; Chen, C.W.; Zhu, N.; Koche, R.; et al. AF10 Regulates Progressive H3K79 Methylation and HOX Gene Expression in Diverse AML Subtypes. Cancer Cell 2014, 26, 896–908. [Google Scholar] [CrossRef] [Green Version]
- Benito, J.M.; Godfrey, L.; Kojima, K.; Hogdal, L.; Wunderlich, M.; Geng, H.; Marzo, I.; Harutyunyan, K.G.; Golfman, L.; North, P.; et al. MLL-Rearranged Acute Lymphoblastic Leukemias Activate BCL-2 through H3K79 Methylation and Are Sensitive to the BCL-2-Specific Antagonist ABT-199. Cell Rep. 2015, 13, 2715–2727. [Google Scholar] [CrossRef] [Green Version]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-Associated IDH1 Mutations Produce 2-Hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef] [Green Version]
- Ye, D.; Ma, S.; Xiong, Y.; Guan, K. R-2-Hydroxyglutarate as the Key Effector of IDH Mutations Promoting Oncogenesis. Cancer Cell 2013, 23, 274–276. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Ward, P.S.; Kapoor, G.S.; Rohie, D.; Turcan, S.; Abdel-Wahab, O.; Edwards, C.R.; Khanin, R.; Figueroa, M.E.; Melnick, A.; et al. IDH Mutations Impairs Histone Demethylation and Results in a Block to Cell Differentiation. Nature 2013, 483, 474–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran-crusio, K.; Reavie, L.; Shih, A.; Abdel-wahab, O.; Ndiaye-lobry, D.; Lobry, C.; Figueroa, M.E.; Vasanthakumar, A.; Patel, J.; Zhao, X.; et al. Tet2 Loss Leads to Increased Hematopoietic Stem Cell Self- Renewal and Myeloid Transformation. Cancer Cell 2012, 20, 11–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.U.; Su, Y.; Zhong, C.; Ming, G.L.; Song, H. Emerging Roles of TET Proteins and 5-Hydroxymethylcytosines in Active DNA Demethylation and Beyond. Cell Cycle 2011, 10, 2662–2668. [Google Scholar] [CrossRef] [Green Version]
- Pollyea, D.A.; DiNardo, C.D.; Arellano, M.L.; Pigneux, A.; Fiedler, W.; Konopleva, M.; Rizzieri, D.A.; Smith, B.D.; Shinagawa, A.; Lemoli, R.M.; et al. Impact of Venetoclax and Azacitidine in Treatment-Naïve Patients with Acute Myeloid Leukemia and IDH1/2 Mutations. Clin. Cancer Res. 2022, 28, 2753–2761. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Ravandi, F.; Agresta, S.; Konopleva, M.; Takahashi, K.; Kadia, T.; Routbort, M.; Patel, K.P.; Brandt, M.; Pierce, S.; et al. Characteristics, Clinical Outcome, and Prognostic Significance of IDH Mutations in AML. Am. J. Hematol. 2015, 90, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.M.; Thomas, D.; Corces-Zimmerman, M.R.; Xavy, S.; Rastogi, S.; Hong, W.J.; Zhao, F.; Medeiros, B.C.; Tyvoll, D.A.; Majeti, R. Isocitrate Dehydrogenase 1 and 2 Mutations Induce BCL-2 Dependence in Acute Myeloid Leukemia. Nat. Med. 2015, 21, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Gao, L.; Teng, L.; Ge, J.; Oo, Z.M.; Kumar, A.R.; Gilliland, D.G.; Mason, P.J.; Tan, K.; Speck, N.A. Runx1 Deficiency Decreases Ribosome Biogenesis and Confers Stress Resistance to Hematopoietic Stem and Progenitor Cells. Cell Stem Cell 2015, 17, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [CrossRef] [Green Version]
- Gary Gilliland, D.; Griffin, J.D. The Roles of FLT3 in Hematopoiesis and Leukemia. Blood 2002, 100, 1532–1542. [Google Scholar] [CrossRef] [Green Version]
- Grafone, T.; Palmisano, M.; Nicci, C.; Storti, S. An Overview on the Role of FLT3-Tyrosine Kinase Receptor in Acute Myeloid Leukemia: Biology and Treatment. Oncol. Rev. 2012, 6, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.T.; Levis, M.; Small, D. Constitutively Activated FLT3 Phosphorylates BAD Partially through Pim-1. Br. J. Haematol. 2006, 134, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Stirewalt, D.L.; Kopecky, K.J.; Meshinchi, S.; Appelbaum, F.R.; Slovak, M.L.; Willman, C.L.; Radich, J.P. FLT3, RAS, and TP53 Mutations in Elderly Patients with Acute Myeloid Leukemia. Blood 2001, 97, 3589–3595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, F.; Towatari, M.; Kiyoi, H.; Tanimoto, M.; Kitamura, T.; Saito, H.; Naoe, T. Tandem-Duplicated Flt3 Constitutively Activates STAT5 and MAP Kinase and Introduces Autonomous Cell Growth in IL-3-Dependent Cell Lines. Oncogene 2000, 19, 624–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiyoi, H.; Towatari, M.; Yokota, S.; Hamaguchi, M.; Ohno, R.; Saito, H.; Naoe, T. Internal Tandem Duplication of the FLT3 Gene Is a Novel Modality of Elongation Mutation Which Causes Constitutive Activation of the Product. Leukemia 1998, 12, 1333–1337. [Google Scholar] [CrossRef] [Green Version]
- Abu-Duhier, F.M.; Goodeve, A.C.; Wilson, G.A.; Care, R.S.; Peake, I.R.; Reilly, J.T. Identification of Novel FLT-3 Asp835 Mutations in Adult Acute Myeloid Leukaemia. Br. J. Haematol. 2001, 113, 983–988. [Google Scholar] [CrossRef]
- Daver, N.; Liu Dumlao, T.; Ravandi, F.; Pierce, S.; Borthakur, G.; Pemmaraju, N.; Nazha, A.; Faderl, S.; Jabbour, E.; Garcia-Manero, G.; et al. Effect of NPM1 and FLT3 Mutations on the Outcomes of Elderly Patients with Acute Myeloid Leukemia Receiving Standard Chemotherapy. Clin. Lymphoma Myeloma Leuk. 2013, 13, 435–440. [Google Scholar] [CrossRef] [Green Version]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- Aldoss, I.; Zhang, J.; Mei, M.; Al Malki, M.M.; Arslan, S.; Ngo, D.; Aribi, A.; Ali, H.; Sandhu, K.; Salhotra, A.; et al. Venetoclax and Hypomethylating Agents in FLT3-Mutated Acute Myeloid Leukemia. Am. J. Hematol. 2020, 95, 1193–1199. [Google Scholar] [CrossRef]
- Konopleva, M.; Thirman, M.J.; Pratz, K.W.; Garcia, J.S.; Recher, C.; Pullarkat, V.; Kantarjian, H.M.; DiNardo, C.D.; Dail, M.; Duan, Y.; et al. Impact of F LT3 Mutation on Outcomes after Venetoclax and Azacitidine for Patients with Treatment-Naïve Acute Myeloid Leukemia. Clin. Cancer Res. 2022, 28, 2744–2752. [Google Scholar] [CrossRef]
- Kasper, S.; Breitenbuecher, F.; Heidel, F.; Hoffarth, S.; Markova, B.; Schuler, M.; Fischer, T. Targeting MCL-1 Sensitizes FLT3-ITD-Positive Leukemias to Cytotoxic Therapies. Blood Cancer J. 2012, 2, e60-10. [Google Scholar] [CrossRef] [Green Version]
- Aldoss, I.; Zhang, J.; Pillai, R.; Shouse, G.; Sanchez, J.F.; Mei, M.; Nakamura, R.; Stein, A.S.; Forman, S.J.; Marcucci, G.; et al. Venetoclax and Hypomethylating Agents in TP53-Mutated Acute Myeloid Leukaemia. Br. J. Haematol. 2019, 187, e45–e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, A.D.; Horvat, T.Z.; Hsu, M.; Devlin, S.M.; Cuello, B.M.; Daley, R.J.; King, A.C.; Buie, L.W.; Glass, J.L.; Mauro, M.J.; et al. Venetoclax Combined with Either a Hypomethylating Agent or Low-Dose Cytarabine Shows Activity in Relapsed and Refractory Myeloid Malignancies. Blood 2017, 130, 1353. [Google Scholar]
- Ball, B.; Hsu, M.; Devlin, S.M.; Famulare, C.; Cai, S.F.; Dunbar, A.; Epstein-Peterson, Z.D.; Menghrajani, K.; Glass, J.L.; Taylor, J.; et al. RAS Mutations Are Independently Associated with Decreased Overall Survival and Event-Free Survival in Patients with AML Receiving Induction Chemotherapy. Blood 2019, 134, 18. [Google Scholar] [CrossRef]
- Stasik, S.; Eckardt, J.N.; Kramer, M.; Röllig, C.; Krämer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. Impact of PTPN11 Mutations on Clinical Outcome Analyzed in 1529 Patients with Acute Myeloid Leukemia. Blood Adv. 2021, 5, 3279–3289. [Google Scholar] [CrossRef] [PubMed]
- Chyla, B.; Daver, N.; Doyle, K.; McKeegan, E.; Huang, X.; Ruvolo, V.; Wang, Z.; Chen, K.; Souers, A.; Leverson, J.; et al. Genetic Biomarkers of Sensitivity and Resistance to Venetoclax Monotherapy in Patients with Relapsed Acute Myeloid Leukemia. Am. J. Hematol. 2018, 93, E202–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Riley-Gillis, B.; Han, L.; Jia, Y.; Lodi, A.; Zhang, H.; Ganesan, S.; Pan, R.; Konoplev, S.N.; Sweeney, S.R.; et al. Activation of RAS/MAPK Pathway Confers MCL-1 Mediated Acquired Resistance to BCL-2 Inhibitor Venetoclax in Acute Myeloid Leukemia. Signal Transduct. Target. Ther. 2022, 7, 51. [Google Scholar] [CrossRef]
- Stevens, B.M.; Jones, C.L.; Winters, A.; Dugan, J.; Abbott, D.; Savona, M.R.; Fesik, S.W.; Pollyea, D.A.; Jordan, C.T. PTPN11 Mutations Confer Unique Metabolic Properties and Increase Resistance to Venetoclax and Azacitidine in Acute Myelogenous Leukemia. Blood 2018, 132, 909. [Google Scholar] [CrossRef]
- Stevens, B.M.; Jones, C.L.; Pollyea, D.A.; Culp-Hill, R.; D’Alessandro, A.; Winters, A.; Krug, A.; Abbott, D.; Goosman, M.; Pei, S.; et al. Fatty Acid Metabolism Underlies Venetoclax Resistance in Acute Myeloid Leukemia Stem Cells. Nat. Cancer 2020, 1, 1176–1187. [Google Scholar] [CrossRef]
- Goemans, B.F.; Zwaan, C.M.; Martinelli, S.; Harrell, P.; de Lange, D.; Carta, C.; Reinhardt, D.; Ha¨hlen, K.; Creutzig, U.; Tartaglia, M.; et al. Differences in the Prevalence of PTPN11 Mutations in FAB M5 Paediatric Acute Myeloid Leukaemia. Br. J. Haematol. 2005, 130, 800–801. [Google Scholar] [CrossRef]
- Pei, S.; Pollyea, D.A.; Gustafson, A.; Stevens, B.M.; Minhajuddin, M.; Fu, R.; Riemondy, K.A.; Gillen, A.E.; Sheridan, R.M.; Kim, J.; et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia. Cancer Discov. 2020, 10, 536–551. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Mysliwski, M.; Serio, J.; Ropa, J.; Abulwerdi, F.A.; Chan, R.J.; Patel, J.P.; Tallman, M.S.; Paietta, E.; et al. Mutated Ptpn11 Alters Leukemic Stem Cell Frequency and Reduces the Sensitivity of Acute Myeloid Leukemia Cells to Mcl1 Inhibition. Leukemia 2015, 29, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Zhang, Q.; Dail, M.; Shi, C.; Cavazos, A.; Ruvolo, V.R.; Zhao, Y.; Kim, E.; Rahmani, M.; Mak, D.H.; et al. Concomitant Targeting of BCL2 with Venetoclax and MAPK Signaling with Cobimetinib in Acute Myeloid Leukemia Models. Haematologica 2020, 105, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastenhuber, E.R.; Lowe, S.W. Putting P53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laptenko, O.; Prives, C. Transcriptional Regulation by P53: One Protein, Many Possibilities. Cell Death Differ. 2006, 13, 951–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivier, M.; Hollstein, M.; Hainaut, P. TP53 Mutations in Human Cancers: Origins, Consequences, and Clinical Use. Cold Spring Harb. Perspect. Biol. 2010, 2, a001008. [Google Scholar] [CrossRef] [Green Version]
- Rücker, F.G.; Schlenk, R.F.; Bullinger, L.; Kayser, S.; Teleanu, V.; Kett, H.; Habdank, M.; Kugler, C.M.; Holzmann, K.; Gaidzik, V.I.; et al. TP53 Alterations in Acute Myeloid Leukemia with Complex Karyotype Correlate with Specific Copy Number Alterations, Monosomal Karyotype, and Dismal Outcome. Blood 2012, 119, 2114–2121. [Google Scholar] [CrossRef]
- Short, N.J.; Montalban-Bravo, G.; Hwang, H.; Ning, J.; Franquiz, M.J.; Kanagal-Shamanna, R.; Patel, K.P.; DiNardo, C.D.; Ravandi, F.; Garcia-Manero, G.; et al. Prognostic and Therapeutic Impacts of Mutant TP53 Variant Allelic Frequency in Newly Diagnosed Acute Myeloid Leukemia. Blood Adv. 2020, 4, 5681–5689. [Google Scholar] [CrossRef]
- Grob, T.; Al Hinai, A.S.A.; Sanders, M.A.; Kavelaars, F.G.; Rijken, M.; Gradowska, P.L.; Biemond, B.J.; Breems, D.A.; Maertens, J.; van Marwijk Kooy, M.; et al. Molecular Characterization of Mutant TP53 Acute Myeloid Leukemia and High-Risk Myelodysplastic Syndrome. Blood 2022, 139, 2347–2354. [Google Scholar] [CrossRef]
- Nechiporuk, T.; Kurtz, S.E.; Nikolova, O.; Liu, T.; Jones, C.L.; D’alessandro, A.; Culp-Hill, R.; D’almeida, A.; Joshi, S.K.; Rosenberg, M.; et al. The TP53 Apoptotic Network Is a Primary Mediator of Resistance to BCL2 Inhibition in AML Cells. Cancer Discov. 2019, 9, 910–925. [Google Scholar] [CrossRef]
- Michels, J.; Johnson, P.W.M.; Packham, G. Mcl-1. Int. J. Biochem. Cell Biol. 2005, 37, 267–271. [Google Scholar] [CrossRef]
- Pan, R.; Ruvolo, V.; Mu, H.; Leverson, J.D.; Nichols, G.; Reed, J.C.; Konopleva, M.; Andreeff, M. Synthetic Lethality of Combined Bcl-2 Inhibition and P53 Activation in AML: Mechanisms and Superior Antileukemic Efficacy. Cancer Cell 2017, 32, 748–760.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schakel, U.; Platzbecker, U.; Wermke, M.; Bornhauser, M.; Ritter, M.; Neubauer, A.; et al. Analysis OfFLT3-Activating Mutations in 979 Patients with Acute Myelogenous Leukemia: Association with FAB Subtypes and Identification Ofsubgroups with Poor Prognosis. Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novershtern, N.; Subramanian, A.; Lawton, L.N.; Mak, R.H.; Haining, W.N.; McConkey, M.E.; Habib, N.; Yosef, N.; Chang, C.Y.; Shay, T.; et al. Densely Interconnected Transcriptional Circuits Control Cell States in Human Hematopoiesis. Cell 2011, 144, 296–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.L.; Stevens, B.M.; Pollyea, D.A.; Culp-Hill, R.; Reisz, J.A.; Nemkov, T.; Gehrke, S.; Gamboni, F.; Krug, A.; Winters, A.; et al. Nicotinamide Metabolism Mediates Resistance to Venetoclax in Relapsed Acute Myeloid Leukemia Stem Cells. Cell Stem Cell 2020, 27, 748–764.e4. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Duncavage, E.J.; Chang, G.S.; Jacoby, M.A.; Miller, C.A.; Shao, J.; Heath, S.; Elliott, K.; Reineck, T.; Fulton, R.S.; et al. Dynamic Changes in the Clonal Structure of MDS and AML in Response to Epigenetic Therapy. Leukemia 2017, 31, 872–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson-Grusby, L.; Laird, P.W.; Magge, S.N.; Moeller, B.J.; Jaenisch, R. Mutagenicity of 5-Aza-2′-Deoxycytidine Is Mediated by the Mammalian DNA Methyltransferase. Proc. Natl. Acad. Sci. USA 1997, 94, 4681–4685. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Jelinek, J.; Si, J.; Shu, J.; Issa, J.P.J. Mechanisms of Resistance to 5-Aza-2’-Deoxycytidine in Human Cancer Cell Lines. Blood 2009, 113, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Ørskov, A.D.; Treppendahl, M.B.; Skovbo, A.; Holm, M.S.; Friis, L.S.; Hokland, M.; Grønbæk, K. Hypomethylation and Up-Regulation of PD-1 in T Cells by Azacytidine in MDS/AML Patients: A Rationale for Combined Targeting of PD-1 and DNA Methylation. Oncotarget 2015, 6, 9612–9626. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, G.S.; Al-Harbi, S.; Mazumder, S.; Hill, B.T.; Smith, M.R.; Bodo, J.; Hsi, E.D.; Almasan, A. MCL-1 and BCL-XL-Dependent Resistance to the BCL-2 Inhibitor ABT-199 Can Be Overcome by Preventing PI3K/AKT/MTOR Activation in Lymphoid Malignancies. Cell Death Dis. 2015, 6, e1593. [Google Scholar] [CrossRef]
- Ramsey, H.E.; Fischer, M.A.; Lee, T.; Gorska, A.E.; Arrate, M.P.; Fuller, L.; Boyd, K.L.; Strickland, S.A.; Sensintaffar, J.; Hogdal, L.J.; et al. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov. 2018, 8, 1566–1581. [Google Scholar] [CrossRef] [Green Version]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Dohner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax Resistance and Acquired BCL2 Mutations in Chronic Lymphocytic Leukemia. Heaematologica 2019, 5, 117–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blombery, P.; Anderson, M.A.; Gong, J.N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birkinshaw, R.W.; Gong, J.N.; Luo, C.S.; Lio, D.; White, C.A.; Anderson, M.A.; Blombery, P.; Lessene, G.; Majewski, I.J.; Thijssen, R.; et al. Structures of BCL-2 in Complex with Venetoclax Reveal the Molecular Basis of Resistance Mutations. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Lew, T.E.; Dengler, M.A.; Thompson, E.R.; Lin, V.S.; Chen, X.; Nguyen, T.; Panigrahi, A.; Handunnetti, S.M.; Carney, D.; et al. Clonal Hematopoiesis, Myeloid Disorders and BAX-Mutated Myelopoiesis in Patients Receiving Venetoclax for CLL. Blood 2021, 139, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qian, J.; Wang, H.; Wang, Y.; Zhang, Y.; Qian, P.; Lou, Y.; Jin, J.; Zhu, H. Not BCL2 Mutation but Dominant Mutation Conversation Contributed to Acquired Venetoclax Resistance in Acute Myeloid Leukemia. Biomark. Res. 2021, 9, 7–10. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Stein, A.S.; Stein, E.M.; Fathi, A.T.; Frankfurt, O.; Schuh, A.C.; Döhner, H.; Martinelli, G.; Patel, P.A.; Raffoux, E.; et al. Mutant Isocitrate Dehydrogenase 1 Inhibitor Ivosidenib in Combination with Azacitidine for Newly Diagnosed Acute Myeloid Leukemia. J. Clin. Oncol. 2021, 39, 57–65. [Google Scholar] [CrossRef]
- Venugopal, S.; Maiti, A.; DiNardo, C.D.; Loghavi, S.; Daver, N.G.; Kadia, T.M.; Rausch, C.R.; Alvarado, Y.; Ohanian, M.; Sasaki, K. Decitabine and Venetoclax for IDH1/2-Mutated Acute Myeloid Leukemia. Am. J. Hematol. 2021, 96, E154–E157. [Google Scholar] [CrossRef]
- Dinardo, C.D.; Schuh, A.C.; Stein, E.M.; Montesinos, P.; Wei, A.; De Botton, S.; Zeidan, A.M.; Fathi, A.T.; Quek, L.; Kantarjian, H.M.; et al. Effect of Enasidenib (ENA) plus Azacitidine (AZA) on Complete Remission and Overall Response versus AZA Monotherapy in Mutant-IDH2 (MIDH2) Newly Diagnosed Acute Myeloid Leukemia (ND-AML). J. Clin. Oncol. 2020, 38, 7501. [Google Scholar] [CrossRef]
- Issa, G.C.; DiNardo, C.D. Acute Myeloid Leukemia with IDH1 and IDH2 Mutations: 2021 Treatment Algorithm. Blood Cancer J. 2021, 11, 107. [Google Scholar] [CrossRef]
- McMurry, H.; Fletcher, L.; Traer, E. IDH Inhibitors in AML—Promise and Pitfalls. Curr. Hematol. Malig. Rep. 2021, 16, 207–217. [Google Scholar] [CrossRef]
- Lachowiez, C.A.; Borthakur, G.; Loghavi, S.; Zeng, Z.; Kadia, T.M.; Masarova, L.; Takahashi, K.; Tippett, G.D.; Smith, S.; Garcia, J.; et al. A Phase Ib/II Study of Ivosidenib with Venetoclax +/- Azacitidine in IDH1-Mutated Myeloid Malignancies. J. Clin. Oncol. 2021, 39, 7012. [Google Scholar] [CrossRef]
- Daver, N.; Venugopal, S.; Ravandi, F. FLT3 Mutated Acute Myeloid Leukemia: 2021 Treatment Algorithm. Blood Cancer J. 2021, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3 -Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Maiti, A.; DiNardo, C.D.; Daver, N.G.; Rausch, C.R.; Ravandi, F.; Kadia, T.M.; Pemmaraju, N.; Borthakur, G.; Bose, P.; Issa, G.C.; et al. Triplet Therapy with Venetoclax, FLT3 Inhibitor and Decitabine for FLT3-Mutated Acute Myeloid Leukemia. Blood Cancer J. 2021, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Kantarjian, H.; Short, N.J.; Reville, P.; Konopleva, M.; Kadia, T.; Dinardo, C.; Borthakur, G.; Pemmaraju, N.; Maiti, A.; et al. Hypomethylating Agent and Venetoclax with FLT3 Inhibitor “Triplet” Therapy in Older/Unfit Patients with FLT3 Mutated AML. Blood Cancer J. 2022, 12, 77. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Q.; Su, Y.; Lv, J.; Gai, Y.; Liu, S.; Lin, H.; Wang, Y.; Wang, G. Cotargeting of Bcl-2 and Mcl-1 Shows Promising Antileukemic Activity against AML Cells Including Those with Acquired Cytarabine Resistance. Exp. Hematol. 2022, 105, 39–49. [Google Scholar] [CrossRef]
- Post, S.M.; Ma, H.; Malaney, P.; Zhang, X.; Aitken, M.J.L.; Mak, P.Y.; Ruvolo, V.R.; Yasuhiro, T.; Kozaki, R.; Chan, L.E.; et al. AXL/MERTK Inhibitor ONO-7475 Potently Synergizes with Venetoclax and Overcomes Venetoclax Resistance to Kill FLT3-ITD Acute Myeloid Leukemia. Haematologica 2021, 107, 1204–1223. [Google Scholar] [CrossRef]
- Hormi, M.; Birsen, R.; Belhadj, M.; Huynh, T.; Cantero Aguilar, L.; Grignano, E.; Haddaoui, L.; Guillonneau, F.; Mayeux, P.; Hunault, M.; et al. Pairing MCL-1 Inhibition with Venetoclax Improves Therapeutic Efficiency of BH3-Mimetics in AML. Eur. J. Haematol. 2020, 105, 588–596. [Google Scholar] [CrossRef]
- Panina, S.B.; Pei, J.; Kirienko, N.V. Mitochondrial Metabolism as a Target for Acute Myeloid Leukemia Treatment. Cancer Metab. 2021, 9, 1–25. [Google Scholar] [CrossRef]
- Bosc, C.; Saland, E.; Bousard, A.; Gadaud, N.; Sabatier, M.; Cognet, G.; Farge, T.; Boet, E.; Gotanègre, M.; Aroua, N.; et al. Mitochondrial Inhibitors Circumvent Adaptive Resistance to Venetoclax and Cytarabine Combination Therapy in Acute Myeloid Leukemia. Nat. Cancer 2021, 2, 1204–1223. [Google Scholar] [CrossRef]
- Niu, X.; Rothe, K.; Chen, M.; Grasedieck, S.; Li, R.; Nam, S.E.; Zhang, X.; Novakovskiy, G.E.; Ahn, Y.H.; Maksakova, I.; et al. Targeting AXL Kinase Sensitizes Leukemic Stem and Progenitor Cells to Venetoclax Treatment in Acute Myeloid Leukemia. Blood 2021, 137, 3641–3655. [Google Scholar] [CrossRef] [PubMed]
- Sharon, D.; Cathelin, S.; Mirali, S.; Di Trani, J.M.; Yanofsky, D.J.; Keon, K.A.; Rubinstein, J.L.; Schimmer, A.D.; Ketela, T.; Chan, S.M. Inhibition of Mitochondrial Translation Overcomes Venetoclax Resistance in AML through Activation of the Integrated Stress Response. Sci. Transl. Med. 2019, 11, aax2863. [Google Scholar] [CrossRef] [PubMed]
- Scotland, S.; Saland, E.; Skuli, N.; De Toni, F.; Boutzen, H.; Micklow, E.; Sénégas, I.; Peyraud, R.; Peyriga, L.; Théodoro, F.; et al. Mitochondrial Energetic and AKT Status Mediate Metabolic Effects and Apoptosis of Metformin in Human Leukemic Cells. Leukemia 2013, 27, 2129–2138. [Google Scholar] [CrossRef]
- Zhou, F.J.; Zeng, C.X.; Kuang, W.; Cheng, C.; Liu, H.C.; Yan, X.Y.; Chen, X.P.; Zhou, G.; Cao, S. Metformin Exerts a Synergistic Effect with Venetoclax by Downregulating Mcl-1 Protein in Acute Myeloid Leukemia. J. Cancer 2021, 12, 6727–6739. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL Receptor Tyrosine Kinase as a Promising Anti-Cancer Approach: Functions, Molecular Mechanisms and Clinical Applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Munoz-Sagredo, L.; Streule, K.; Muschong, P.; Bayer, E.; Walter, R.J.; Gutjahr, J.C.; Greil, R.; Concha, M.L.; Müller-Tidow, C.; et al. CD44 Loss of Function Sensitizes AML Cells to the BCL-2 Inhibitor Venetoclax by Decreasing CXCL12-Driven Survival Cues. Blood 2021, 138, 1067–1080. [Google Scholar] [CrossRef]
- Chesnokov, M.S.; Borhani, S.; Halasi, M.; Arbieva, Z.; Khan, I.; Gartel, A.L. FOXM1-AKT Positive Regulation Loop Provides Venetoclax Resistance in AML. Front. Oncol. 2021, 11, 696532. [Google Scholar] [CrossRef]
| Risk Stratification (%) | Overall Outcome | CR/CRi Outcome (Months) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Stage | n | Median Age (Range) | Favorable | Intermediate | Adverse | NA | Combination Therapy | mOS (Months) | CR/CRi (%) | mOS | PFS |
| Asghari (2019) [36] | ND | 41 | 75 (47–86) | 24.4 | 58.5 | 17.1 | HMA | 13.8 | 56 | NA | 5.2 | |
| DiNardo (2019) [31] | ND | 145 | 75 (65–86) | - | 51 | 49 | - | HMA | 17.5 | 67 | NA | 11.3 |
| DiNardo (2020) [37] | ND | 58 | 74 (62–87) | - | 62.1 | 34.5 | 3.4 | HMA | 15.1 | 69 | NA | 22.4 |
| 23 | 73 (66–78) | 4.3 | 60.9 | 26.1 | 8.7 | LDAC | 10.7 | 52 | NA | 17.1 | ||
| DiNardo (2020) [38] | ND | 286 | 76 (49–91) | - | 64 | 36 | - | AZA | 14.7 (11.9–18.7) | 37 | NA | NA |
| DiNardo (2021) [44] | ND | 29 | 45 (20–65) | 17.2 | 44.8 | 37.9 | - | FLAG-IDA | NR | 90 | NA | NR |
| Morsia (2020) [39] | ND | 44 | 65 (18–79) | 7.1 | 26.2 | 66.7 | - | HMA | 11 (8–23) | 50 | 17 (9-NR) | NR (11-NR) |
| Pollyea (2020) [40] | ND | 84 | 75 (61–90) | - | 60 | 39 | 1 | AZA | 16.4 (11.3–24.5) | 71 | NA | 21.9 (15.1–30.2) |
| 31 | 72 (65–86) | - | 52 | 48 | - | DEC | 16.2 (9.1–27.8) | 74 | NA | 15.0 (7.2–30.0) | ||
| Wei (2019) [41] | ND | 82 | 74 (63–90) | - | 60 | 32 | 8 | LDAC | 10.1 (5.7–14.2) | 54 | 18.4 (14.0-NR) | 8.1 (5.3–14.9) |
| Wei (2020) [42] | ND | 143 | 76 (36–93) | 1 | 63 | 33 | 3 | LDAC | 8.4 (5.9–10.1) | 34 | NA | 4.7 (3.7–6.4) |
| Winters (2019) [43] | ND | 33 | 72 (33–85) | - | - | 67 | 33 | AZA | 12.7 (5.8-NR) | 63 | NA | 10.7 (4.5-NR) |
| Aldoss (2018) [47] | R/R | 33 | 62 (19–81) | 9.1 | 33.3 | 54.5 | 3 | HMA | NR | 51 | NR | 8.9 (3.2–10.6) |
| Aldoss (2019) [48] | R/R | 90 | 59 (18–81) | 8 | 26 | 66 | - | HMA | 7.8 (5.9–15.5) | 46 | 16.6 (13.5–26.8) | 8.9 (5.9–15.2) |
| Asghari (2019) [36] | R/R | 31 | 63 (25–77) | 25.8 | 45.2 | 29.0 | HMA | 4.9 | 28 | NR | 5.2 | |
| Byrne (2020) [49] | R/R | 21 | 65 (35–74) | 4.8 | 47.6 | 47.6 | - | HMA | 7.8 (0.2–12.1) | 38 | NR | NA |
| DiNardo (2018) [50] | R/R | 43 | 68 (25–83) | - | 53 | 47 | - | HMA/LDAC | 3.0 (0.5–8.0) | 12 | 4.8 | NA |
| DiNardo (2021) [44] | R/R | 23 | 47 (22–66) | 26 | 13 | 61 | - | FLAG-IDA | NR | 61 | NA | 11 (2-NR) |
| Gaut (2020) [51] | R/R | 14 | 58 (41–79) | - | 28.6 | 71.4 | - | HMA/LDAC | 4.7 (NA) | 21 | NR | NR |
| Konopleva (2016) [52] | R/R | 32 | 71 (19–84) | - | - | - | 100 | 4.7 (2.3–6.0) | 19 | NA | 2.3 (1.0–2.7) | |
| Morsia (2020) [39] | R/R | 42 | 65 (18–79) | 7.1 | 26.2 | 66.7 | - | HMA | 5 (3–9) | 33 | 15 (5-NR) | 8 (1–20) |
| Ram (2019) [53] | R/R | 23 | 76 (41–92) | 9 | 48 | 43 | - | HMA/LDAC | 5.6 (4.9–6.2) | 43 | 10.8 (6.2–15.4) | NA |
| Wang (2020) [54] | R/R | 40 | 63 (20–88) | 12.5 | 17.5 | 70 | - | HMA/LDAC | 6.6 (0.7–16.3) | 23 | NR | NA |
| Adverse Events ≥ Grade 3 (%) | Common Adverse Events (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Therapy | Febrile Neutropenia | Neutropenia | Anemia | Pneumonia | Nausea | Vomiting | Diarrhea |
| Dombret (2015) [14] | AZA | 28 | 36 | 16 | 19 | 27 | 14 | 12 |
| LDAC | 30 | 25 | 23 | 19 | 22 | 11 | 5 | |
| IC | 31 | 33 | 14 | 5 | 43 | 7 | 21 | |
| DiNardo (2019) [31] | AZA + VEN (400 mg) | 38 | NA | 31 | NA | 62 | 31 | 52 |
| AZA + VEN (800 mg) | 35 | NA | 24 | NA | 62 | 27 | 49 | |
| DiNardo (2020) [38] | AZA + placebo | 19 | 28 | 20 | 25 | 35 | 23 | 33 |
| AZA + VEN (400 mg) | 42 | 42 | 26 | 56 | 44 | 30 | 41 | |
| Wei (2020) [42] | LDAC + placebo | 29 | 16 | 22 | 16 | 31 | 13 | 16 |
| LDAC + VEN (600 mg) | 32 | 46 | 25 | 20 | 42 | 25 | 28 | |
| Konopleva (2016) [52] | VEN (800 mg) | 31 | NA | NA | 19 | 59 | 41 | 56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Griffioen, M.S.; de Leeuw, D.C.; Janssen, J.J.W.M.; Smit, L. Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies. Cancers 2022, 14, 3456. https://doi.org/10.3390/cancers14143456
Griffioen MS, de Leeuw DC, Janssen JJWM, Smit L. Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies. Cancers. 2022; 14(14):3456. https://doi.org/10.3390/cancers14143456
Chicago/Turabian StyleGriffioen, Mila S., David C. de Leeuw, Jeroen J. W. M. Janssen, and Linda Smit. 2022. "Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies" Cancers 14, no. 14: 3456. https://doi.org/10.3390/cancers14143456
APA StyleGriffioen, M. S., de Leeuw, D. C., Janssen, J. J. W. M., & Smit, L. (2022). Targeting Acute Myeloid Leukemia with Venetoclax; Biomarkers for Sensitivity and Rationale for Venetoclax-Based Combination Therapies. Cancers, 14(14), 3456. https://doi.org/10.3390/cancers14143456







