- Article
Process Differences in Phosphorus Release Between Wetland and River Sediments in a Plain River Network
- Yinan Liu,
- Xin Xu and
- Shanshan Zhao
- + 2 authors
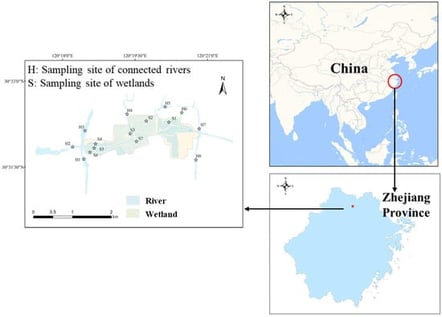
The release process of endogenous phosphorus (P) in the sediments of large ecological wetlands and their connected rivers in the plain river network area shows temporal and spatial differences. This study investigated P dynamics of the sediments in a large ecological wetland and its connected rivers in a plain river network area. Sample collection occurred across three periods (October 2024, March 2025, and July 2025). P source-sink characteristics and microbial regulatory mechanisms were analyzed to clarify differences in the P release processes between wetland (SS) and river (SH) sediments. The results showed that the total phosphorus (TP) concentration in overlying water was highest in July (0.16 mg/L), while the TP content in SS was relatively low, with a mean value of 514.1 mg/kg. SS generally acted as a P sink, with its zero equilibrium P concentrations (EPC0) significantly lower than those of river sediments (SH), reaching a minimum of 0.01 mg/L, and its maximum P sorption capacity (Qmax) higher, with a maximum value of 1.413 mg/g. In contrast, SH mainly served as a P source, with a particularly high release risk in spring and summer. Seasonal changes significantly influenced P behavior, and sorption capacity was highest in spring (March), while the high EPC0 of SH still facilitated P release under actual water conditions. In autumn, elevated microbial diversity enhanced organic matter mineralization to increase EPC0 and P release risk (p < 0.05), while in summer, specific functional phyla (Proteobacteria and Bacteroidota) simultaneously regulated both adsorption capacity (Qmax) and release threshold (EPC0) through organic matter mineralization, iron reduction, and competitive sorption (p < 0.05). This study provides scientific support for internal pollution control in ecological wetlands and watershed phosphorus management in plain river network areas.
9 March 2026