- Article
Activity of Natural Substances and n-Undecyl-α/β-l-Fucopyranoside Against the Formation of Pathogenic Biofilms by Pseudomonas aeruginosa
- Christian Dietrich Vogel,
- Anne Christine Aust and
- Raffael Christoph Wende
- + 2 authors
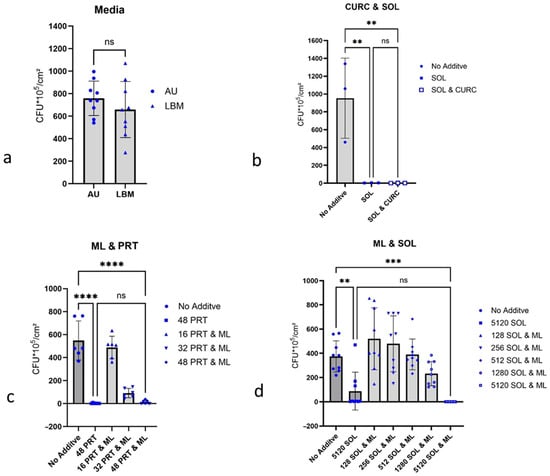
Background/Objectives: Emerging biofilms of uropathogenic bacteria, particularly P. aeruginosa, on medical devices such as urinary catheters, lead to complications in the treatment of urinary tract infections (UTI). Considering the spread of antibiotic resistance, the search for alternative efficient control options for biofilms is of great medical interest. Methods: Curcumin, 1-monolaurin, n-undecyl-α/β-l-fucopyranoside, and the fungal metabolite terrein were investigated for their influence on biofilm formation by P. aeruginosa on latex catheter pieces in artificial urine (AU), monitoring the number of colony-forming units per cm Latex-Catheter (CFU/cm Latex-Catheter). Results: Significant inhibition of P. aeruginosa biofilm formation [55.6% CFU reduction/cm2] was observed with the fungal metabolite terrein at 256 µg/mL AU. At a concentration of 512 µg/mL AU, terrein achieved almost complete inhibition of biofilm formation. n-undecyl-α/β-l-fucopyranoside inhibited biofilm formation [58.3% CFU reduction/cm2] by P. aeruginosa ATCC 27853 at 512 µg/mL AU. Compared to that, it caused an increase in biofilm formation [87.0% CFU increase/cm2] by P. aeruginosa PA 01 at 256 µg/mL AU. This study is limited by the fact that no investigations into the possible cytotoxicity of the two active substances, terrein and n-undecyl-α/β-l-fucopyranoside, on healthy eukaryotic cells have been carried out. Conclusions: Natural substances may be a promising approach to prevent the formation of P. aeruginosa biofilms. For antibacterial applications, fungal metabolites, such as terrein, offer a novel approach to prevent biofilms in urological practice.
10 January 2026