Abstract
Raw biomass presents challenges for energy use due to its high moisture content, low bulk density, and susceptibility to biological degradation, which hinder storage, transport, and utilization. An experimental setup was developed to investigate exothermic behavior during torrefaction of agricultural and food industry wastes. Exothermic reactions were observed between 190 °C and 450 °C, with more prominent effects in corn waste, sugarcane bagasse, and straw compared to sunflower husks, palm residues, and coffee skin. A series of tests performed on a torrefaction reactor with a core-type wall heating system showed that the heat generated by exothermic reactions makes it possible to reduce the torrefaction time by a factor of 1.5 (from 120 to 80 min) to obtain biochar of the required quality, with only a slight process temperature increase (15%, from 200 to 230 °C). These findings offer practical pathways for transforming waste into valuable biochar, fostering environmental resilience and socio-economic benefits in communities reliant on biomass resources.
1. Introduction
The search for alternative and renewable energy sources is driven, on the one hand, by the increasing energy demand, which is projected to grow by approximately 1.3% per year during the period from 2020 to 2030 [1], and, on the other hand, by the intensifying environmental issues associated with carbon dioxide emissions from the use of fossil fuels [2]. Bioenergy represents the largest source of renewable energy, and it is anticipated that bioenergy will account for up to 14% of industrial energy demand by 2026 [3].
The global annual biomass production is such that only 5% of this volume would be sufficient to cover half of the total primary energy demand [4]. Nevertheless, at present, bioenergy accounts for only 9% of the global primary energy consumption, with half of this share being utilized in rural communities for cooking and heating [1,3].
This situation underscores the growing need to develop technologies for converting non-food biomass and bio-waste into biofuels [5,6,7].
In their raw state, many types of biomass exhibit high moisture content, low bulk density, and a fibrous structure that impedes comminution. Moreover, biomass is prone to decay, and its storage without significant losses in thermophysical properties presents a considerable challenge.
Therefore, raw biomass, including agricultural and food industry residues, undergoes pre-treatment processes such as drying, pelletization, briquetting, and pyrolysis. The by-products of pyrolysis may also have direct applications either as fuels or as feedstocks for the production of chemical products.
Torrefaction is a thermochemical pre-treatment of biomass aimed at improving its fuel properties, while retaining up to 90% of its original energy content [8,9]. The process represents a form of low-temperature pyrolysis, carried out at slow heating rates and at temperatures up to 350 °C. The characteristics of the product obtained through torrefaction (biochar) depend on the type of the initial biomass as well as on various process parameters, including reaction temperature, heating rate, residence time, and the reaction environment.
Torrefaction has the potential to become a key technology for enhancing the properties of biomass for a wide range of applications, including its storage and transportation. Torrefied biomass can be utilized in subsequent thermochemical conversion processes such as gasification and pyrolysis, with the aim of producing higher-calorific syngas [10]. It can also be applied in combustion processes, including co-firing with coal, owing to its higher heating value and lower burning rate compared to raw biomass, which ensures more complete combustion within the furnace volume [11].
Since the emergence of torrefaction technology, several research laboratories and companies in North America and Europe have been actively seeking to establish its commercial application. Commercial-scale torrefaction plants are being introduced in different parts of the world; however, only a limited number of these facilities achieve a capacity exceeding 2 t/h [12].
The difficulty of controlling exothermic reactions during the torrefaction process hinders the widespread industrial deployment of this technology. When critical temperatures are reached, uncontrolled heat release may occur, leading to the transition to carbonization or, in severe cases, to hazardous situations such as feedstock ignition, equipment damage, or even system failure.
The available literature data on the thermal effects observed during biomass pyrolysis are largely qualitative, and in some cases, contradictory. Comparisons are further complicated by the differences in experimental conditions under which these data were obtained [13,14,15,16,17,18].
The aim of the present study is to investigate the exothermic processes occurring during biomass torrefaction, including in the context of a core-type reactor, which we have previously employed in the study of torrefaction processes for various types of biomass [19,20].
2. Materials and Methods
2.1. Materials
The study was conducted within the framework of the project “Dry and wet torrefaction of agricultural waste for production of biochar as a multifunctional product”, carried out in collaboration with S3Bio-USMS–Laboratory of Biotechnology, Bioresources and Bioinformatics (3 BIO) of Sultan Moulay Slimane University (USMS), Morocco; the Bio and Emerging Technology Institute, Ethiopia; and the Djibouti Study and Research Center, Djibouti. Therefore, the torrefaction process was studied using wheat straw, corn stalks and cobs, sunflower husks, as well as wastes more prevalent in African countries, such as olive pomace, coffee husks, date palm waste, and sugarcane bagasse.
2.2. Experimental Setup
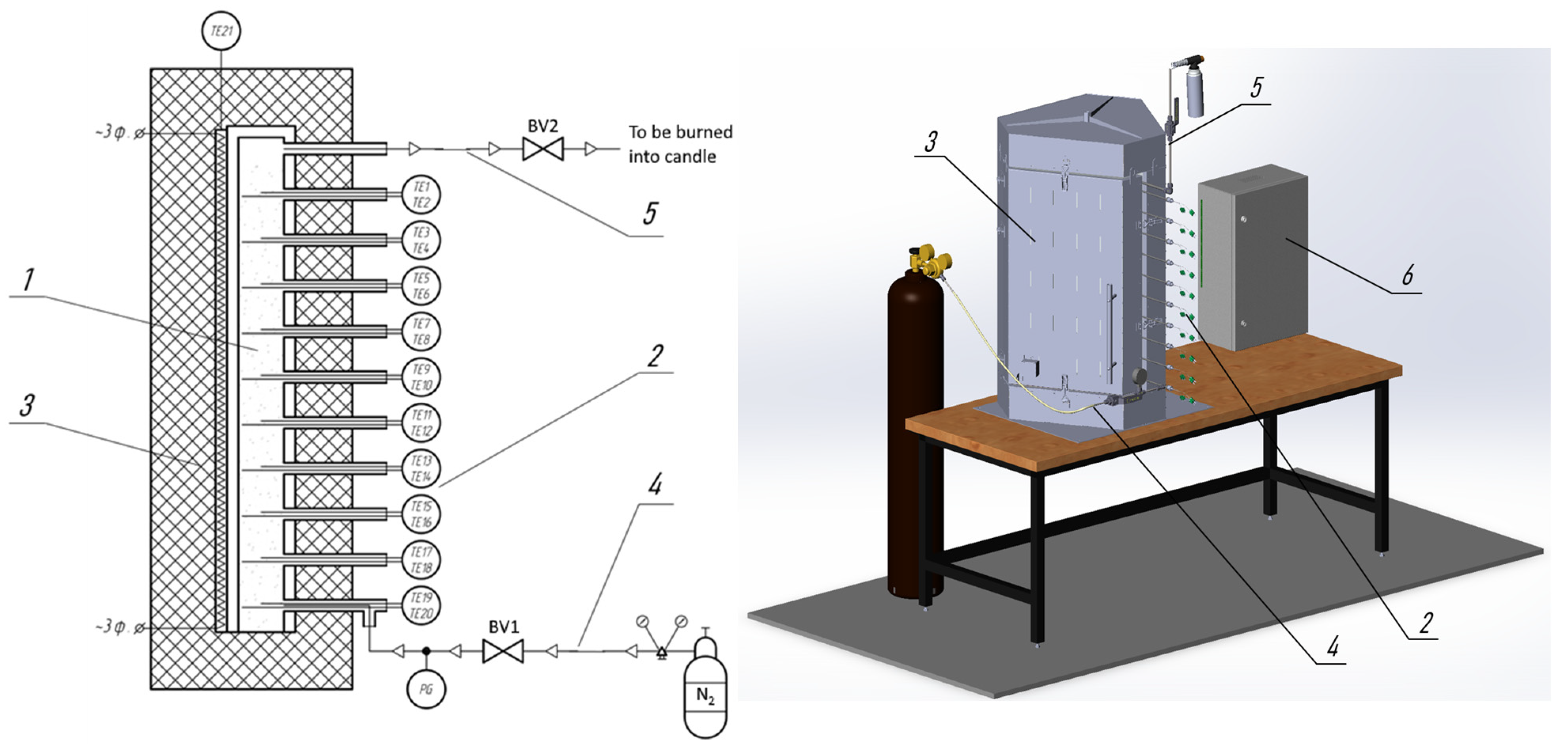
Figure 1 shows the schematic diagram and 3D model of the experimental setup designed to study the exothermic processes of thermochemical biomass conversion (pyrolysis and dry torrefaction). The aim of the research is to obtain the temperature profile at various points within the fixed bed of biomass.
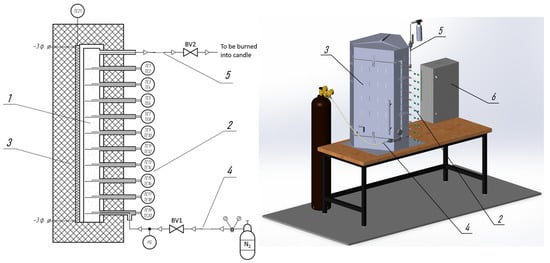
Figure 1.
Schematic diagram and 3D model of the experimental setup. 1—Pyrolysis reactor, 2—Thermocouple set, 3—Electric furnace, 4—Inert gas supply system, 5—Outlet for pyrolysis volatiles to the combustion torch, 6—Control cabinet, BV1, BV2—Ball valves, TE1–TE21—Thermocouples, PG—Pressure gauge.
The studied material, with a volume of up to 2.2 dm3, is placed in the pyrolysis reactor (1). Various samples of thermally untreated biomass, biochar samples (thermally treated biomass), as well as inert materials (ceramic beads), can be used as the experimental material.
A set of ten dual-zone thermocouples (2) is placed in guiding tubes that are evenly distributed along the entire height of the pyrolysis reactor at equal intervals of 80 mm. The dual-zone thermocouples in each guiding tube make it possible to measure the material layer temperature simultaneously at two coordinates (along the axis and near the reactor wall). Thus, comprehensive information on the evolution of the temperature field inside the pyrolysis reactor was obtained during the experiments.
The filled pyrolysis reactor is placed inside an electric furnace (3), which has a gap between the door and the body to accommodate the thermocouple tubes.
The laboratory setup is equipped with an inert gas supply system (4) to ensure an oxygen-free atmosphere inside the reactor. The inert gas supply system consists of a nitrogen cylinder, a pressure regulator, a ball valve (BV1), and a pressure gauge.
The removal of volatile pyrolysis products (6) from the reactor is carried out to a combustion torch through the ball valve (BV2).
The heating rate and maintenance of the set temperature in the furnace is carried using Proportional Integral (PI) control based on the readings of the furnace thermocouple. PI control was created programmatically using the CODESYS 2.3 development system. Adjusted coefficients increase the accuracy of the obtained experimental data and minimize the difference between the set value and the furnace temperature.
Temperature data from all thermocouples are transmitted to a controller and processed in a specially developed program. The program provides data acquisition, archiving, visualization, and analysis of the recorded signals. All processing is performed on a personal computer.
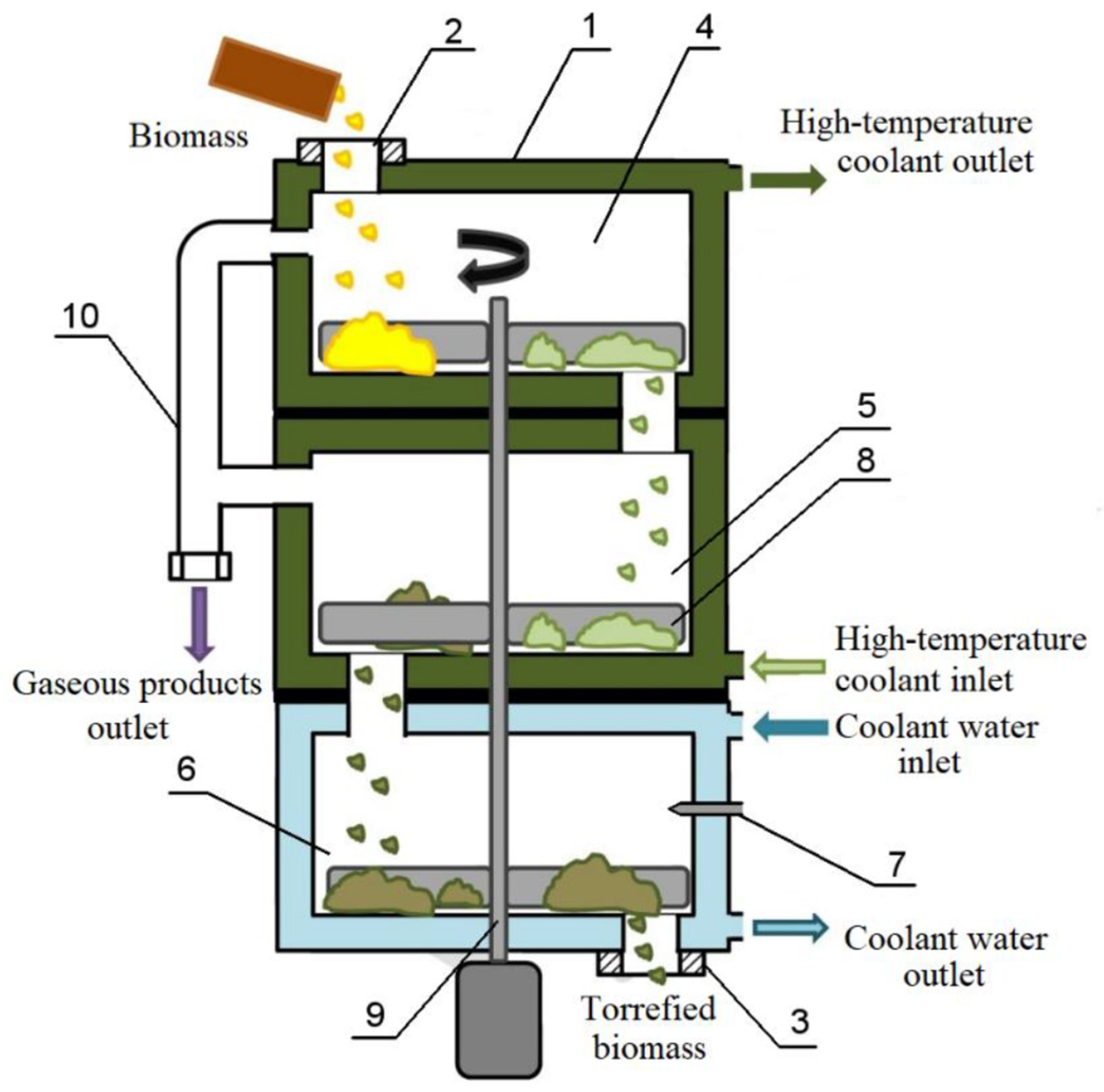
Biomass torrefaction was also carried out in a hearth-type reactor, the schematic diagram of which is shown in Figure 2.
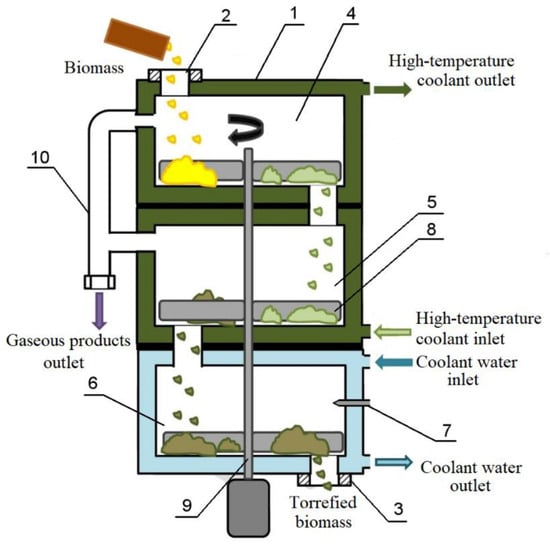
Figure 2.
Schematic diagram of the reactor for dry biomass torrefaction. 1—Torrefaction reactor body, 2—feed unit for raw biomass, 3—discharge unit for torrefied biomass, 4, 5—torrefaction trays, 6—cooling tray for torrefied biomass, 7—nozzle for cooling water supply, 8—stirrer, 9—stirrer shaft with electric drive, 10—pipeline for the removal of gaseous torrefaction products.
2.3. Experimental Methodology
The experimental procedure was identical for all materials. Pre-dried samples (1.9 L volume) were loaded into the reactor (Figure 1), which contained a mesh 70 mm above the bottom to separate the material from the gas inlet. The bed height was 700 mm, with sample mass (137–580 g) depending on bulk density.
Material loading alternated with thermocouple installation: an 80 mm material layer was added, followed by dual thermocouples with one tip against the reactor wall and the other along the axis. This ensured proper thermocouple positioning and material distribution.
The sealed reactor was purged with nitrogen until 0.0% oxygen concentration was achieved. Heating occurred in two stages: initial drying at 110 °C (30–50 min) followed by pyrolysis at 5 °C/min to 600 °C with 5–15 min holding time. This slow heating rate enabled precise detection of exothermic effects between 200 and 600 °C.
The reactor (Figure 2) features a thermal oil jacket (300 °C) and an automated feeding system. Biomass moves along heated trays (4, 5) while stirred, with torrefaction occurring in CO/CO2 atmosphere. Final cooling combines jacket cooling and direct water injection, where instant evaporation prevents moisture increase.
Process parameters were varied (180–250 °C, 30–120 min). Resulting biochar was analyzed for moisture, ash, elemental composition, and heating value.
3. Results and Discussion
Sample analysis was performed according to the following standards: EN 14775:2009 [21], EN 14774-3:2009 [22], EN 15104:2011 [23], and EN 15148:2009 [24]. The following equipment was used for biomass characterization: low-temperature laboratory electric furnace SNOL 67/350, electric muffle furnace SNOL 10/11-B (SnolTherm UAB, Narkünai, Lithuania), CHNS analyzer TruSpec Micro (LECO, St Joseph, MI, USA), and bomb calorimeter ABK-1 (Retech, Moscow, Russia).
The oxygen content was determined as the difference between 100% and the sum of the percentages of moisture, ash, sulfur, carbon, nitrogen, and hydrogen in the analytical sample. The mass of the sample in each experiment was no less than 50 g.
Chemical characteristics of raw biomass samples are presented in Table 1.

Table 1.
Physicochemical and Thermal Properties of Agricultural Waste.
As shown in Table 1, the untreated biomass samples exhibit moisture contents ranging from 5.67% to 9.3%. The ash content of the researched biomass varies widely, from 2.44% in corn residues to 9.63% in straw. Sulfur content generally does not exceed 0.1%, although it reaches 0.19% in date palm leaves and branches. Chlorine content is highest in straw and sunflower husks (1.7% and 1.9%, respectively), while in the other biomass samples, it ranges from 0.8% to 1.3%. Regarding the lower heating value, many of the studied samples have values within the range of 14.15–17.96 MJ/kg, with the minimum observed in sugarcane bagasse and the maximum in olive leaves.
Thus, the investigated biomass samples can be considered relatively low-quality, low-calorific fuels, the combustion of which is likely to result in high levels of sulfur compound emissions.
Differential thermogravimetry (DTG), and differential scanning calorimetry (DSC), were determined using a thermal analyzer (SDTQ 600, TA Instruments, New Castle, DE, USA). Thermograms were recorded in a mixture of argon, nitrogen, and oxygen. The heating rate was 5 °C/min.
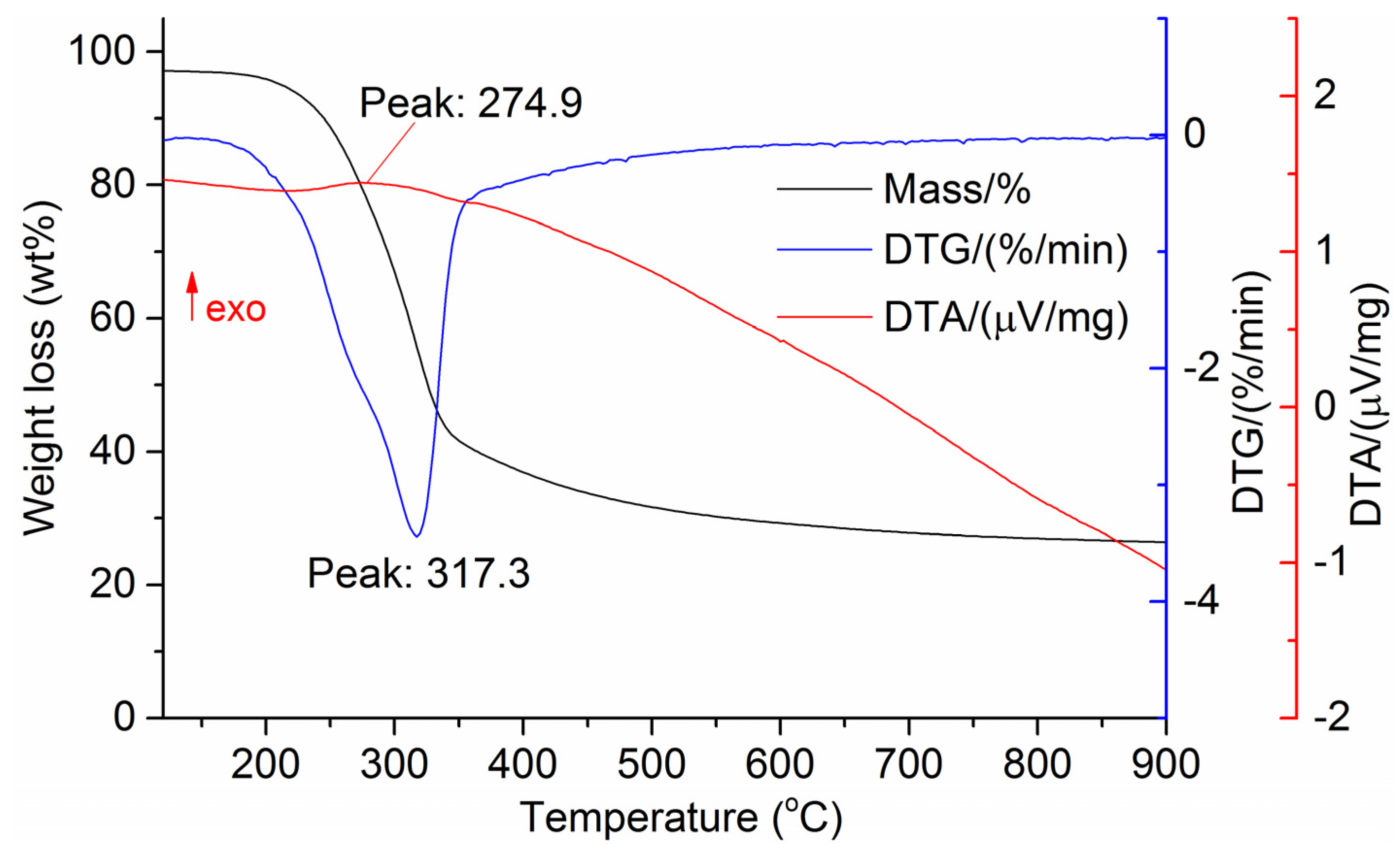
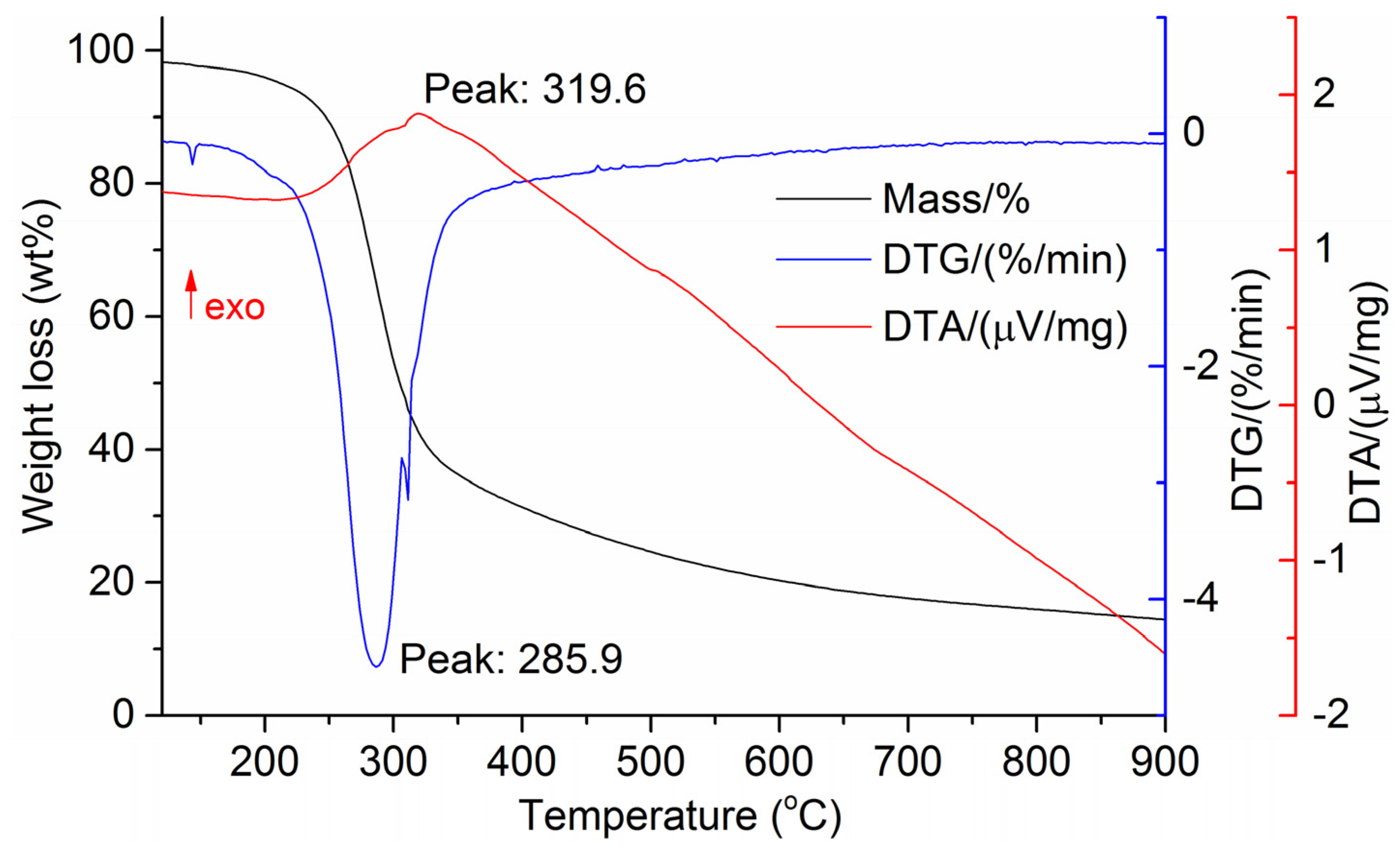
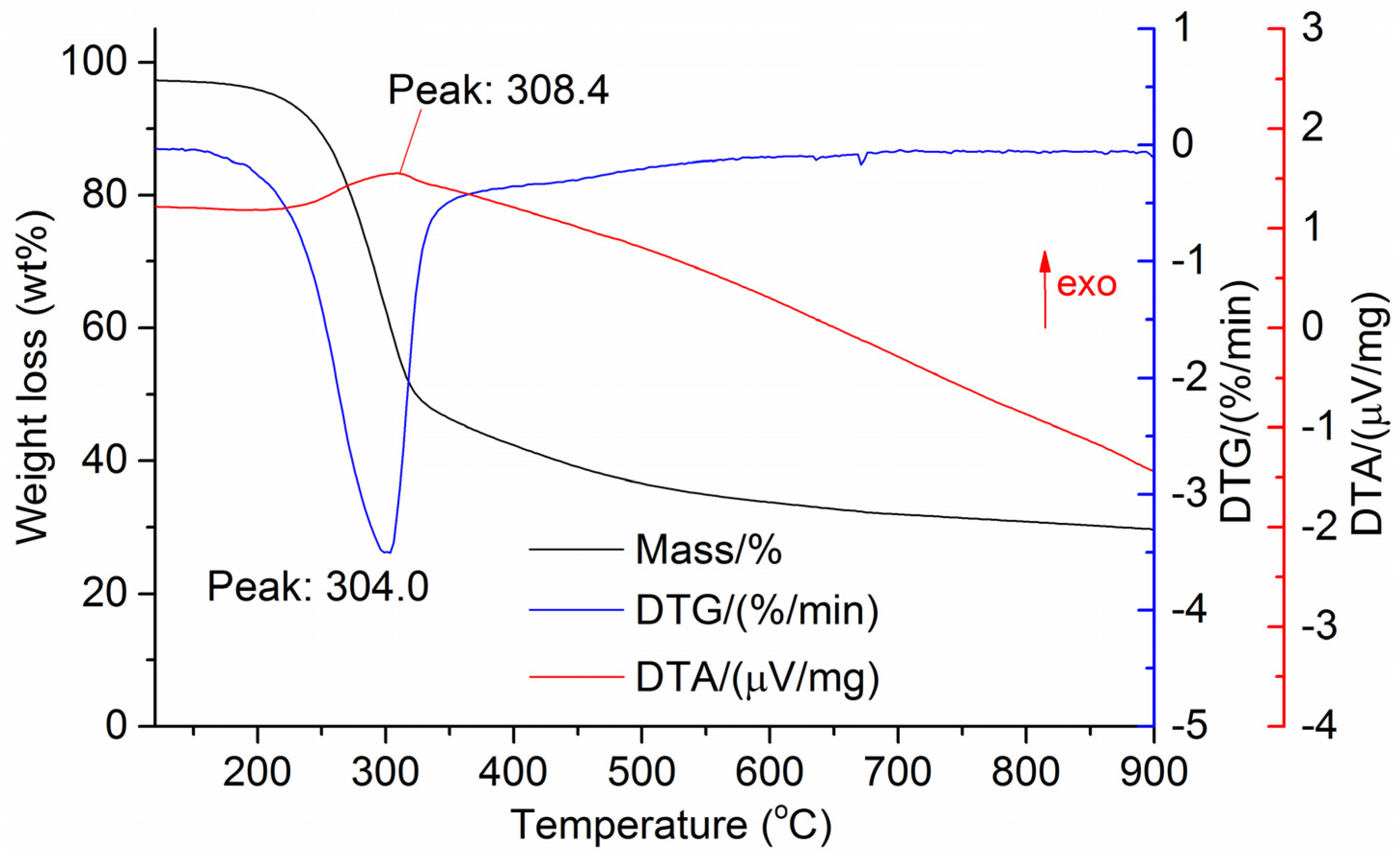
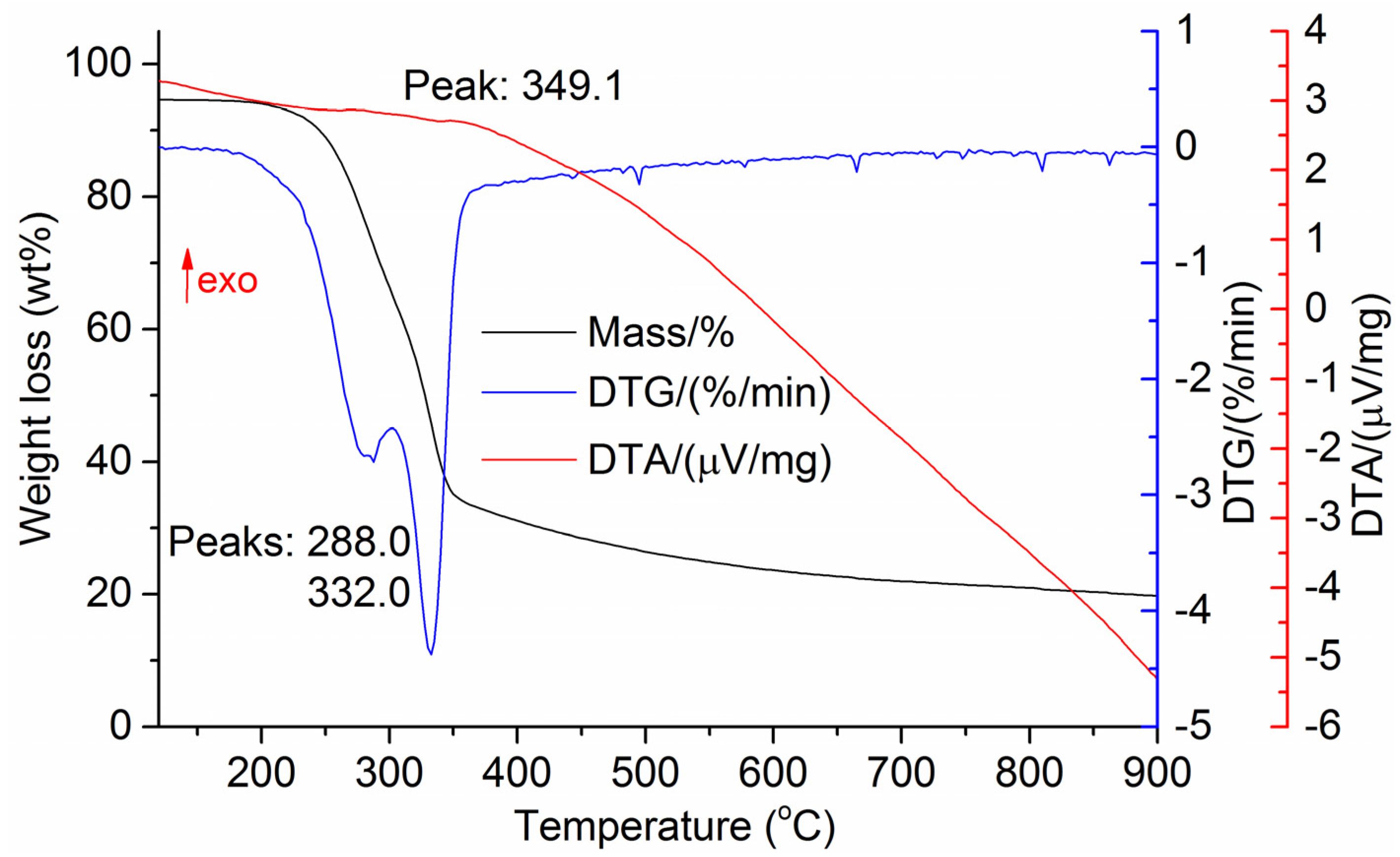
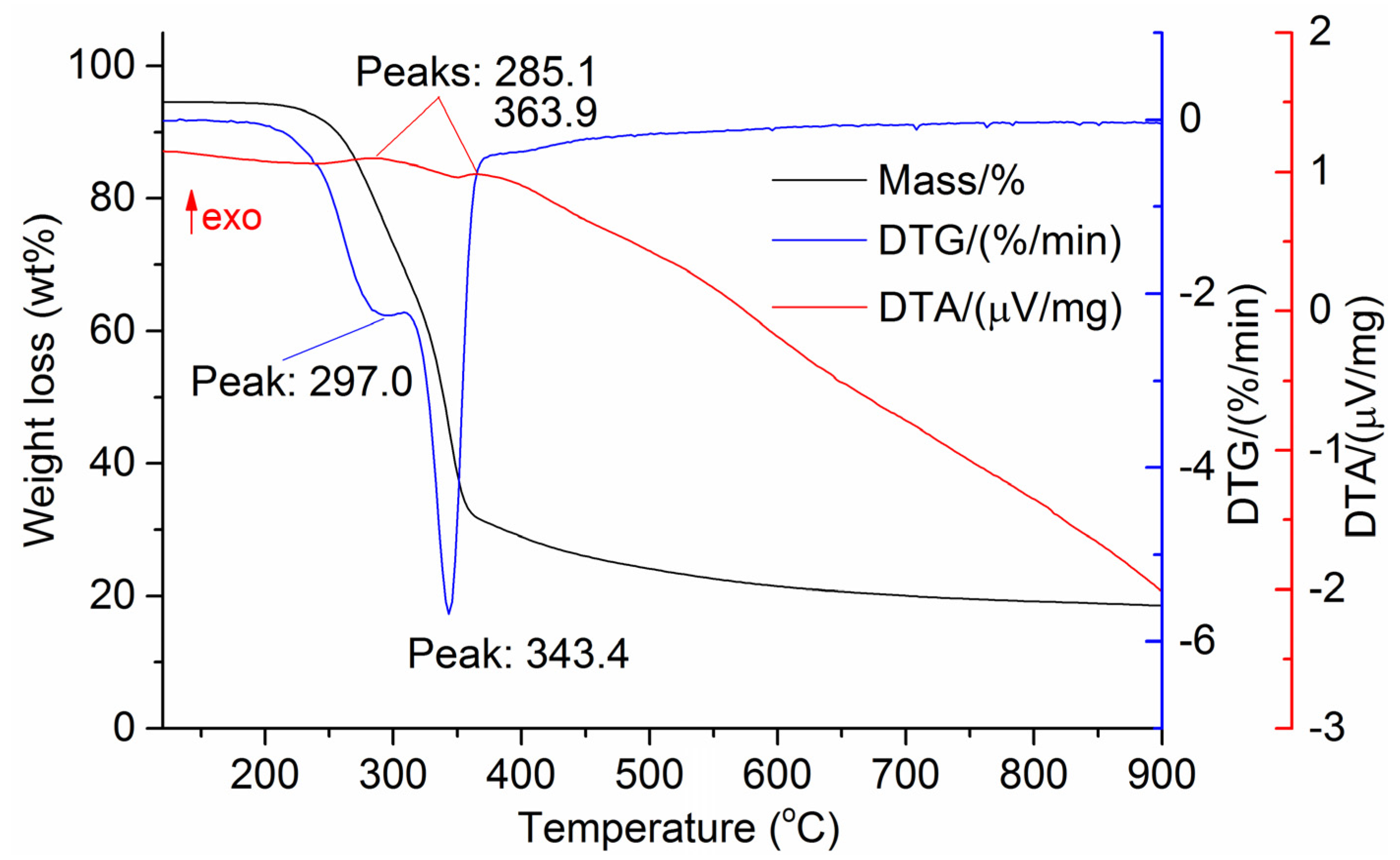
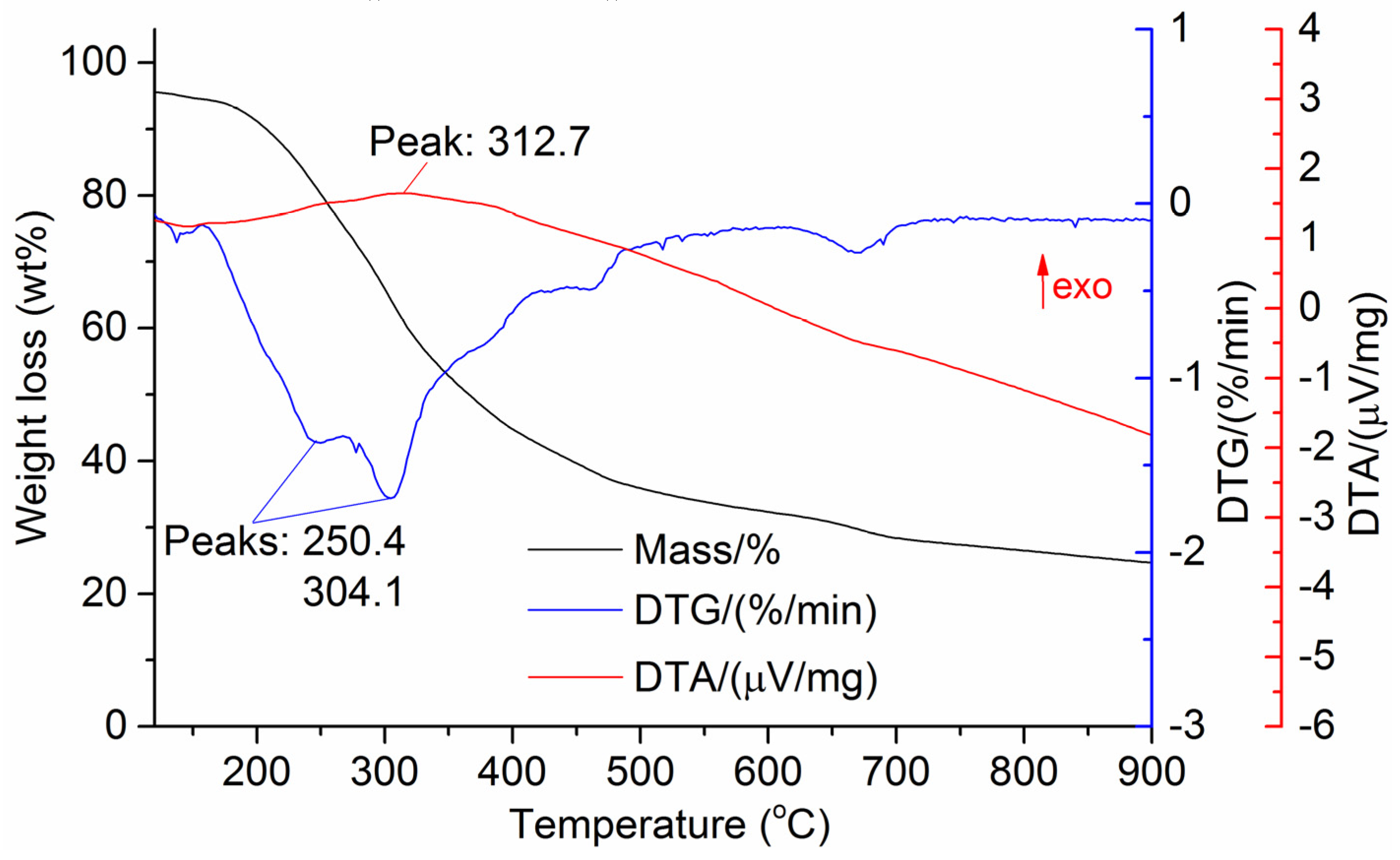
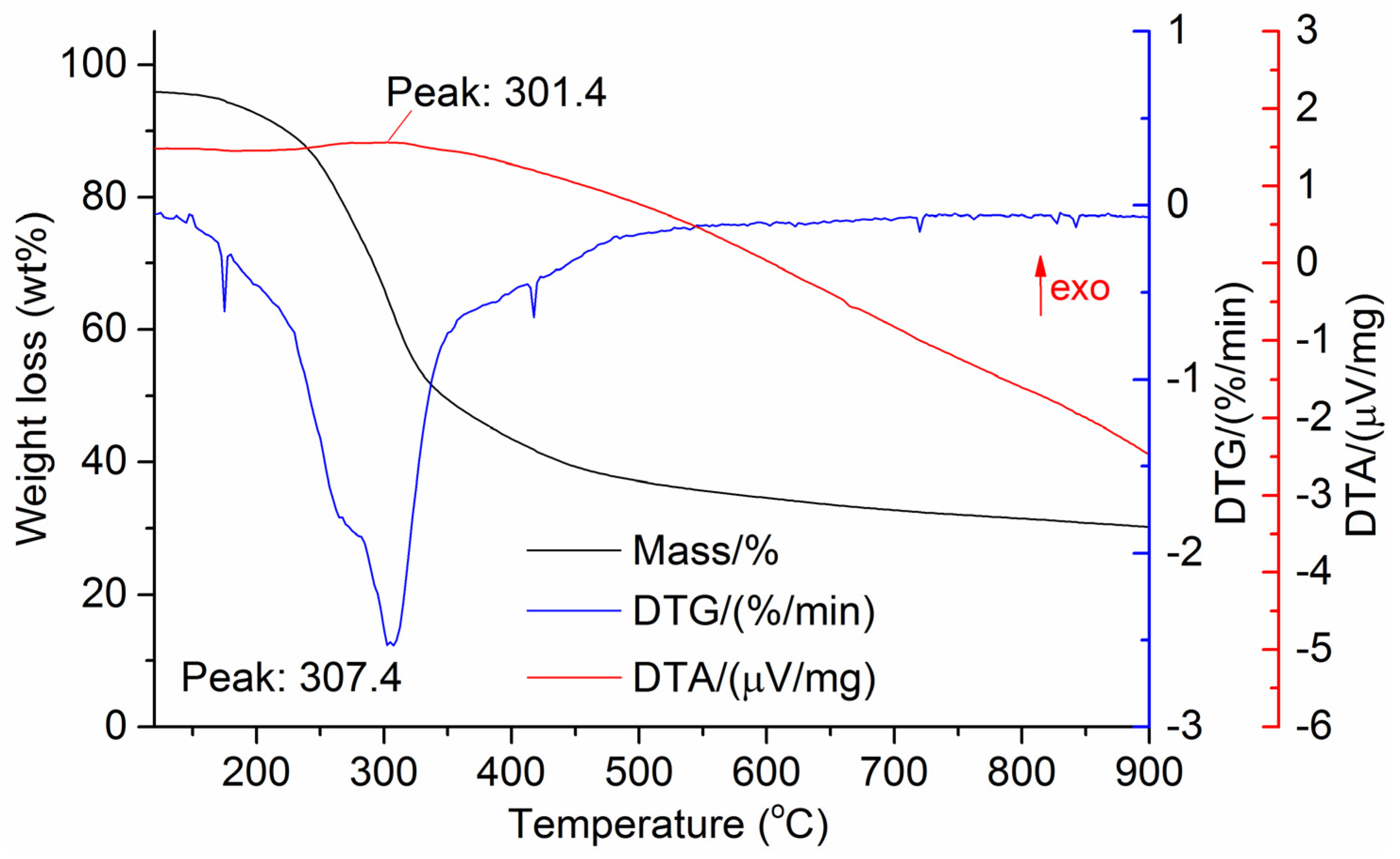
The DTG results of the studied materials are presented in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7. For each type of feedstock, the onset temperatures of decomposition, the peak mass loss temperatures, and the intervals of intense volatile release accompanied by thermal effects vary considerably. For several materials, the mass loss rate is characterized by a single peak at the following temperatures: 317.3 °C for sunflower husks, 285.9 °C for corn, 304.0 °C for wheat straw, and 307.4 °C for date palm leaves. The pyrolysis of coffee skin, olive leaves, and sugarcane bagasse exhibited two peaks at 297.0 and 343.4 °C, 250.4 and 304.1 °C, and 288.0 and 332.0 °C, respectively. The decomposition of sugarcane bagasse was characterized by three peaks at 228, 265.4, and 320.6 °C.
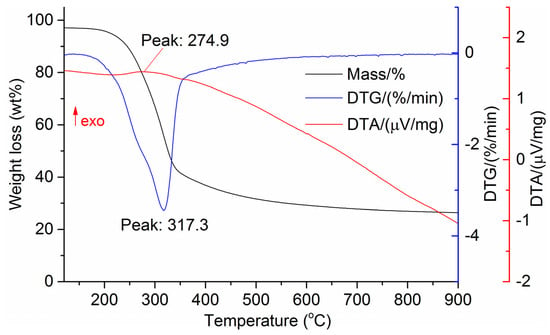
Figure 3.
DTG and DTA of sunflower husks. The arrow “exo” indicates the exothermic direction DTA.
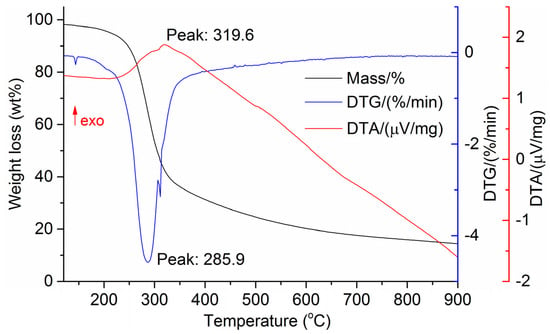
Figure 4.
DTG and DTA of corn cobs and stalks.
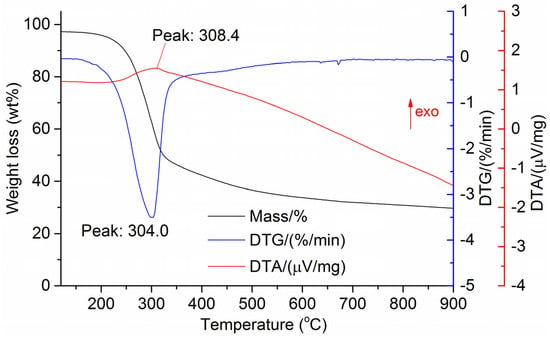
Figure 5.
DTG and DTA of wheat straw.
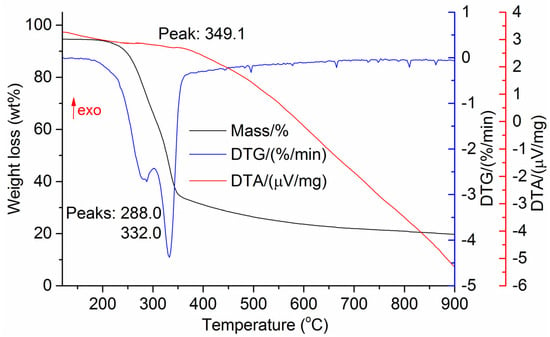
Figure 6.
DTG and DTA of sugarcane bagasse.
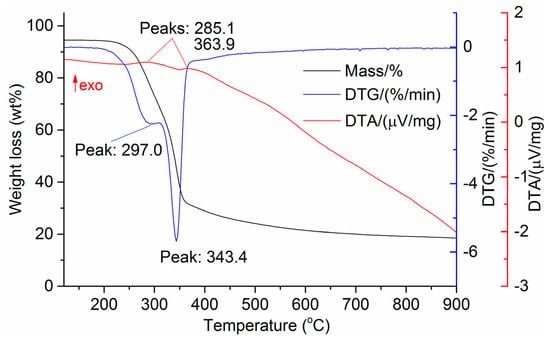
Figure 7.
DTG and DTA of coffee skin.
The DTA peaks indicate the occurrence of chemical reactions within the sample over specific temperature intervals. The DTA results revealed minor exothermic peaks for sunflower husks, coffee skin, sugarcane bagasse, and date palm leaves. In contrast, the pyrolysis of corn cobs and stalks, wheat straw, sugar beet pulp, and olive leaves was accompanied by obvious exothermic reactions within the temperature range of 200–400 °C. It should be noted that in the DTG and DTA experiments, the sample mass was only 15–20 mg, and the evolved volatiles were carried away by the purge gas flow, minimizing the occurrence of secondary reactions.
As can be seen from Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9, a sharp mass loss in the samples begins when heated to 240–260 °C and is completed at temperatures of 450–600 °C.
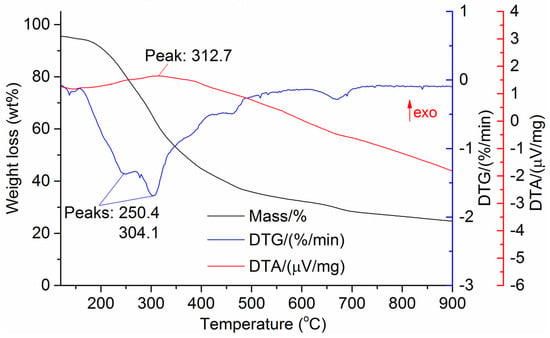
Figure 8.
DTG and DTA of olive leaves.
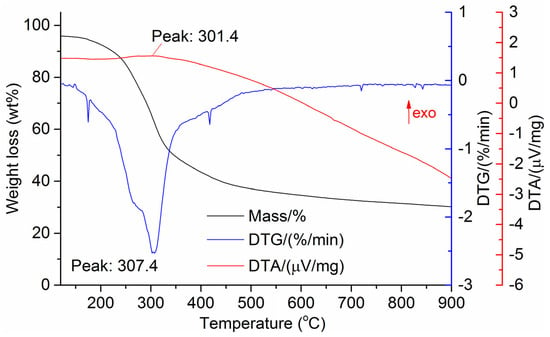
Figure 9.
DTG and DTA of date palm leaves.
Basu [25] demonstrated that in the temperature range of 250–300 °C, the decomposition of hemicellulose is nearly complete, while at temperatures above 350 °C, intensive cellulose degradation and noticeable lignin decomposition occur. Therefore, the temperature range of 250–400 °C can be expected to correspond to the maximum exothermic effect during biomass heating.
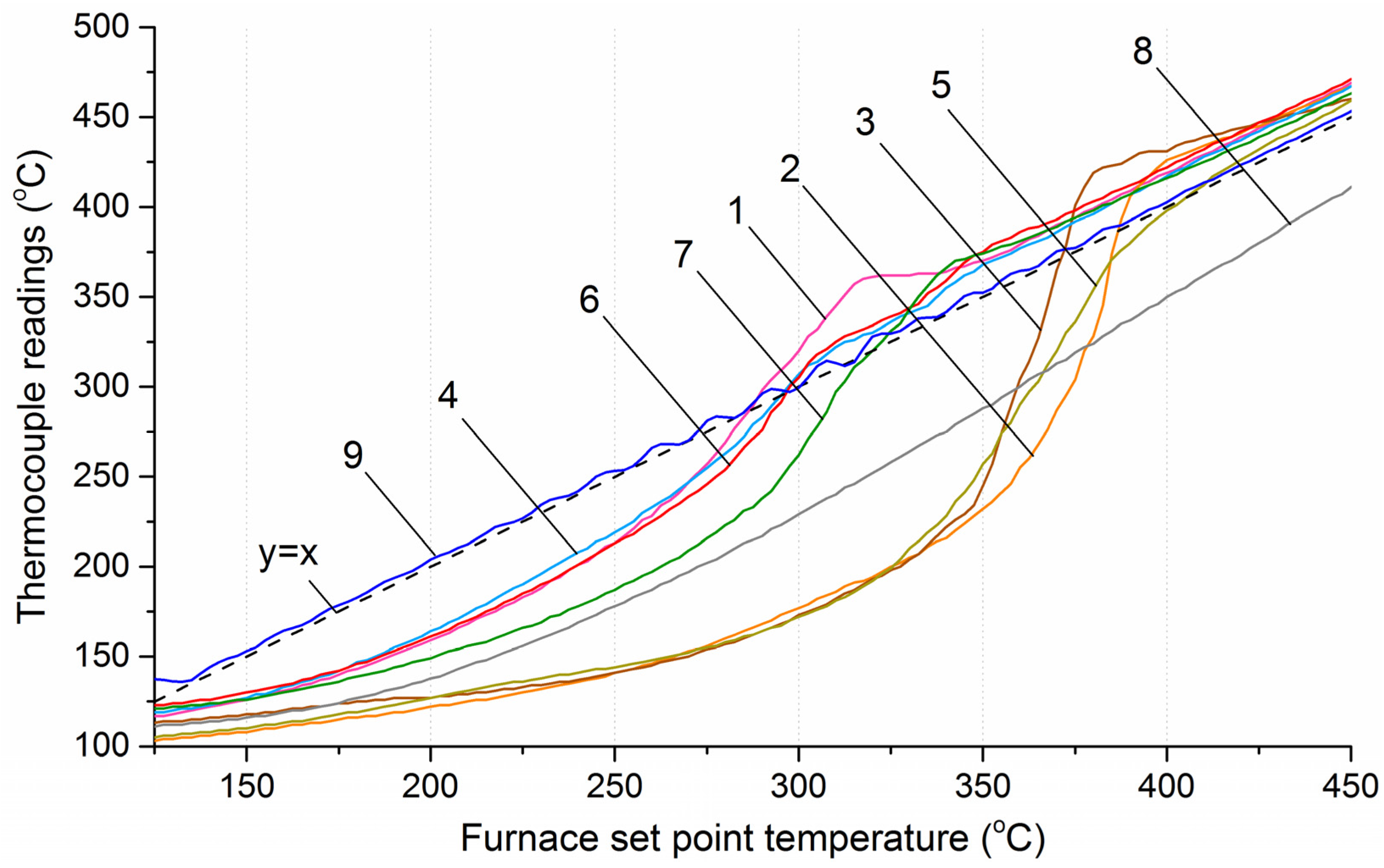
Figure 10 shows the temperature profiles along the reactor axis at section № 4 for all studied materials, as well as for an inert material (biochar from wood pellets), depending on the furnace temperature. Section № 4 is located at the center of both the reactor and the furnace, which allows minimizing the influence of the reactor’s massive covers on the temperature distribution and ensures a maximal heat flux, primarily generated by radiation from the heating coils. By comparing the temperature values along the reactor axis in experiments with biomass and inert material, it can be noted that heating of the center of the biomass layer initially lagged behind, followed by a rapid temperature increase. From the data presented in Figure 10, it is evident that upon reaching temperatures of 400–450 °C, the sharp rise in thermocouple readings ceased, and the temperature profile showed uniform heating at the rate set by the program controller −5 °C/min. Thus, it can be concluded that no heat-releasing reactions occur above 450 °C. Similar results were obtained in other studies [26,27] on the pyrolysis of wood, as well as coffee grounds, garden chaff and rapeseed straw [28].
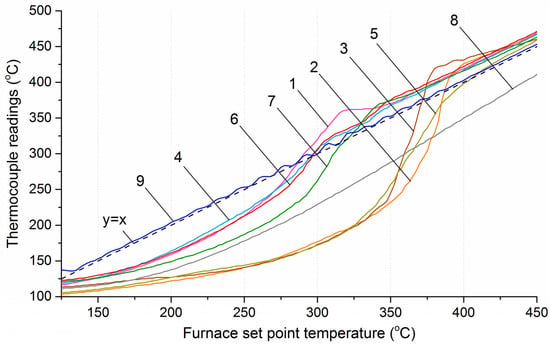
Figure 10.
Temperature profiles along the reactor axis at section № 4. 1—sunflower husks, 2—corn stalks and cobs, 3—wheat straw, 4—coffee skin, 5—olive leaves, 6—sugarcane bagasse, 7—date palm leaves, 8—charcoal, 9—furnace temperature.
During heating, the temperature distribution within the layer of each of the eight investigated materials varied due to differences in thermal conductivity, heat capacity, and bed mass. For corn cobs, wheat straw, sugarcane bagasse, and olive leaves, more time was required to heat the material layer, resulting in prolonged lag of the temperature values along the reactor axis compared to those at the inner reactor wall. Consequently, at the moment when the temperature along the reactor axis reached values corresponding to the onset of intensive volatile release, the temperature difference between the axis and the inner wall was approximately 80–100 °C. At the same time, the subsequent sharp increase in the heating rate at the center of the biomass layer, due to the development of the exothermic reaction, led to a rapid rise in temperature at the center.
For more precise identification of the exothermic effects and determination of the characteristic temperature ranges corresponding to their maximum manifestation, the difference between the first derivatives of the temperature versus time along the reactor axis and at the reactor wall was calculated using the following formula:
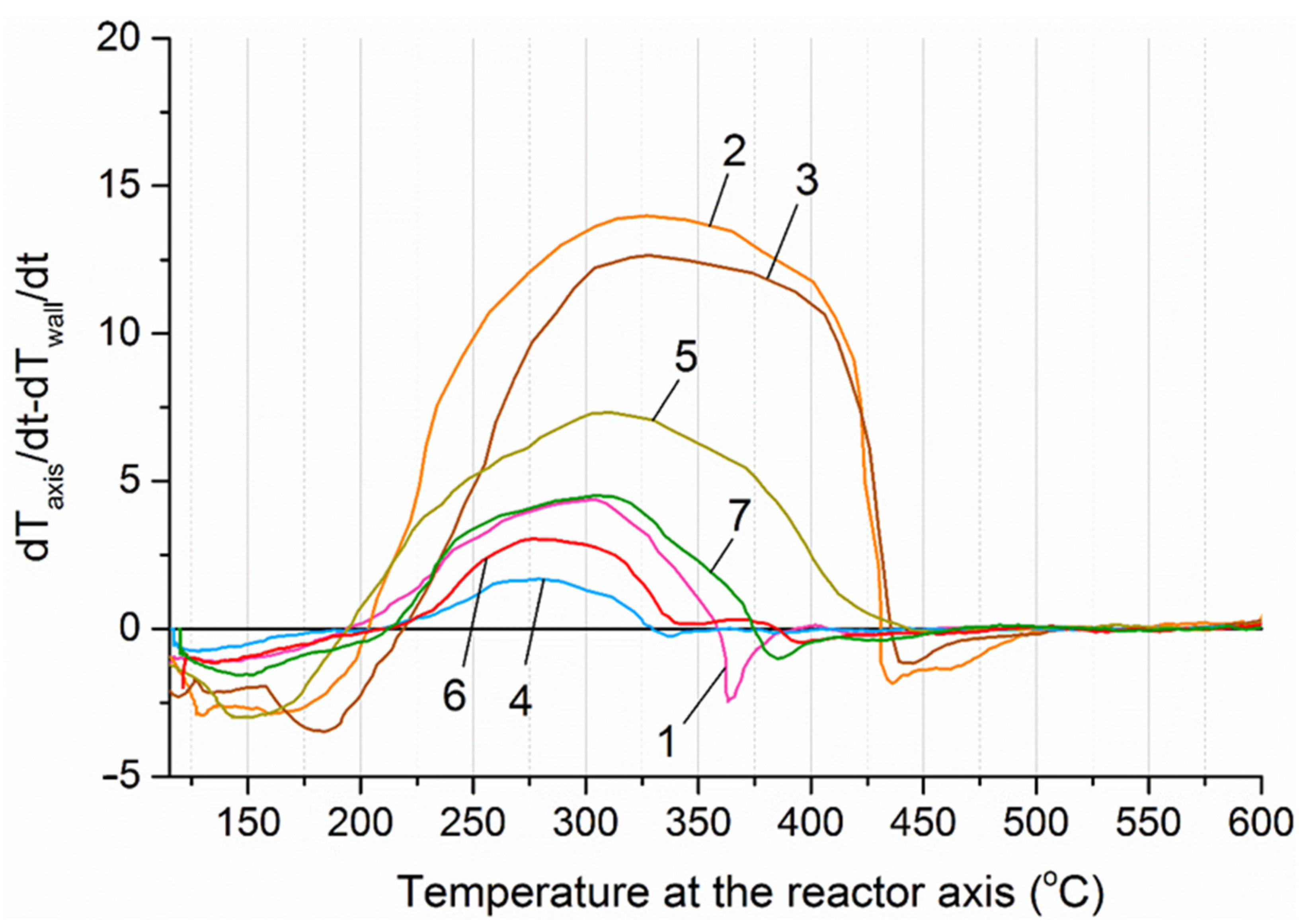
where dTaxis/dt—the first derivative of the temperature along the reactor axis with respect to time, dTw/dt—the first derivative of the temperature at the reactor wall with respect to time. The first derivative represents the rate of temperature change over time. The change in temperature of charcoal in the absence of chemical or phase reactions approximately corresponds to the change in temperature of the heating elements of the furnace, which is set using PI control (Figure 10). However, the rate of temperature change in the sample is directly proportional to the energy of the exothermic and endothermic reactions occurring during thermal decomposition of the biomass. A positive difference between the rates of temperature change along the reactor axis and at the reactor wall indicates the presence of heat-releasing processes within the material layer. The dependence of this difference on the temperature along the reactor axis at section № 4 is shown in Figure 11.
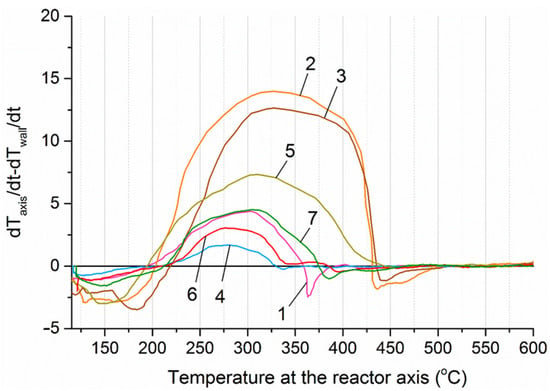
Figure 11.
Difference in the rates of temperature change along the reactor axis and at the reactor wall at section № 4. 1—sunflower husks, 2—corn stalks and cobs, 3—wheat straw, 4—coffee skin, 5—olive leaves, 6—sugarcane bagasse, 7—date palm leaves.
The temperature intervals at which the heating rate of the material core exceeded that of the reactor wall varied across the studied samples and were as follows: sunflower husks—195–360 °C, corn cobs and stalks—205–430 °C, wheat straw—220–435 °C, sugar beet pulp—190–420 °C, coffee skin—205–330 °C, olive leaves—195–445 °C, sugarcane bagasse—210–385 °C, and date palm leaves—210–375 °C.
Thus, for all the studied biomass samples, an exothermic effect was observed during heating: for corn residues, sugarcane bagasse, and wheat straw, this effect was the most pronounced, covering the temperature range from 180 to 220 °C to 425–450 °C; whereas for palm leaves, sunflower husks, and coffee skin, the effect was minimal, within the range of 200–375 °C. However, in all cases, the exothermic effect must be taken into account when selecting the temperature and duration of the torrefaction process. For example, a relatively modest increase in torrefaction temperature from 200 to 230 °C, due to the manifestation of the exothermic effect, should reduce the required processing time to obtain biochar of the desired quality. This hypothesis was tested using the unit whose schematic diagram is shown in Figure 11.
A series of torrefaction experiments was carried out using flax straw, wheat straw, sunflower husks, olive leaves, corn residues, date palm leaves and branches, coffee skin, olive pomace, and sugarcane bagasse at temperatures of 200 °C (processing time of 80 and 120 min) and 230 °C (processing time of 60 and 80 min). After processing, the resulting biochar samples were analyzed for moisture, ash, sulfur, carbon, hydrogen, nitrogen, and oxygen content. In addition, the lower heating value of the biochar samples was determined. The results of the study are presented in Table 2.

Table 2.
Summary table of test protocols for biomass samples before and after dry torrefaction in a hearth-type reactor.
It was previously shown that the exothermic effect is most shown during the torrefaction of corn residues, sugarcane bagasse, and wheat straw. Therefore, in the case of corn residues, torrefaction at 230 °C for 80 min increases the lower heating value (LHV) by 28.14% compared to the initial corn residues, reaching 19.26 MJ/kg. In contrast, torrefaction at 200 °C for 120 min results in only a 17.8% increase, with the LHV reaching 18.05 MJ/kg. Similarly, for sugarcane bagasse, increasing the torrefaction temperature from 200 to 230 °C makes it possible to obtain biochar with a higher LHV of 19.63 MJ/kg after 80 min, compared to the LHV obtained at 200 °C and 120 min of treatment. For wheat straw, raising the torrefaction temperature to 230 °C enables achieving an LHV of 16.58 MJ/kg after 80 min, whereas at 200 °C, biochar with an LHV of 16.29 MJ/kg can only be obtained after 120 min of processing.
For biomass types in which the exothermic effect is less, a smaller increase in the heating value of biochar may be observed when the torrefaction temperature is raised from 200 to 230 °C while simultaneously reducing the residence time from 120 to 80 min. For instance, in the case of sunflower husks, biochar obtained through torrefaction at 230 °C for 80 min exhibits a lower heating value (LHV) of 19.47 MJ/kg, which is slightly lower than the LHV of 19.85 MJ/kg achieved after torrefaction at 200 °C for 120 min
Thus, the utilization of the exothermic effect makes it possible, with a relatively moderate increase in process temperature (by approximately 15%), to reduce the required residence time of biowaste in the reactor and to enhance the energy efficiency of torrefaction by a factor of 1.5. This improvement, in turn, provides a substantial reduction in the overall energy consumption and, consequently, in the operational costs of the torrefaction process, which is of critical importance for its large-scale industrial implementation. Substantial enhancement of torrefaction process efficiency has likewise been shown in publications [29,30].
4. Conclusions
Torrefaction is a thermochemical biomass treatment technology that converts moist, biodegradable bio-waste into high-quality, environmentally friendly fuel with characteristics similar to lignite. This technology not only provides a valuable energy resource but also contributes to solving the problem of waste disposal, the accumulation of which poses a significant environmental burden. However, torrefaction is a lengthy and energy-intensive process, which limits its widespread application.
This study investigated the torrefaction of seven types of agricultural and food waste with varying moisture content, ash content, and calorific value. The main focus was on analyzing the exothermic effects that occur during biomass heating for their subsequent practical application.
Experiments conducted using a specially designed setup and an original methodology revealed that exothermic reactions are characteristic of all biomass types studied within the temperature range of 200–450 °C. The most pronounced thermal effect was observed in corn residues, sugarcane bagasse, and straw, while sunflower husk, palm leaves, and coffee skin exhibited a less noticeable effect.
A series of tests demonstrated that utilizing the heat from exothermic reactions allows for a reduction in torrefaction time by a factor of 1.5 while maintaining target biochar quality, with only a modest temperature increase (15%).
It is important to emphasize, however, that the utilization of exothermic effects requires careful control, as uncontrolled temperature escalation within the biomass layer may result in spontaneous ignition and reactor damage. This underscores the need for further research aimed at developing methods to manage these processes effectively.
Author Contributions
Conceptualization, R.I. and A.S.; methodology, O.M. and V.L.; validation, D.K., M.J.M. and K.M.; investigation, S.K. and Y.F.; data curation, A.M.; writing—original draft preparation, R.I. and A.S.; writing—review and editing, F.T. and A.M.; visualization, K.M. and Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was carried out with the financial support of the Ministry of Science and Higher Education of the Russian Federation (№ 075-15-2024-650, 12 September 2024; project title: “Dry and Wet Torrefaction of Agricultural Waste for the Production of Biochar as a Multifunctional Product”; lead institution: Tambov State Technical University).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Author Fouzi Tabet was employed by the company Opti’Tech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DTG | Differential thermogravimetry |
| DSC | Differential scanning calorimetry |
| DTA | Differential thermal analysis |
| LHV | Lower heating value |
References
- World Energy Outlook (WEO). World Energy Outlook 2021; International Energy Agency: Paris, France, 2021; p. 386. Available online: https://www.iea.org/weo (accessed on 22 May 2025).
- Ghoniem, A.F. Needs, resources and climate change: Clean and efficient conversion technologies. Prog. Energy Combust. Sci. 2011, 37, 15–51. [Google Scholar] [CrossRef]
- International Energy Agency. Renewables 2021: Analysis and Forecast to 2026; International Energy Agency: Paris, France, 2021; Available online: https://www.iea.org/renewables2021 (accessed on 22 May 2025).
- World Bioenergy Association. Global Bioenergy Statistics 2020; World Bioenergy Association: Stockholm, Sweden, 2020. [Google Scholar]
- Singh, R.; Krishna, B.B.; Kumar, J.; Bhaskar, T. Opportunities for utilization of nonconventional energy sources for biomass pretreatment. Bioresour. Technol. 2016, 199, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Thengane, S.K. Assessment of different technologies for managing yard waste using Analytic Hierarchy Process. Process. Integr. Optim. Sustain. 2019, 3, 255–572. [Google Scholar] [CrossRef]
- Robbins, M.P.; Evans, G.; Valentine, J.; Donnison, I.S.; Allison, G.G. New opportunities for the exploitation of energy crops by thermochemical conversion in northern Europe and the UK. Prog. Energy Combust. Sci. 2012, 38, 138–155. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Kiel, J.H.A.; Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction for entrained-flow gasification of biomass. In Proceedings of the 2nd World Conference and Technology Exhibition, Rome, Italy, 10–14 May 2004; pp. 1–7. [Google Scholar]
- Prins, M.J. Thermodynamic Analysis of Biomass Gasification and Torrefaction. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2005. [Google Scholar] [CrossRef]
- Couhert, C.; Salvador, S.; Commandr’e, J.M. Impact of torrefaction on syngas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Li, J.; Brzdekiewicz, A.; Yang, W.; Blasiak, W. Co-firing based on biomass torrefaction in a pulverized coal boiler with aim of 100% fuel switching. Appl. Energy 2012, 99, 344–3654. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.S.; Gomez-Barea, A.; Ghoniem, A.F. Advances in biomass torrefaction: Parameters, models, reactors, applications, deployment, and market. Prog. Energy Combust. Sci. 2022, 93, 101040. [Google Scholar] [CrossRef]
- Nguyen, T.; Zavarin, E.; Barrall, E.M. Thermal Analysis of Lignocellulosic Materials Part 1. Unmodified Materials. J. Marcomol. Sci. Rev. Macromol. Chem. 1981, 20, 1–65. [Google Scholar] [CrossRef]
- Kislytsyn, A.N. Wood Pyrolysis: Chemistry, Kinetics, Products, New Processes; Forest Industry: Moscow, Russia, 1980; 313p. [Google Scholar]
- Agroskin, A.A.; Gleybman, V.B. Thermophysics of Solid Fuels; Nedra: Moscow, Russia, 1980; 256p. [Google Scholar]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- Ohliger, A.; Förster, M.; Kneer, R. Torrefaction of beechwood: A parametric study including heat of reaction and grindability. Fuel 2013, 104, 607–613. [Google Scholar] [CrossRef]
- Van der Stelt, M.J.C. Chemistry and Reaction Kinetics of Biowaste Torrefaction. Ph.D. Thesis, Eindhoven University of Technology, Eindhoven, The Netherlands, 2011. [Google Scholar]
- Isemin, R.; Tabet, F.; Nebyvaev, A.; Kokh-Tatarenko, V.; Kuzmin, S.; Milovanov, O.; Klimov, D.; Mikhalev, A.; Dobkin, S.; Zhulaev, Y. Prediction of the Behavior of Sunflower Husk Ash after Its Processing by Various Torrefaction Methods. Energies 2022, 15, 7483. [Google Scholar] [CrossRef]
- Isemin, R.; Mikhalev, A.; Kuzmin, S.; Brule, M.; Ainane, T.; Milovanov, O.; Klimov, D.; Milovanov, K. Comparison of Dry and Wet Torrefaction for Biochar Production from Olive Leaves and Olive Pomace. Processes 2025, 13, 2155. [Google Scholar] [CrossRef]
- EN 14775:2009; Solid Biofuels—Determination of Ash Content. SIST: Brussels, Belgium, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/6887c746-63c5-43b4-bc7d-05b2687fbd9f/en-14775-2009?srsltid=AfmBOorx9-sLrrYDqkV2_p5ESgJMmgJinwRcJvwbJnEa7H_w5lQmx_Kq (accessed on 22 May 2025).
- EN 14774-3:2009; Solid Biofuels—Determination of Moisture Content—Oven Dry Method—Part 3: Moisture in General Analysis Sample. SIST: Brussels, Belgium, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/518eb6db-5f1c-4681-9355-8f93f0eeed06/en-14774-3-2009?srsltid=AfmBOoqY_JDNmpZmnaVY_wH2nswcTm64b8JO56-eysIKMDwuLr_mHygj (accessed on 22 May 2025).
- EN 15104:2011; Solid Biofuels—Determination of Total Content of Carbon, Hydrogen and Nitrogen—Instrumental Methods. SIST: Brussels, Belgium, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/8284c803-422b-409a-891e-5d4859ba52a5/en-15104-2011?srsltid=AfmBOooY0tWNQQi7wryedVT2mZICGzZ3ACtujwcj9lORWrcn5ppp56mE (accessed on 22 May 2025).
- EN 15148:2009; Solid Biofuels—Determination of the Content of Volatile Matter. SIST: Brussels, Belgium, 2015. Available online: https://standards.iteh.ai/catalog/standards/cen/7535bbc8-6cd4-4062-96c6-55a383dfd9a1/en-15148-2009?srsltid=AfmBOoqc6_XfqaJjr2n7XYlLusSsa279J7OsgnHIA7waWTHJnpnO6K7U (accessed on 22 May 2025).
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction. Practical Design and Theory; Academic Press: Cambridge, MA, USA, 2013; 530p. [Google Scholar]
- Huang, H.; Jiao, Z.; Shu, Z.; Zeng, Y. Experimental Study on Impact of Heating Temperature and Heating Rate During Carbonization of Woody Biomass. In Proceedings of the 2nd International Conference on Green Materials and Environmental Engineering (GMEE 2015), Phuket, Thailand, 20–21 December 2015; pp. 174–178. [Google Scholar]
- Wannapeera, J.; Fungtammasan, B.; Worasuwannarak, N. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrolysis 2011, 92, 99–105. [Google Scholar] [CrossRef]
- Jezerska, L.; Sassmanova, V.; Prokes, R.; Gelnar, D. The pelletization and torrefaction of coffee grounds, garden chaff and rapeseed straw. Renew. Energy 2023, 210, 346–354. [Google Scholar] [CrossRef]
- Zaichenko, V.M.; Sychev, G.A.; Shevchenko, A.L. Thermal effects of plant origin biomass torrefaction: Experiment and mathematical modeling. Therm. Eng. 2023, 70, 346–353. [Google Scholar] [CrossRef]
- Direktor, L.B.; Zaichenko, V.M.; Shevchenko, A.L.; Is’emin, R.L.; Chernyavskii, A.A. Comparison of the efficiency of the reactors for low-temperature pyrolysis of biomass. Therm. Eng. 2020, 67, 296–303. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).