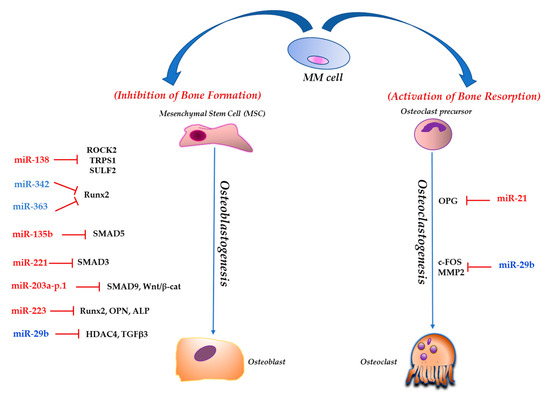
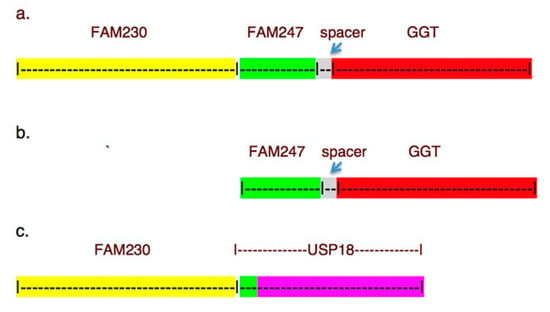
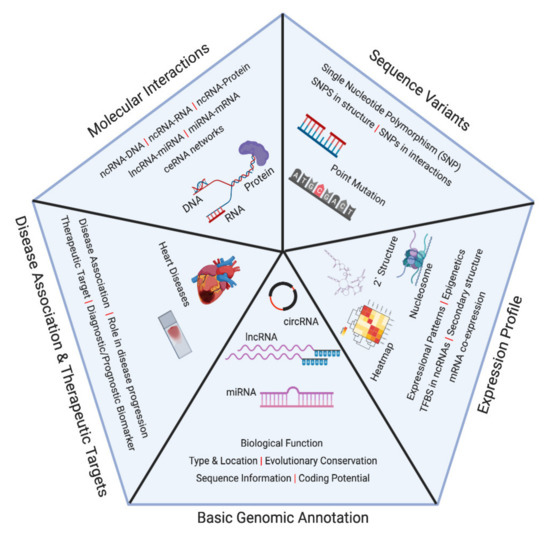
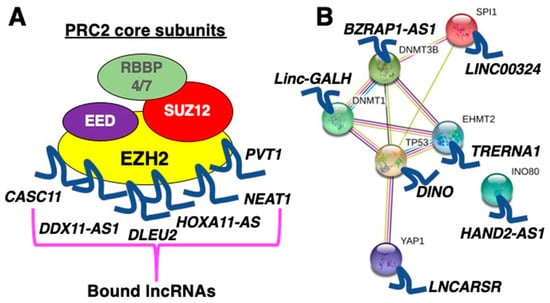
Feature Papers in Non-Coding RNA
A topical collection in Non-Coding RNA (ISSN 2311-553X). This collection belongs to the section "Computational Biology".
Viewed by 188616Editor
Interests: non-coding RNA; microRNA; cancer; metastasis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection “Feature Papers in Non-coding RNA” aims to collect high-quality research articles, review articles, and communications in all the fields of non-coding RNA research and their regulatory roles. Since the aim of this Topical Collection is to illustrate, through selected works, frontier research in noncoding RNA, we encourage Editorial Board Members of Non-coding RNA to contribute papers reflecting the latest progress in their research field, or relevant experts and colleagues to do so. Please kindly note that only invited papers can be published online once accepted in this collection.
Topics include, without being limited to, the following:
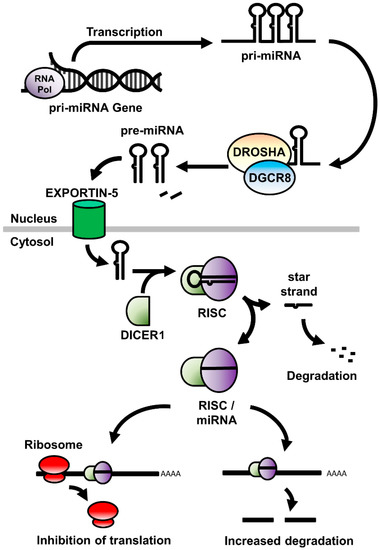
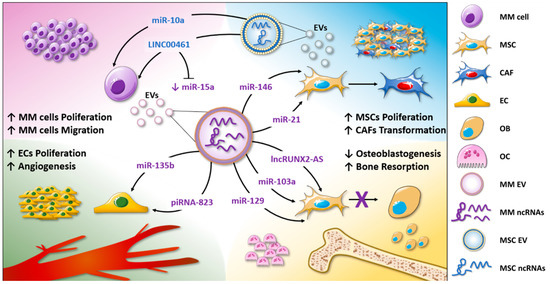
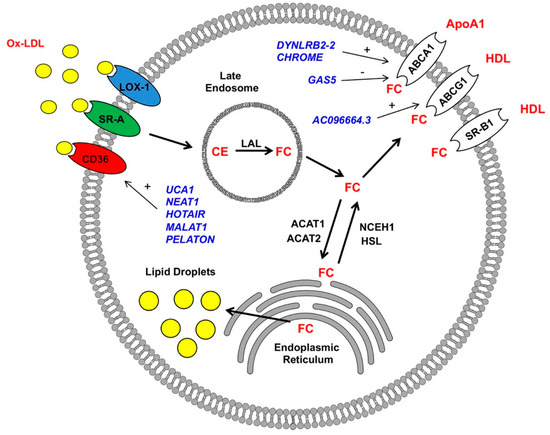
- Functional studies dealing with the identification, structure–function relationships, or biological activity of miRNAs, siRNAs, piRNAs, tRNAs, long noncoding RNAs, and other classes of RNAs;
- Analysis of RNA processing, RNA binding proteins, RNA signaling, and RNA interaction pathways;
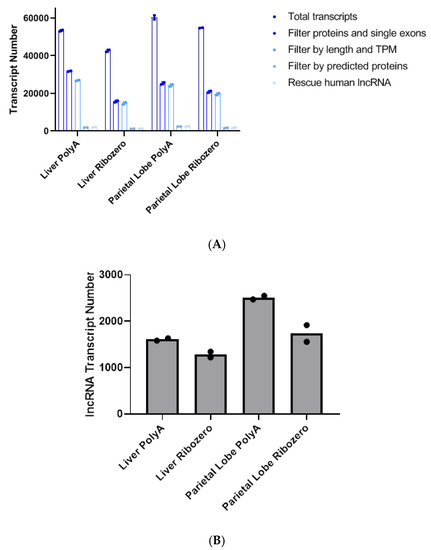
- RNA analyses, informatics, tools, and technologies;
- Translational studies involving long and short noncoding RNAs.
Prof. Dr. Georges A. Calin
Guest Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Non-Coding RNA is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.