Abstract
Lung cancer is associated with a high mortality, with around 1.8 million deaths worldwide in 2018. Non-small-cell lung cancer (NSCLC) accounts for around 85% of cases and, despite improvement in the management of NSCLC, most patients are diagnosed at advanced stage and the five-year survival remains around 15%. This highlights a need to identify novel ways to treat the disease to reduce the burden of NSCLC. Long non-coding RNAs (lncRNAs) are non-coding RNA molecules longer than 200 nucleotides in length which play important roles in gene expression and signaling pathways. Recently, lncRNAs were implicated in cancer, where their expression is dysregulated resulting in aberrant functions. LncRNAs were shown to function as both tumor suppressors and oncogenes in a variety of cancer types. Although there are a few well characterized lncRNAs in NSCLC, many lncRNAs remain un-characterized and their mechanisms of action largely unknown. LncRNAs have success as therapies in neurodegenerative diseases, and having a detailed understanding of their function in NSCLC may guide novel therapeutic approaches and strategies. This review discusses the role of lncRNAs in NSCLC tumorigenesis, highlighting their mechanisms of action and their clinical potential.
1. Introduction
Lung cancer is the leading cause of cancer related deaths worldwide, with 1.8 million deaths in men and women in 2018 [1]. Lung cancer is classified histologically into two main subtypes: small-cell lung cancer (SCLC) which accounts for around 15% of cases and non-small-cell lung cancer (NSCLC) which makes up about 85% of cases [2]. NSCLC is further grouped into adenocarcinoma (AC), squamous cell carcinoma (SCC), and large cell carcinoma (LCC), where ACs are the most prevalent accounting for around 40% of cases [3]. NSCLC is regarded as a heterogeneous disease, even within histological subgroups, owing to different molecular mechanisms driving lung tumorigenesis [4]. The development of targeted molecular therapies greatly improved patient response to the therapy, and their success demonstrates the benefit of treating biologically relevant alterations [3]. However, 40% of patients still present with stage IV lung cancer at diagnosis, with a five-year survival of 10–15%. This highlights a need to identify novel targets that may direct future therapies to reduce the burden of NSCLC [2,4].
In the last decade, the role of non-coding RNAs (ncRNA) as key regulators in a wide range of cellular processes became increasingly apparent [5]. NcRNAs are mainly grouped by size, and those smaller than 200 nucleotides (nt) are classified as small non-coding RNAs (sncRNAs), including the well-known housekeeping ncRNAs transfer RNAs (tRNAs), ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), and small nucleolar RNAs (snoRNAs), as well as small interfering RNAs (siRNA), microRNAs (miRNAs), and piwi-interacting RNAs (piRNAs) [6,7]. Long non-coding RNAs (lncRNAs) are defined as non-protein coding RNA transcripts more than 200 nt in length [8,9]. Although lncRNAs are principally classified by length, they can also be categorized by their genomic location [10]. Based on their position relative to coding genes, lncRNAs can be classed as long intergenic RNAs (lincRNAs), which include enhancer RNAs (eRNAs) that are transcribed from distal enhancer regions, intronic lncRNAs, overlapping lncRNAs, sense lncRNAs, antisense lncRNAs, and bidirectional lncRNAs [11,12]. Through their interaction with chromatin, proteins, and RNA targets, lncRNAs can regulate gene expression and signaling pathways at the epigenetic, transcriptional, and post-transcriptional level, as reviewed in Schmitt et al. [9].
The processing of lncRNAs is similar to that of messenger RNAs (mRNAs) in that they are transcribed by RNA polymerase type II (RNAP2), showing similar methylation patterns across the gene body, and they undergo post-transcriptional modifications such as splicing, polyadenylation, and 5′ capping [10]. Although lncRNAs are less conserved than protein-coding genes, the promoter regions have high sequence conservation, suggesting the importance of lncRNA regulation [9,11]. The expression of lncRNAs is regulated at the transcriptional and epigenetic level and tightly controlled [13,14]. Although their expression is relatively low, lncRNAs are differentially expressed in tissues and have high tissue specificity [14]. Although tightly regulated in physiological tissues, lncRNAs are commonly dysregulated in disease, leading to aberrant expressions and functions [15,16].
Recently, lncRNAs were implicated in cancer, where they can function as oncogenes and tumor suppressors, and their dysregulation is associated with tumor cell growth, apoptosis, invasion, and metastasis [14,17]. Due to the function of lncRNAs in key pathophysiological pathways, they are gaining increasing attention as novel anti-cancer therapeutic targets [18]. Some lncRNAs are well characterized in NSCLC; however, the precise mechanisms of action for most lncRNAs remains largely unknown [14]. Here, we review the role of lncRNAs in NSCLC, focusing on their precise function in NSCLC tumorigenesis, their mechanism of action, and their clinical potential.
2. LncRNA Mechanisms of Action
The function of lncRNAs is largely reflected by their subcellular localization, where nuclear lncRNAs mainly function in transcriptional processes, whereas cytoplasmic lncRNAs tend to function post-transcriptionally and influence signaling cascades [9,19]. There are three general mechanisms for lncRNA regulation: localization and interaction with chromatin, interaction with RNA targets, and protein modulation (Figure 1). A single lncRNA can function via different mechanisms, suggesting that their regulation of gene expression is complex [9]. The ability of lncRNAs to interact with distinct biological molecules has important implications in cancer, and understanding these complex networking interactions may provide novel therapeutic targets [20].
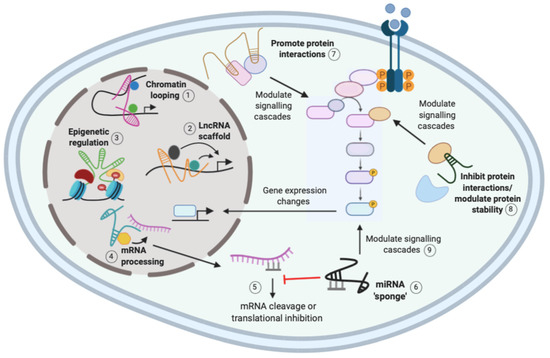
Figure 1.
Mechanisms of action of long non-coding RNAs (lncRNAs). The function of lncRNAs is largely reflected by subcellular localization, and their mechanisms include interaction with chromatin, proteins, and RNA. Nuclear lncRNAs can induce chromatin looping (1) or act as a scaffold (2) to recruit multiple regulatory molecules to a gene promoter to activate or repress gene expression. Nuclear lncRNAs can recruit epigenetic regulatory complexes to gene promoters to induce methylation changes to regulate gene expression (3). Nuclear lncRNAs can also recruit regulatory molecules to mRNAs to regulate messenger RNA (mRNA) processing (4). Upon nuclear export for translation, mRNAs can be bound by miRNAs that promote mRNA degradation or inhibition of translation to inhibit mRNA function (5). Cytoplasmic lncRNAs can act as miRNA sponges to competitively bind the miRNAs and release the inhibition on the mRNA (6). Cytoplasmic lncRNAs also modulate protein interactions (7) and stability (8) to regulate signaling cascades and subsequent changes in gene expression. Micro RNA (miRNA) sponging can also modulate signaling cascades by regulating the activity of mRNAs (9). Created with Biorender.com.
2.1. Chromatin Interactions and Transcriptional Regulation
By localizing to chromatin, lncRNAs can regulate pre-transcriptional processes such as gene imprinting, dosage compensation, and epigenetic regulation such as histone modification [19]. For example, the well-characterized lncRNA X-inactive-specific transcript (XIST) localizes to the X chromosome and recruits factors for chromosome inactivation [21]. As X aneuploidy exists in many human cancers, it suggests that dysregulation of X-linked genes is required for malignant transformation [9]. Furthermore, XIST was shown to be upregulated in NSCLC tissues and cell lines and act as an oncogene by recruiting enhancer of zeste homolog 2 (EZH2), a member of the polycomb repressive complex 2 (PRC2), to the promoter of the tumor suppressor Krüppel-like factor 2 (KLF2) to initiate histone methylation to repress KLF2 expression [22]. As KLF2 silencing partly reversed the tumorigenic effects of XIST silencing, it suggests that XIST acts as an oncogene by epigenetically silencing KLF2 by directly binding to EZH2. However, as the pro-oncogenic effects were only partly reversed by KLF2, it suggests that XIST may regulate other possible targets [22].
LncRNAs can regulate transcription by controlling chromatin architecture and recruiting regulatory molecules such as transcription factors to specific sites [9,11]. For example, chromatin looping by lncRNAs induces changes in chromatin structure to promote interactions with key regulatory molecules to activate gene expression, as shown in Figure 1 [11]. HoxA distal transcript antisense RNA (HOTTIP) is a lincRNA located in the HoxA locus that binds to the transcriptional activator WD repeat-containing protein 5 (WDR5) and the mixed lineage leukemia (MLL) complex. HOTTIP facilitates chromatin looping to recruit the two regulatory factors to the HoxA locus which activates gene expression [23]. In NSCLC, HOTTIP expression is significantly higher in malignant tissues than normal adjacent tissues, and it was shown to function as an oncogene by regulating Homeobox Protein A3 (HOXA3 expression to increase cell proliferation and invasion [24]. However, further functional studies are required to determine if chromatin looping is responsible for HOTTIP regulation of HOXA3 in NSCLC.
LncRNAs can also regulate gene expression by acting as scaffolds, to allow the assembly of regulatory complexes for transcriptional activation or repression, depending on the molecules recruited (Figure 1) [11]. The lncRNA antisense noncoding RNA in the INK4 locus (ANRIL) functions as a scaffold for different chromatin remodeling complexes, such as PRC1, PRC2, and WDR5 [17,25]. In NSCLC, ANRIL was shown to interact with PRC2 to repress the expression of KLF2 and p21. This led to reduced NSCLC cell proliferation and apoptosis, suggesting that scaffold lncRNAs such as ANRIL may be important in NSCLC tumorigenesis [26].
2.2. RNA Interactions
LncRNAs are known to function in gene expression through their interaction with different RNA targets [9]. Interactions between mRNAs and lncRNAs can regulate RNA metabolism through the recruitment of factors involved in splicing, mRNA stability, and translation (Figure 1) [9,19]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) is a well-characterized lncRNA that is thought to be involved in the alternative splicing of mRNAs. Indeed, nuclear-retained MALAT1 was shown to interact with and influence the distribution of serine/arginine splicing factors (SRSF), and MALAT1 depletion led to changes in the alternative splicing pattern of a set of pre-mRNAs [27]. MALAT1 is overexpressed in NSCLC and contributes to lung tumorigenesis, as suppression of MALAT1 in A549 NSCLC cells suppressed clonogenic growth and migration [16,28]. MALAT1 was shown to increase SRSF7 levels by inhibiting miR-347b-5p to enhance NSCLC tumorigenesis in vitro [29]. Therefore, MALAT1 may affect the splicing of key tumor suppressor and oncogenic mRNAs to promote tumorigenesis [16,27].
Furthermore, lncRNAs can modulate miRNAs by acting as “miRNA sponges” or competing endogenous RNAs (ceRNAs) that competitively bind to and sequester miRNAs, reducing their regulatory effect on the destined mRNA [Figure 1] [9,10]. Nuclear-enriched abundant transcript 1 (NEAT1), which is highly expressed in NSCLC patients, was shown to function as a ceRNA for miR-377-3p. Direct binding of NEAT1 to miR-377-3p led to de-repression of oncogenic E2F transcription factor (E2F) 3, a direct target of miR-377-3p [30]. In vitro studies showed that NEAT1 promotes NSCLC cell growth by inhibiting the miR-377-3p/E2F3 axis leading to activation of the E2F3 pathway [30,31]. NEAT1 was also shown to “sponge” the miR-181a-5p, upregulating its target gene high-mobility group protein B2 (HMGB2) and increasing NSCLC cell proliferation and invasion [32]. The ability of NEAT1 to interact with distinct miRNAs and modulate NSCLC tumorigenesis via distinct mechanisms suggests a complex regulatory network exists. It is worth noting that, to confirm that NEAT1 acts as a ceRNA, Sun et al. and Li et al. used siRNAs to knockdown NEAT1 and investigate the expression levels of miR-377-3p and miR-181a-5p, respectively [30,32]. However, Zhang et al. failed to check miR-377-3p expression levels, highlighting a limitation for many studies when investigating the function of lncRNAs as “miRNA sponges” [31].
2.3. Protein Regulation
LncRNAs can modulate protein functions by promoting the assembly of protein complexes, disrupting protein–protein interactions and affecting their cellular localization [9]. As mentioned above, lncRNAs can modulate protein interactions and the formation of regulatory complexes to regulate all stages of gene expression [23,27,33]. LncRNAs are also able to influence important signaling pathways by modulating proteins directly or indirectly through miRNAs (Figure 1) [9]. In a similar mechanism to NEAT1 modulation of the E2F3 pathway, MALAT1 was shown to directly interact and “sponge” miR-206, leading to activation of the Protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway to promote epithelial-to-mesenchymal transition (EMT) and migration of A549 and H1299 NSCLC cells [34].
3. LncRNA Dysregulation in NSCLC
Many large-scale analyses investigated differentially expressed lncRNAs in NSCLC [35,36]. As shown in Table 1, most upregulated lncRNAs in NSCLC function as oncogenes, whereas less commonly downregulated lncRNAs function as tumor suppressors (Table 2). However, for some lncRNAs, there is controversy over their role as tumor promoters or suppressors. This review further discusses mechanisms behind well-characterized lncRNAs in NSCLC, as well as the role of novel and less well-established lncRNAs.

Table 1.
Oncogenic lncRNAs upregulated in non-small-cell lung cancer (NSCLC).

Table 2.
Tumor suppressor lncRNAs downregulated in NSCLC.
3.1. Oncogenic LncRNAs
3.1.1. Proliferation and Survival
Dysregulation of the cell cycle is common in cancer to confer a proliferative advantage to cells, where key checkpoint regulators such as cyclins and cyclin dependent kinases (CDKs) are commonly dysregulated [117]. For example, lncRNA JPX transcript and XIST activator (JPX) promoted cell-cycle progression and NSCLC cell proliferation by acting as a ceRNA for miR-145-5p to upregulate its mRNA target, cyclin D2 [54]. The lncRNAs XIST, small nucleolar RNA host gene 15 (SNHG15), and HNF1A antisense RNA 1 (HNF1A-AS1) led to the upregulation of CDK8, CDK14, and CDK6, respectively, to increase NSCLC growth and proliferation, by acting as ceRNAs to sponge the inhibitory miRNAs miR-16, miR-486, and miR-149-5p, respectively [50,87,93]. Cell-cycle progression proteins can also be regulated by lncRNAs. For example, Cip1 interacting with zinc finger protein 1 (Ciz1) was recently shown to regulate the Gap 1 (G1) to synthesis (S) phase (G1-to-S) transition in cancer and lncRNA differentiation antagonizing nonprotein coding RNA (DANCR) was shown to sponge miR-214-5p, upregulating its target Ciz1 and increasing NSCLC cell proliferation [38,118]. Furthermore, GTPase G1-to-S phase transition 1 (GSPT1) was upregulated by lncRNA DLX6 antisense gene 1 (DLX6-AS1) due to inhibition of miR-27-3p, resulting in increased NSCLC cell proliferation [40]. In addition, LINC00339 promoted NSCLC proliferation and progression by sponging miR-145 to upregulate the cell-cycle regulator Forkhead box protein 1 (FOXM1) [56]. Therefore, lncRNA regulation of inhibitory miRNAs is important for NSCLC cell-cycle progression and proliferation.
Oncogenic E2F family members, E2F1, E2F2, and E2F3 play important roles in G1-to-S cell-cycle transition [117]. LncRNA HOXA transcript induced by transforming growth factor (TGF)-β (HIT) was shown to interact directly with E2F1 and modulate E2F1 promoter binding to increase expression of its target genes to enhance NSCLC cell proliferation [48]. LINC00461 also led to E2F1 upregulation by sponging miR-4478, where E2F1 could also bind to the promoter of LINC00461 to induce its transcription and initiate a positive feedback loop for NSCLC progression [57]. LncRNA FLVCR1 antisense gene 1 (FLVCR1-AS1) upregulated E2F3 by acting as a ceRNA for miR-573, promoting NSCLC proliferation and progression [44]. Therefore, lncRNA regulation of the E2F pathway directly or indirectly through miRNAs is also important for NSCLC growth and progression.
As well as upregulating positive cell-cycle regulators, lncRNAs can also epigenetically repress the expression of cell-cycle inhibitors such as cyclin-dependent kinase inhibitor 1A (CDKN1A or p21) and tumor protein 53 (p53) [117]. Three lncRNAs upregulated in NSCLC, AFAP1 antisense gene 1 (AFAP1-AS1), small nucleolar RNA host gene 20 (SNHG20), and LINC01088, were shown to directly interact with EZH2, increasing binding and methylation of the p21 promoter and silencing of p21 expression [37,61,119]. AFAP1-AS1 knockdown reduced growth in vitro and in vivo and silencing of SNHG20, and LINC01088 reduced NSCLC proliferation and cell-cycle progression [37,61,119]. LncRNAs plasmacytoma variant translocation 1 (PVT1) and prostate cancer-associated transcript 6 (PCAT-6) were also shown to bind to EZH2, and recruit it to the large tumor suppressor kinase 2 (LATS2) promoter, repressing LATS2 transcription [75,78]. PCACT-6 promoted NSCLC growth in vitro and in vivo through epigenetic silencing of LATS2, but further mechanistic insight needs to be discussed [75]. Downregulation of PVT1 or overexpression of LATS2, however, decreased E3 ubiquitin ligase (MDM2) expression and subsequent inhibition of p53. Therefore, lncRNA repression of LATS2 may promote cell proliferation and growth through inhibition of the MDM2–p53 pathway [78]. PVT1 also sponged miR-526b, increasing EZH2 levels and generating a positive feedback mechanism to enhance NSCLC progression [79]. LncRNA lung cancer associated transcript 1 (LUCAT-1) was shown to repress both p21 and p57 expression through interaction with the PRC2 complex to promote NSCLC cell proliferation [67]. Furthermore, LINC01234 acted as a scaffold to bind LSD1 and EZH2 and repress BTG anti-proliferation factor 2 (BTG2) to enhance NSCLC tumorigenesis [62]. Therefore, epigenetic silencing of cell cycle and proliferative inhibitors is a common mechanism for lncRNA regulation of NSCLC proliferation.
In addition to regulation of NSCLC proliferation, lncRNAs are implicated in suppression of apoptosis. For example, lncRNA small nucleolar RNA host gene 6 (SNHG6) competitively sponged miR-490-3p to increase remodeling and splicing factor 1 (RSF1) to promote proliferation and inhibit apoptosis, suggesting the importance of pre-transcriptional complexes in NSCLC cell proliferation and survival [89]. XIST, as well as regulating CDK8, can upregulate the anti-apoptotic factor B-cell lymphoma type 2 (BCL-2) to inhibit apoptosis by sponging miR-449a [93,94]. LncRNAs XLOC-008446 and prostate cancer associated transcript 1 (PCAT-1) can act as ceRNAs for miR-874 and miR-149-5p, respectively, to increase the anti-apoptotic regulators x-linked inhibitor of apoptosis (XIAP) and leucine-rich repeats and immunoglobulin-like domains 2 (LRIG2), respectively [74,97]. Therefore, lncRNA regulation of miRNAs is also important to promote cell survival, as well as proliferation.
3.1.2. Invasion and Migration
Matrix metalloproteinases (MMPs) are key enzymes in tumor dissemination, largely through their role in ECM degradation and proteolytic breakdown of tissue barriers to invasion [120]. In addition to epigenetic silencing of LATS2, PVT1 also acts as a ceRNA for tumor suppressor miRNAs miR-200a and miR-200b to increase their target MMP-9, which promoted the invasive ability of NSCLC cells [78,80]. PVT1 also sponged miR-148 to increase Ras-related protein Rab-34 (RAB34), a GTPase that regulates surface proteins for invasion, to enhance the migration and invasion of NSCLC cells [81]. In a similar mechanism, XLOC-008466 and lncRNA 1308 increase MMP-2 and a disintegrin and metalloproteinase domain 15 (ADAM15) by competitively sponging miR-874 and miR-124, respectively, to increase the invasion of NSCLC cells [65,97]. XLOC-008466 can, therefore, regulate the invasion, as well as apoptosis, of NSCLC cells, through regulation of miR-874 [97]. CeRNA sponging by lncRNAs can also increase transcription factors that regulate MMPs. For example, lncRNA HOXD cluster antisense RNA 1 (HOXD-AS1) sponging of miR-133b upregulates its target HOXAD, leading to the upregulation of MMP-9 [53]. SNHG6 was also shown to upregulate MMP-9, as well as MMP-2, by competitively binding miR-994 and miR-181d-5p and increasing ETS proto-oncogene transcription factor 1 (ETS1) activity [90]. MMP-9 expression was also shown to be epigenetically regulated by lncRNA myocardial infarction associated transcript (MIAT), which interacts with the MLL complex to reduce silencing of the MMP-9 promoter [70]. Therefore, various lncRNAs can regulate MMP expression via a variety of mechanisms to promote NSCLC invasion.
XIST was shown to positively regulate paxillin, a focal adhesion protein, by acting as a ceRNA for miR-137 to modulate attachments between the cell and the ECM for NSCLC migration [95]. Due to functions in proliferation, apoptosis, and invasion, XIST may be a promising therapeutic target in NSCLC [93,94,95]. In addition to changes in ECM attachments, cellular gene expression changes are required for NSCLC invasion and migration. LncRNAs SNHG15 and LINC01234, two lncRNAs that also function in cell-cycle regulation, act as ceRNAs to increase oncogenes zinc finger protein 217 (ZNF217) and Vav guanine nucleotide exchange factor 3 (VAV3), respectively, to enhance proliferation and invasion [62,88]. Similar to LINC01234, lncRNA DUXAP8 acts as a scaffold to recruit histone modifiers EZH2 and LSD1 to repress early growth response protein 1 (EGR1) and Rho-related GTP binding protein (RHOB), respectively. EGR1 and RHOB are both tumor suppressors where loss of expression is associated with invasion and metastasis, whereas DUXAP8-induced suppression in NSCLC reduced proliferation and invasion [41]. Therefore, lncRNA regulation of proteins for invasion and migration is important in NSCLC.
3.1.3. EMT and Metastasis
EMT is a key process that allows cancer cells to adopt a more invasive and migratory phenotype for tumor migration, invasion, and metastasis [9] Zinc finger E-box-binding homeobox (ZEB) proteins are key inducers of EMT, and several lncRNAs in NSCLC can regulate their expression and activity. For example, XIST and lncRNA SOX-2 overlapping transcript (SOX2OT) act as ceRNAs for miR-367/miR-141 and miR-132, respectively, to increase ZEB2 expression and EMT [91,96]. LncRNAs LINC00673, human testis developmental related gene 1 (TDRG1), and ZEB1 antisense RNA 1 (ZEB1-AS1) increase ZEB1 expression and EMT by sponging mir-150-5p, miR-873-5p, and miR-409-3p, respectively [59,92,98]. ZEB1 can also bind to the promoter of ZEB-AS1 to increase its expression, acting in a positive feedback loop to regulate EMT [98]. This is a similar mechanism to E2F1 feedback on LINC00461 expression to regulate cell-cycle progression and proliferation [57]. HIT can interact directly with ZEB1, increasing its stability and binding to the CDH1 promoter to repress E-cadherin, a marker of EMT [49]. Furthermore, lncRNA HOXA11 antisense 1 (HOXA11-AS1) can interact with EZH2 and DNMT1 to repress miR-200b expression and increase miR-200b targets ZEB1 and ZEB2 for enhanced EMT [51]. HOXA11-AS1 also sponged miR-148a-3p, increasing DNA methyltransferase 1 (DNMT1) expression and generating a feedback loop for EMT, similar to PVT1 upregulation of EZH2 [52,79]. Twist family BHLH transcription factor 1 (TWIST), another inducer of EMT, was shown to be increased by LINC01296 inhibition of miR-598, and TWIST enhanced the expression of LINC01296 to enhance EMT [64]. Therefore, feedback mechanisms are important in and add to the complexity of lncRNA regulation in NSCLC EMT.
YES-associated protein 1 (YAP1), a regulator of the hippo pathway, is another inducer of EMT, and lncRNA nicotinamide nucleotide transhydrogenase-antisense RNA1 (NNT-AS1) led to an increase in YAP1 expression by sponging miR-22-3p, increasing NSCLC migration, invasion, and EMT [73]. SRY-box transcription factor 4 (SOX4), a novel epigenetic regulator of EMT, was increased by two lncRNAs DANCR and LEF1 antisense RNA 1 (LEF1-AS1) by sponging mir-138 and miR-489, respectively, increasing NSCLC migration and metastasis [39,55]. E-cadherin was downregulated by EZH2 induced epigenetic silencing mediated by FEZF1 antisense RNA 1 (FEZF1-AS1), decreasing cell-to-cell adhesions and increasing EMT [43]. Therefore, lncRNAs can regulate multiple inducers of EMT in NSCLC.
LncRNAs were also shown to regulate proteins associated with metastasis. For example, lncRNA long stress-induced non-coding transcript 5 (LSINCT5) interacted with metastasis-associated transcription factor, high-mobility group AT-hook 2 (HMGA2), protecting it from proteasome-mediated degradation and increasing migration of NSCLC cells [66]. Furthermore, lncRNA ZNFX1 antisense RNA 1 (ZFAS1) acted as a ceRNA for miR-150 to increase HMGA2, resulting in increased NSCLC invasion [99]. LINC00673 modulated EZH2 epigenetic silencing of HOXA5, a tumor suppressor that inhibited NSCLC metastasis by regulating cytoskeletal remodeling [60]. Metaherin, another oncogene implicated in metastasis in NSCLC, was upregulated by small nucleolar RNA host gene 1 (SNHG1) and prostate cancer non-coding RNA 1 [PRNCR1] by sponging miR-145-4p and miR-126-5p, respectively, increasing EMT, migration, and invasion of NSCLC cells [77,84]. LINC00525 also acts as a ceRNA, for miR-338-3p, increasing NSCLC cell invasion and migration by increasing insulin receptor substrates 2 (IRS2), a signaling adapter protein that is implicated in cancer progression and metastasis [58]. Therefore, lncRNAs can act via multiple mechanisms to regulate EMT and metastasis in NSCLC.
3.1.4. Regulation of Key Oncogenic Signaling Pathways
LncRNAs can also regulate oncogenic signaling pathways that control growth, proliferation, apoptosis, and metastasis by modulating pathway activation and key signaling components [9]. For example, LINC01288 can interact directly with and increase the stability of interleukin 6 (IL-6) mRNA, leading to upstream activation of the signal transducer and activator of transcription 3 (STAT3) pathway, promoting NSCLC proliferation, growth, and invasion [63]. Furthermore, MALAT1 and H19 were shown to act as ceRNAs for miR-124 and miR-17, respectively, to increase their target mRNA STAT3 [46,68]. Therefore, direct upregulation of STAT3 by lncRNAs can also enhance NSCLC cell tumorigenesis.
H19 was also shown to regulate pyruvate dehydrogenase 1 (PDK1) by sponging miR-138 and increasing NSCLC proliferation through activation of the phosphatidylinositol 3-kinase (PI3K)/AKT pathway [47]. Insulin-like growth factor 2 (IGF-2), an upstream activator of the PI3K/AKT pathway, was increased by lncRNAs NEAT1 and GM15290 to enhance NSCLC cell proliferation, invasion, and migration. IGF-2 expression was increased by NEAT1 sponging of the tumor suppressor miR-let7a and lncRNA GM15290 sponging of miR-615-5p [45,72]. In highly metastatic NSCLC SPCA-1scl cells, lncRNA Meta-LNC9 interacted directly with phosphoglycerate kinase 1 (PGK1), protecting it from degradation and leading to activation of the AKT/mTOR pathway to enhance cell migration, invasion, and metastases. Meta-LNC9 also regulated its own transcription by modulating cAMP response element-binding protein (CREB1), generating a positive feedback loop to enhance metastasis [69]. Furthermore, focally amplified lncRNA on chromosome 1 (FAL1) lncRNA increased BMI1 polycomb ring finger oncogene (BMI1) levels, which downregulated phosphatase and tensin homolog (PTEN), an inhibitor of the PI3K/AKT pathway, increasing AKT activation and NSCLC tumorigenesis [42]. Therefore, lncRNAs can also regulate pathway inhibitors to upregulate NSCLC oncogenic signaling.
PRKCZ antisense RNA 1 (PRKCZ-AS1) led to enhanced mitogen-activated protein kinase (MAPK) signaling by inhibiting mir-766-5p and increasing its target MAPK1 to enhance NSCLC proliferation and migration [76]. PVT1 can also regulate activation of MAPK signaling by sponging miR-145-5p and increasing integrin subunit beta 8 (ITGB8), as well as activate the wingless (Wnt)/β-catenin pathway by sponging mir-361 and increasing SRY-box transcription factor 9 (SOX9) for enhanced NSCLC tumorigenesis [82,83]. As discussed previously, PVT1 was shown to have roles in cell-cycle regulation and the regulation of proteins for invasion and migration and, therefore, be a promising therapeutic target due to its multiple functions in NSCLC progression. SNHG1 also increased SOX9 by sponging miR-101-3p to increase Wnt signaling and NSCLC proliferation and invasion [85]. SNHG1 also could sponge miR-361-3p to increase FRAT regulator of WNT signaling Pathway 1 (FRAT1) to enhance NSCLC cell tumorigenesis, suggesting that SNHG1 activation of the Wnt pathway through regulation of distinct lncRNAs is important and complex in NSCLC [86].
3.2. Tumor Suppressor LncRNAs
3.2.1. Proliferation and Apoptosis
LncRNA growth arrest specific 5 (GAS5) was shown to act as a tumor suppressor in NSCLC by post-transcriptionally regulating p53, p21, and E2F1 to inhibit tumor growth and increase NSCLC cell apoptosis, but not migration and invasion [105]. GAS5 is downregulated in NSCLC, and low expression correlates with a poor prognosis, in contrast to oncogenic lncRNAs such as HIT and LUCAT-1 that are upregulated in NSCLC and positively regulate proliferation [48,67,105]. Long intergenic non-protein coding RNA, p53-induced transcript (LINC-PINT) acts as a ceRNA of miR-208-3p to upregulate programmed cell death protein 4 (PCDC4), reducing NSCLC cell proliferation and cell-cycle progression [107]. Although the exact mechanism was not defined, lncRNA MIR503-HG also reduced NSCLC proliferation by downregulating cyclin D1 expression [114]. However, MIR503-HG was shown to have a contradictory role as an oncogene by sponging miR-489-3p and mir-625-5p and promoting NSCLC cell proliferation and reduced apoptosis [71]. As a defined mechanism for MIR503-HG’s role in NSCLC is not yet elucidated, further work is required to understand its exact role as a promoter or suppressor of NSCLC.
LncRNAs LINC00702 and MAGI2 antisense RNA 3 (MAGI2-AS3) can act as ceRNAs for miR-510 and miR-23a-3p, respectively, to increase PTEN expression and reduce NSCLC proliferation [109,111]. Both lncRNAs are downregulated in NSCLC, and their function opposes oncogenic lncRNA FAL1-BMI1 that downregulates PTEN to enhance AKT-induced tumorigenesis [42,109,111]. LncRNA maternally expressed 3 (MEG3) acts as a ceRNA for miR-7-5p to inhibit BCL-2 and promote BCL-2-like factor 4 (BAX), enhancing NSCLC apoptosis [112]. In contrast, XIST acts as a ceRNA to increase BCL-2, and it may be upregulated to reverse the tumor suppressor function of MEG3 [94,112]. Therefore, tumor suppressor lncRNAs can regulate proliferation via similar mechanisms to oncogenic lncRNAs, albeit to suppress NSCLC progression.
3.2.2. EMT and Metastasis
Tumor suppressor lncRNAs can reduce EMT by downregulating key inducers and reducing EMT induction and metastasis. For example, NF-κB (NF-κB)-interacting lncRNA (NKILA) interacts directly with NF-κB to decrease NF-κB inhibitor alpha (IKBa) phosphorylation, inhibiting NF-κB nuclear translocation and zinc finger protein SNAI1 (SNAIL1)-induced EMT, inhibiting NSCLC cell migration and invasion [115]. NKILA could also reduce EMT by inhibiting interleukin 11 (IL-11) mRNA levels, reducing STAT3 phosphorylation and EMT induction [116]. Therefore, NKILA may function transcriptionally and post-transcriptionally to regulate EMT inhibition in NSCLC. LncRNA BRE antisense RNA 1 (BRE-AS1) directly interacted with STAT3 to reduce its binding to and derepress the nuclear receptor subfamily 4 group A member 3 (NR4A3) promoter to reduce NSCLC cell growth, survival, and EMT [100]. Furthermore, LINC8150 reduced STAT3 activation by sponging miR-199b-5p and increasing caveolin-1 (CAV1), causing a reduction in NSCLC cell migration and invasion in vitro and EMT and metastases in vivo [110]. Therefore, negative regulation of the STAT3 pathway by tumor suppressor lncRNAs is important for inhibition of NSCLC EMT and metastasis.
LncRNA Fork-head box F1 (FOXF1) antisense RNA 1 (FOXF1-AS1) also inhibited EMT by directing EZH2 repression of FOXF1 expression to inhibit migration and invasion of NSCLC cells [103]. LncRNA FTX transcript, XIST regulator (FTX) acted as a ceRNA for miR-200a-3p to increase Fork-head box protein A2 (FOXA2) to inhibit NSCLC cell EMT and metastasis [104]. Interestingly, the miR-200 family is generally associated with tumor suppressors and oncogenic lncRNAs such as PVT1 silence miR-200a and miR-200b to enhance NSCLC tumorigenesis [80]. As FTX also inhibits miR-200 family member miR-200a-3p to suppress NSCLC progression, it suggests that miRNA regulation by lncRNAs is complex in NSCLC [104]. GAS5 antisense RNA 1 (GAS5-AS1) also inhibited NSCLC cell EMT to inhibit migration and invasion of NSCLC cells [106]. As GAS5 sense lncRNA inhibited NSCLC cell proliferation but not migration and invasion, transcription of lncRNAs in both the sense and antisense direction may be important for inhibition of NSCLC tumorigenesis [105,106].
In addition to a role in inhibiting apoptosis, MEG3 can increase NSCLC migration, invasion, and metastasis by sponging miR-650 and increase metastatic suppressor solute carrier family 34 member 2 (SLC34A2) [112,113]. LncRNA DiGeorge syndrome critical region gene 5 (DGCR5) also acts as a ceRNA to sponge miR-211-5p to increase EPH receptor B6 (EPHB6) to reduce NSCLC cell growth, invasion, and migration [101]. Furthermore, LINC00261 acted as a ceRNA for miR-552-3p to increase Wnt pathway suppressor secreted Frizzled-related protein 2 (SRFP2) to increase NSCLC cell apoptosis and inhibit invasion [108]. In contrast to oncogenic lncRNAs that upregulate MMPs to enhance invasion, lncRNA FOXF1 adjacent non-coding developmental regulatory RNA (FENDRR) upregulates tissue inhibitors of MMP-2 (TIMP2) by sponging mir-176 to suppress NSCLC migration, invasion, and metastasis [102]. Tumor suppressor lncRNAs can, therefore, suppress NSCLC EMT and metastasis via distinct mechanisms.
4. Clinical Potential of LncRNAs in NSCLC
4.1. Diagnostic and Prognostic Potential
With a five-year survival of around 15%, NSCLC is associated with a poor prognosis, which is largely attributable to poor detection and late diagnosis [121]. Current traditional detection methods typically have low sensitivity and specificity, limiting early detection, and novel biomarkers are required for improved molecular diagnosis and prognosis [122]. Although proteins are commonly used as markers for diagnosis, lncRNAs are advantageous as clinical indicators as they are stable and highly tissue-specific, and they can be detected in various bodily fluids [122,123]. Compared to traditional biopsies, lncRNA-based biomarkers may also be better endured by patients and minimally invasive [123]. As reviewed in Shi et al., lncRNAs could be used to distinguish early-stage disease from healthy patients at a high sensitivity and specificity, as well as providing prognostic insight into the risk of metastases and recurrence [124]. For example, prostate cancer antigen 3 (PCA3) is an overexpressed lncRNA in prostate cancer (PCa) that contributes to PCa progression by modulating androgen signaling, and PCA3 urine levels were successfully used as a biomarker for PCa diagnosis [125].
In NSCLC, MALAT1 was shown to function in many aspects of NSCLC tumorigenesis, and its potential as a biomarker detected in fluids was studied. MALAT1 detection in NSCLC blood samples was shown to have a high specificity compared to cancer-free controls, as well as minimal invasiveness, suggesting its promise as a diagnostic tool. However, with a low sensitivity of 56%, it suggests that the use of MALAT1 as a single diagnostic biomarker may not be feasible in NSCLC [126]. XIST and HIF1A-AS1 both play important roles in NSCLC and are upregulated in NSCLC tissues and serums. Interestingly, the combination of XIST and HIF1A-AS1 yielded a higher positive diagnostic rate compared to either lncRNA alone [127]. Therefore, the use of multiple lncRNAs may improve the sensitivity and diagnostic efficiency of NSCLC; however, a commonly recognized group of lncRNAs is required. Li et al. performed a meta-analysis investigating the prognostic potential of MALAT1 and, although MALAT1 sensitivity was low for individual diagnostic testing, they found that MALAT1 could be used significantly as an independent prognostic factor for overall survival in NSCLC [128]. Zhang et al. investigated the clinical potential of multiple lncRNAs in NSCLC patients and found that a poor prognosis was associated with high expression of H19, MALAT1, and Hox antisense intergenic RNA (HOTAIR) and low expression of taurine upregulated gene 1 (TUG1) and p21-associated ncRNA DNA damage activated (PANDA) [129]. Therefore, individual lncRNAs, as well as combinations, may be important in investigating the prognosis of NSCLC.
4.2. Therapeutic Potential
The main form of treatment for NSCLC is curative intent surgery; however, with patients typically presenting at late-stage disease, surgery has limited efficacy, and platinum-based chemotherapy is the standard of care [121]. Furthermore, with increasing resistance to chemotherapies and targeted treatments, novel approaches need to be explored [122]. Due to the function of lncRNAs in all aspects of NSCLC tumorigenesis and the regulation of key signaling pathways, lncRNAs may be promising therapeutic targets. Furthermore, many lncRNAs are associated with enhanced chemoresistance and, therefore, their targeting may also restore cancer cell sensitivity to chemotherapeutic drugs [122].
There are various different approaches for targeting lncRNAs in cancer including RNA interference (RNAi)-based gene silencing, antisense oligonucleotide (ASO)-based treatment, small-molecule modulators of lncRNA–protein interactions, and the delivery of tumor suppressor lncRNAs [122,130]. For example, siRNA interference of HOTAIR decreased the migration and invasion of NSCLC cells in vitro and reduced metastases in a HOTAIR siRNA xenograft mouse model [131]. Furthermore, HOTAIR siRNA-mediated knockdown also increased the sensitivity of NSCLC cells to cisplatin treatment (Table 3) [132]. However, RNAi can have off-target effects and can be problematic for nuclear RNAs, with many lncRNAs functioning in the nucleus in NSCLC. In contrast, ASOs are advantageous due to their high affinity and reduced toxicity due to relatively low off-target effects [133]. In a MALAT1 knockout mouse model, mice injected with MALAT1 ASOs had reduced lung tumor nodules and volume compared to untreated mice (Table 3). Therefore, ASO inhibition of MALAT1 inhibited NSCLC metastasis and may provide a promising therapeutic approach in NSCLC [134]. Many lncRNAs function in NSCLC by interacting with or regulating the epigenetic regulator EZH2 (Table 1), to promote NSCLC tumorigenesis. High-throughput screening methods to identify small-molecule inhibitors of the lncRNA–EZH2 interaction were developed. which may lead to the identification of general inhibitors targeting the RNA-binding pocket, as well as specific inhibitors targeting particular lncRNA interactions [135]. Targeting lncRNA interactions with proteins, such as EZH2, may be a strategy to reduce off-target effects and increase targeted specificity (Table 3). MEG3 was shown to be downregulated in NSCLC, and it inhibits NSCLC cell apoptosis, invasion, migration, and metastasis, as well as increasing sensitivity to cisplatin [112,113,136]. Overexpression of MEG3 inhibited tumorigenesis in vivo and reduced NSCLC cell proliferation, as well as induced apoptosis in vitro, suggesting that delivery of tumor suppressor lncRNAs such as MEG3 may potentially be an alternative therapeutic option in NSCLC (Table 3) [137]. However, further work is required to assess the effectiveness of tumor suppressor lncRNA delivery as a therapeutic option in the clinic.

Table 3.
Therapeutic targeting approaches in NSCLC.
4.3. Limitations and Future Prospectives
Despite evidence supporting the role of lncRNAs as therapeutic targets in NSCLC, there are limitations to targeting and studying the effectiveness of lncRNAs. For example, the lack of a protein product restricts treatment approaches largely to nucleic acid-based therapies [138]. Although successful, their limitations include off-target effects, difficulty crossing the cellular plasma membrane, and reduced bioavailability [139]. Furthermore, unlike proteins, the three-dimensional (3D) structure of lncRNAs remains largely unknown, and a lack of conserved domains could hinder the design of small-molecule inhibitors. A lack of full understanding of the mechanisms and regulatory networks for many lncRNAs in NSCLC may also limit specific targeting strategies to reduce toxicities [138]. Although some lncRNAs in NSCLC are conserved, such as MALAT1, NEAT1, and H19, many lack conservation across species, which can impede investigations and pre-clinical studies in animal models [138,139]. Furthermore, the lungs are a difficult site for siRNA and ASO administration in vivo, which may hinder pre-clinical studies for lncRNA therapies in NSCLC. There is difficulty in initial lung delivery due to physical barriers such as mucosa and cilia, as well as avoiding the immune system. Furthermore, entry past the target cell membrane into the correct subcellular compartment and escaping endosome destruction is difficult to achieve [140]. Further work is, therefore, required to improve drug delivery to the lungs in vivo to accurately study the effectiveness of lncRNA-based therapies in NSCLC.
The use of ASOs in the treatment of neurodegenerative disorders showed promise, with the FDA approval in 2016 of Nusinersen and Eteplirsen for the treatment of spinal muscular atrophy and Duchenne muscular dystrophy, respectively [141,142]. The success of ASO-based therapies paved the way for investigating lncRNA-driven ASO treatments in cancer and, despite the limitations of lncRNAs as therapeutic targets, there is hope that they can become a reality in the future for NSCLC.
5. Conclusions
LncRNAs were shown to function in many important cellular processes, with their role in cancer becoming increasingly more apparent [9]. The localization of lncRNAs largely reflects their function, and they can interact with chromatin, protein, and RNA to regulate all stages of gene expression and influence important signaling cascades [9]. Although normally tightly regulated, large-scale analyses revealed that lncRNAs are commonly dysregulated in NSCLC [36]. Many lncRNAs are upregulated and function as oncogenes to enhance NSCLC proliferation, survival, invasion, migration, EMT, and metastasis. Some lncRNAs, such as MALAT1, PVT1, and XIST, can function in multiple aspects of NSCLC tumorigenesis through a variety of different mechanisms. Although the most common mechanism of lncRNA regulation in NSCLC appears to be miRNA sponging, many lncRNAs can also modulate gene expression and protein interactions and stability, suggesting that lncRNA regulation in NSCLC is complex. Tumor suppressor lncRNAs that are downregulated in NSCLC act via similar mechanisms to oncogenes, albeit to suppress cancer progression. As many of the pathways and genes regulated by suppressor lncRNAs are also regulated by oncogenic lncRNAs, oncogenic lncRNAs may be upregulated to reverse the effects of suppressor lncRNAs. Although the mechanisms of action are detailed for the majority of lncRNAs within this review, the mechanisms of action for many lncRNAs are not fully elucidated and are required to ascertain their function as tumor promoters or suppressors.
As the poor prognosis for NSCLC is largely attributable to late diagnosis and the lack of efficient treatments for late-stage disease, novel approaches are required for the management of NSCLC [121]. Combinations of multiple lncRNAs show promise as diagnostic biomarkers, as well as individual or multiple lncRNAs as prognostic indicators. However, further study is required to bring lncRNAs to the clinic as diagnostic tools in NSCLC. Recently, ASOs were successful in the treatment of neurodegenerative diseases, and they may pave the way for lncRNA-based therapies in NSCLC [141,142]. Despite the limitations in studying and designing treatments for lncRNAs, having a deeper understanding of their mechanisms may direct novel treatment approaches to reduce the burden of NSCLC.
Author Contributions
M.G. conceived the structure of this review. L.G., L.S., and M.L.M. investigated key resources for the review. L.G. wrote the manuscript and designed and created the figure and tables. L.S. and M.L.M. reviewed the text. All authors reviewed and edited the text and have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Cancer Research MI core grant (C5759/A20971).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AC | Adenocarcinoma |
| ADAM15 | A disintegrin and metalloproteinase domain 15 |
| AFAP-AS1 | AFAP antisense gene 1 |
| AKT | Protein kinase B |
| ANRIL | Antisense noncoding RNA in the INK4 locus |
| ASO | Antisense oligonucleotide |
| BAX | BCL-2-like factor 4 |
| BCL-2 | B-cell lymphoma type 2 |
| BMI1 | BMI1 polycomb ring finger oncogene |
| BRE-AS1 | BRE antisense RNA 1 |
| BTG2 | BTG anti-proliferation factor 2 |
| CAV1 | Caveolin-1 |
| CDKs | Cyclin-dependent kinases |
| ceRNAs | Competing endogenous RNAs |
| CIZI | Cip1-interacting zinc finger protein 1 |
| CREB1 | cAMP response element-binding protein |
| DANCR | Differentiation antagonizing nonprotein coding RNA |
| DGCR5 | DiGeorge syndrome critical region gene 5 |
| DLX6-AS1 | DLX6 antisense gene 1 |
| DNMT1 | DNA methyltransferase 1 |
| E2F | E2F transcription factor |
| EGR1 | Early growth response protein 1 |
| EMT | Epithelial-to-mesenchymal transition |
| EPHB6 | EPH receptor B6 |
| eRNA | Enhancer RNA |
| ETS1 | ETS proto-oncogene transcription factor 1 |
| EZH2 | Enhancer of zeste homolog 2 |
| FAL1 | Focally amplified lncRNA on chromosome 1 |
| FENDRR | FOXF1 adjacent non-coding developmental regulatory RNA |
| FEZF1-AS1 | FEZF1 antisense RNA 1 |
| FLVCR1-AS1 | LncRNA FLVCR1 antisense gene 1 |
| FOXA2 | Fork-head box protein A2 |
| FOXFF1-AS1 | Fork-head box F1 (FOXF1) antisense RNA 1 |
| FOXM1 | Fork-head box protein 1 |
| FRAT1 | FRAT regulator of WNT signaling Pathway 1 |
| FTX | FTX transcript, XIST regulator |
| GAS5 | Growth arrest specific 5 |
| GAS5-AS1 | GAS5 antisense RNA 1 |
| GSTP1 | G1-to-S phase transition 1 |
| G1-to-S | Gap 1 to synthesis |
| HIT | HOXA transcript induced by transforming growth factor (TGF)-β |
| HMGA2 | High-mobility group AT-hook 2 |
| HMGB2 | High-mobility group protein B2 |
| HNF1A-AS1 | HNF1A antisense RNA 1 |
| hnRNP K | Heterogeneous nuclear ribonucleoprotein K |
| HOTAIR | Hox antisense intergenic RNA |
| HOTTIP | HoxA distal transcript antisense RNA |
| HOXA3 | Homeobox Protein A3 |
| HOXA11-AS1 | HOXA11 antisense 1 |
| HOXAD-AS1 | HOXD cluster antisense RNA 1 |
| IGF-2 | Insulin-like growth factor 2 |
| IKBa | NF-κB inhibitor alpha |
| IL-11 | Interleukin 11 |
| IL-6 | Interleukin 6 |
| IRS2 | Insulin receptor substrates 2 |
| ITGB8 | Integrin subunit beta 8 |
| KLF2 | Krüppel-like factor 2 |
| JPX | JPX transcript and XIST activator |
| LATS2 | Large tumor suppressor kinase 2 |
| LCC | Large-cell carcinoma |
| LEF1-AS1 | LEF1 antisense RNA 1 |
| LINC-PINT | Long intergenic non-protein-coding RNA, p53-induced transcript |
| lincRNA | Long intergenic RNA |
| lncRNA | Long noncoding RNA |
| LRIG2 | Leucine-rich repeats and immunoglobulin-like domains 2 |
| LSD1 | Lysine-specific histone demethylase 1A |
| LSINCT5 | Long stress-induced non-coding transcript 5 |
| LUCAT-1 | Lung cancer-associated transcript 1 |
| MAGI2-AS3 | MAGI2 antisense RNA 3 |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| mTOR | Mammalian target of rapamycin |
| MAPK | Mitogen-activated protein kinase |
| MDM2 | E3 ubiquitin ligase |
| MEG3 | Maternally expressed 3 |
| MIAT | Myocardial infarction-associated transcript |
| miRNA | microRNA |
| MLL | Mixed lineage leukemia |
| MMPs | Matrix metalloproteinases |
| ncRNAs | Non-coding RNAs |
| NEAT1 | Nuclear-enriched abundant transcript 1 |
| NFKb | NF-κB |
| NKILA | NF-κB-interacting lncRNA |
| NNT-AS1 | Nicotinamide nucleotide transhydrogenase-antisense RNA1 |
| NR4A3 | Nuclear receptor subfamily 4 group A member 3 |
| NSCLC | Non-small-cell lung cancer |
| nt | Nucleotides |
| p21 | Cyclin-dependent kinase inhibitor 1A |
| p53 | Tumor protein 53 |
| PANDA | P21-associated ncRNA DNA damage activated |
| PCa | Prostate cancer |
| PCA3 | Prostate cancer antigen 3 |
| PCAT-1 | Prostate cancer-associated transcript 1 |
| PCAT-6 | Prostate cancer-associated transcript 6 |
| PCDC4 | Programmed cell death protein 4 |
| PRC2 | Polycomb repressive complex 2 |
| PDK1 | Pyruvate dehydrogenase 1 |
| PGK1 | Phosphoglycerate kinase 1 |
| PI3K | Phosphatidylinositol 3-kinase |
| piRNA | Piwi-interacting RNA |
| PRKCZ-AS1 | PRKCZ antisense RNA 1 |
| PRNCR1 | Prostate cancer non-coding RNA 1 |
| PTEN | Phosphatase and tensin homolog |
| PVT1 | Plasmacytoma variant translocation 1 |
| RAB34 | Ras-related protein rab-34 |
| RHOB | Rho-related GTP-binding protein |
| RNAi | RNA interference |
| RNAP2 | RNA polymerase type II |
| RSF1 | Remodeling and splicing factor 1 |
| SCC | Squamous cell carcinoma |
| SCLC | Small-cell lung cancer |
| siRNA | Small interfering RNA |
| SLC34A2 | Solute carrier family 34 member 2 |
| SNAIL1 | Zinc finger protein SNAI1 |
| sncRNA | Small non-coding RNA |
| SNHG1 | Small nucleolar RNA host gene 1 |
| SNHG15 | Small nucleolar RNA host gene 15 |
| SNHG20 | Small nucleolar RNA host gene 20 |
| SNHG6 | Small nucleolar RNA host gene 6 |
| snoRNA | Small nucleolar RNA |
| SOX2OT | SOX-2 overlapping transcript |
| SOX4 | SRY-box transcription factor 4 |
| SOX9 | SRY-box transcription factor 9 |
| SRFP2 | Secreted Frizzled-related protein 2 |
| SRSF | Serine/arginine splicing factors |
| STAT3 | Signal transducer and activator of transcription 3 |
| TDRG1 | Human testis developmental related gene 1 |
| TIMP2 | Tissue inhibitors of MMP 2 |
| TUG1 | Taurine upregulated gene 1 |
| TWIST | Twist family BHLH transcription factor 1 |
| VAV3 | Vav guanine nucleotide exchange factor 3 |
| WDR5 | Repeat-containing protein 5 |
| Wnt | Wingless |
| XIAP | X-linked inhibitor of apoptosis |
| XIST | X-inactive-specific transcript |
| YAP1 | YES-associated protein 1 |
| ZEB | Zinc finger E-box-binding homeobox |
| ZEB1-AS1 | ZEB1 antisense RNA 1 |
| ZFAS1 | ZNFX1 antisense RNA 1 |
| ZNF217 | Zinc finger protein 217 |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Zappa, C.; Mousa, S. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Pikor, L.A.; Ramnarine, V.R.; Lam, S.; Lam, W.L. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer 2013, 82, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-coding RNA networks in cancer. Nat. Rev. Cancer 2017, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; Chen, Y. Small and Long Non-Coding RNAs: Novel Targets in Perspective Cancer Therapy. Curr. Genom. 2015, 16, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, W.; Chen, Q.; Chen, M. Non-Coding RNAs and their Integrated Networks. J. Integr. Bioinform. 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.Y.; Colognori, D.; Lee, J.T. Long Noncoding RNAs: Past, Present, and Future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef]
- Schmitt, A.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef]
- Mongelli, A.; Martelli, F.; Farsetti, A.; Gaetano, C. The Dark That Matters: Long Non-coding RNAs as Master Regulators of Cellular Metabolism in Non-communicable Diseases. Front. Physiol. 2019, 10, 369. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Enfield, K.S.S.; Pikor, L.A.; Martinez, V.; Lam, W.L. Mechanistic Roles of Noncoding RNAs in Lung Cancer Biology and Their Clinical Implications. Genet. Res. Int. 2012, 2012, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2015, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Roth, A.; Diederichs, S. Long Noncoding RNAs in Lung Cancer. Curr. Top. Microbio. Immunol. 2015, 394, 57–110. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; An, L.; Wang, Y.; Zhang, Z.; Guo, Y.; Liu, C. Analysis of Long Non-Coding RNA Expression Profiles in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2016, 38, 2389–2400. [Google Scholar] [CrossRef]
- Zhan, Y.; Zang, H.; Feng, J.; Lu, J.; Chen, L.; Fan, S. Long non-coding RNAs associated with non-small cell lung cancer. Oncotarget 2017, 8, 69174–69184. [Google Scholar] [CrossRef]
- Balas, M.M.; Johnson, A.M. Exploring the mechanisms behind long noncoding RNAs and cancer. Non-Coding RNA Res. 2018, 3, 108–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, L. The Application of lncRNAs in Cancer Treatment and Diagnosis. Recent Pat. AntiCancer Drug Discov. 2018, 13, 292–301. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Wang, H.; Xin, Y.; Jiao, W. Long non-coding RNAs in non-small cell lung cancer: Functions and distinctions from other malignancies. Transl. Cancer Res. 2019, 8, 2636–2653. [Google Scholar] [CrossRef]
- Anastasiadou, E.; Faggioni, A.; Trivedi, P.; Slack, F.J. The Nefarious Nexus of Noncoding RNAs in Cancer. Int. J. Mol. Sci. 2018, 19, 2072. [Google Scholar] [CrossRef]
- Lee, J.T.; Bartolomei, M.S. X-Inactivation, Imprinting, and Long Noncoding RNAs in Health and Disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef]
- Fang, J.; Sun, C.-C.; Gong, C. Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem. Biophys. Res. Commun. 2016, 478, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Giles, K.; Woolnough, J.L.; Atwood, B. ncRNA function in chromatin organization. In Epigenetic Gene Expression and Regulation; Huang, S., Litt, M., Blakey, C.A., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 117–148. [Google Scholar]
- Sang, Y.; Zhou, F.; Wang, D.; Bi, X.; Liu, X.; Hao, Z.; Li, Q.; Zhang, W. Up-regulation of long non-coding HOTTIP functions as an oncogene by regulating HOXA13 in non-small cell lung cancer. Am. J. Transl. Res. 2016, 8, 2022–2032. [Google Scholar] [PubMed]
- Zhang, C.; Ge, S.; Gong, W.; Xu, J.; Guo, Z.; Liu, Z.; Gao, X.; Wei, X.; Ge, S. LncRNA ANRIL acts as a modular scaffold of WDR5 and HDAC3 complexes and promotes alteration of the vascular smooth muscle cell phenotype. Cell Death Dis. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.-Q.; Sun, M.; Yang, J.-S.; Xie, M.; Xu, T.-P.; Xia, R.; Liu, Y.-W.; Liu, X.-H.; Zhang, E.-B.; Lu, K.-H.; et al. Long Noncoding RNA ANRIL Promotes Non-Small Cell Lung Cancer Cell Proliferation and Inhibits Apoptosis by Silencing KLF2 and P21 Expression. Mol. Cancer Ther. 2014, 14, 268–277. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The Nuclear-Retained Noncoding RNA MALAT1 Regulates Alternative Splicing by Modulating SR Splicing Factor Phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; Hillejan, L.; et al. The Long Noncoding MALAT-1 RNA Indicates a Poor Prognosis in Non-small Cell Lung Cancer and Induces Migration and Tumor Growth. J. Thorac. Oncol. 2011, 6, 1984–1992. [Google Scholar] [CrossRef]
- Song, J.; Su, Z.-Z.; Shen, Q.-M. Long non-coding RNA MALAT1 regulates proliferation, apoptosis, migration and invasion via miR-374b-5p/SRSF7 axis in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1853–1862. [Google Scholar]
- Sun, C.; Li, S.; Zhang, F.; Xi, Y.; Wang, L.; Bi, Y.; Li, D. Long non-coding RNA NEAT1 promotes non-small cell lung cancer progression through regulation of miR-377–3p-E2F3 pathway. Oncotarget 2016, 7, 51784–51814. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Dong, M.; Wu, D. Long non-coding RNA NEAT1 regulates E2F3 expression by competitively binding to miR-377 in non-small cell lung cancer. Oncol. Lett. 2017, 14, 4983–4988. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Xia, Y.; Fan, Q.; Yang, K.-P. Long Noncoding RNA NEAT1 Promotes Proliferation and Invasion via Targeting miR-181a-5p in Non-Small Cell Lung Cancer. Oncol. Res. 2018, 26, 289–296. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Tang, Y.; Xiao, G.; Chen, Y.; Deng, Y. LncRNA MALAT1 promotes migration and invasion of non-small-cell lung cancer by targeting miR-206 and activating Akt/mTOR signaling. AntiCancer Drugs 2018, 29, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, J.; Liu, T.; Chen, T.; Pan, S.; Huang, W.; Li, S. Analysis of lncRNA expression profiles in non-small cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer 2014, 85, 110–115. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, T.; Saren, G.; Liao, L.; Fang, W.; Zhao, H. Comprehensive analysis of differentially expressed long non-coding RNAs in non-small cell lung cancer. Oncol. Lett. 2019, 18, 1145–1156. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Chen, P.; Yu, S.; Nie, F.; Lu, B.; Zhang, T.; Zhou, Y.; Chen, Q.; Wei, C.; et al. Long non-coding RNA SNHG20 promotes non-small cell lung cancer cell proliferation and migration by epigenetically silencing of P21 expression. Cell Death Dis. 2017, 8, e3092. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Wu, Y.S.; Wang, W.S.; Zhang, J.S.; Wu, Q.G. Upregulation of lncRNA DANCR functions as an oncogenic role in non-small lung cancer by regulating miR-214–5p/CIZ1 axis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2539–2547. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, G.; Chu, H.; Li, P.; Li, J. The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am. J. Cancer Res. 2019, 9, 270–284. [Google Scholar]
- Sun, W.; Zhang, L.; Yan, R.; Yang, Y.; Meng, X. LncRNA DLX6-AS1 promotes the proliferation, invasion, and migration of non-small cell lung cancer cells by targeting the miR-27b-3p/GSPT1 axis. Onco. Targets Ther. 2019, 12, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Nie, F.-Q.; Zang, C.; Wang, Y.; Hou, J.; Wei, C.; Li, W.; He, X.; Lu, K.-H. The Pseudogene DUXAP8 Promotes Non-small-cell Lung Cancer Cell Proliferation and Invasion by Epigenetically Silencing EGR1 and RHOB. Mol. Ther. 2017, 25, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Yao, G.; Liu, B.; Ma, T.; Xia, Y.; Wei, K.; Wang, J.; Xu, J.; Chen, L.; Chen, Y. Long Noncoding RNA FAL1 Promotes Cell Proliferation, Invasion and Epithelial-Mesenchymal Transition Through the PTEN/AKT Signaling Axis in Non-Small Cell Lung Cancer. Cell. Physiol. Biochem. 2017, 43, 339–352. [Google Scholar] [CrossRef]
- He, R.; Zhang, F.H.; Shen, N. LncRNA FEZF1-AS1 enhances epithelial-mesenchymal transition (EMT) through suppressing E-cadherin and regulating WNT pathway in non-small cell lung cancer (NSCLC). Biomed. Pharmacother. 2017, 95, 331–338. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, S.; Yang, X.; Zang, S.; Yuan, X. Long non-coding RNA FLVCR1-AS1 contributes to the proliferation and invasion of lung cancer by sponging miR-573 to upregulate the expression of E2F transcription factor 3. Biochem. Biophys. Res. Commun. 2018, 505, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Huo, X.; Sun, R.; Liu, Z.; Huang, M.; Yang, S. LncRNA Gm15290 Promotes Cell Proliferation and Invasion in Non-Small Cell Lung Cancer Through Directly Interacting With and Suppressing the Tumor Suppressor miR-615-5p. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2017. [Google Scholar] [CrossRef]
- Huang, Z.; Lei, W.; Hu, H.-B.; Zhang, H.; Zhu, Y.-H. H19 promotes non-small-cell lung cancer (NSCLC) development through STAT3 signaling via sponging miR-17. J. Cell. Physiol. 2018, 233, 6768–6776. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Wen, Y.; Peng, B.; Ding, G.; Yang, L.; Wang, Z. Upregulated lncRNA H19 promotes non-small cell lung cancer cell proliferation through miR-138/PDK1 axis. Int. J. Clin. Exp. Pathol. 2017, 10, 9012–9020. [Google Scholar] [PubMed]
- Yu, L.; Fang, F.; Lu, S.; Li, X.; Yang, Y.; Wang, Z. lncRNA-HIT promotes cell proliferation of non-small cell lung cancer by association with E2F1. Cancer Gene Ther. 2017, 24, 221–226. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Z.; Qiu, L.; Yang, Y.; Wang, Y.; Chen, Z.; Liu, Z.; Yu, L. Upregulation of LncRNA-HIT promotes migration and invasion of non-small cell lung cancer cells by association with ZEB1. Cancer Med. 2016, 5, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, Y.; Li, Q.; Duan, P. lncRNA HNF1A-AS1 modulates non-small cell lung cancer progression by targeting miR-149–5p/Cdk6. J. Cell Biochem. 2019, 120, 18736–18750. [Google Scholar] [CrossRef]
- Chen, J.-H.; Zhou, L.-Y.; Xu, S.; Zheng, Y.-L.; Wan, Y.-F.; Hu, C.-P. Overexpression of lncRNA HOXA11-AS promotes cell epithelial–mesenchymal transition by repressing miR-200b in non-small cell lung cancer. Cancer Cell Int. 2017, 17, 64. [Google Scholar] [CrossRef]
- Bai, Y.; Lang, L.; Zhao, W.; Niu, R. Long Non-Coding RNA HOXA11-AS Promotes Non-Small Cell Lung Cancer Tumorigenesis Through microRNA-148a-3p/DNMT1 Regulatory Axis. OncoTargets Ther. 2019, 12, 11195–11206. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Jing, H.; Li, Y.; Lv, X. Long noncoding RNA HOXD-AS1 promotes non-small cell lung cancer migration and invasion through regulating miR-133b/MMP9 axis. Biomed. Pharmacother. 2018, 106, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Ren, J.; Luo, M.; You, Z.; Fang, Y.; Han, Y.; Li, G.; Liu, H. Long noncoding RNA JPX correlates with poor prognosis and tumor progression in non-small cell lung cancer by interacting with miR-145–5p and CCND2. Carcinogenesis 2019, bgz125. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, X.; Jiang, W.; Wu, J.; Lin, L. lncRNA LEF1-AS1 Promotes Malignancy in Non-Small-Cell Lung Cancer by Modulating the miR-489/SOX4 Axis. DNA Cell Biol. 2019, 38, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Haiying, G.; Zhuo, L.; Ying, L.; Xin, H. Long non-coding RNA LINC00339 facilitates the tumorigenesis of non-small cell lung cancer by sponging miR-145 through targeting FOXM1. Biomed. Pharmacother. 2018, 105, 707–713. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, M.; Cheng, R. LINC00461/miR-4478/E2F1 feedback loop promotes non-small cell lung cancer cell proliferation and migration. Biosci. Rep. 2020, 40, BSR20191345. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, X.; Zhang, P.; Liu, Y.; Liu, Z.; Qian, B.; Liu, X.; Shao, G. Long non-coding RNA LINC00525 promotes the non-small cell lung cancer progression by targeting miR-338-3p/IRS2 axis. Biomed. Pharmacother. 2020, 124, 109858. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, H.; Niu, Y.; Wu, Y.; Sun, W.; Li, H.; Kong, J.; Ding, K.; Shen, H.-M.; Wu, H.; et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol. Cancer 2017, 16, 118. [Google Scholar] [CrossRef]
- Ma, C.; Wu, G.; Zhu, Q.; Liu, H.; Yao, Y.; Yuan, D.; Liu, Y.; Lv, T.; Song, Y. Long intergenic noncoding RNA 00673 promotes non-small-cell lung cancer metastasis by binding with EZH2 and causing epigenetic silencing of HOXA5. Oncotarget 2017, 8, 32696–32705. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Feng, Y.-H.; Zeng, S.; Zhong, M.-Z. linc01088 promotes cell proliferation by scaffolding EZH2 and repressing p21 in human non-small cell lung cancer. Life Sci. 2020, 241, 117134. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Lu, B.; Gu, Y.; Chen, Q.; Lei, T.; Nie, F.; Gu, J.; Huang, J.; Wei, C.; et al. Up-regulated LINC01234 promotes non-small-cell lung cancer cell metastasis by activating VAV3 and repressing BTG2 expression. J. Hematol. Oncol. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Bian, C.; Yuan, L.; Gai, H. A long non-coding RNA LINC01288 facilitates non-small cell lung cancer progression through stabilizing IL-6 mRNA. Biochem. Biophys. Res. Commun. 2019, 514, 443–449. [Google Scholar] [CrossRef]
- Xu, L.; Wei, B.; Hui, H.; Sun, Y.; Liu, Y.; Yu, X.; Dai, J. Positive feedback loop of lncRNA LINC01296/miR-598/Twist1 promotes non-small cell lung cancer tumorigenesis. J. Cell Physiol. 2019, 234, 4563–4571. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, X.; Li, Q.; Ran, P.; Xiang, X.; Yuan, Y.; Dong, T.; Zhu, B.; Wang, L.; Li, F.; et al. Long non-coding RNA 1308 promotes cell invasion by regulating the miR-124/ADAM 15 axis in non-small-cell lung cancer cells. Cancer Manag. Res. 2018, 10, 6599–6609. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, N.; Chen, S.; Ma, Y.; Liu, Y.; Zhang, L. The long non-coding RNA LSINCT5 promotes malignancy in non-small cell lung cancer by stabilizing HMGA2. Cell Cycle 2018, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Jin, S.-D.; Zhu, Q.; Han, L.; Feng, J.; Lu, X.-Y.; Wang, W.; Wang, F.; Guo, R.-H. Long non-coding RNA LUCAT1 is associated with poor prognosis in human non-small cell lung cancer and regulates cell proliferation via epigenetically repressing p21 and p57 expression. Oncotarget 2017, 8, 28297–28311. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mei, Z.; Hu, H.-B.; Zhang, X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J. Cell Physiol. 2018, 233, 6679–6688. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Zhao, Y.; Hu, Z.; Li, J.; Chu, D.; Zhang, J.; Li, Z.; Chen, B.; Zhang, X.; Pan, H.; et al. MetaLnc9 Facilitates Lung Cancer Metastasis via a PGK1-Activated AKT/mTOR Pathway. Cancer Res. 2017, 77, 5782–5794. [Google Scholar] [CrossRef] [PubMed]
- Lai, I.-L.; Yang, C.-A.; Lin, P.-C.; Chan, W.-L.; Lee, Y.-T.; Yen, J.-C.; Chang, Y.-S.; Chang, J.-G. Long noncoding RNA MIAT promotes non-small cell lung cancer proliferation and metastasis through MMP9 activation. Oncotarget 2017, 8, 98148–98162. [Google Scholar] [CrossRef]
- Dao, R.; Wudu, M.; Hui, L.; Jiang, J.; Xu, Y.; Ren, H.; Qiu, X. Knockdown of lncRNA MIR503HG suppresses proliferation and promotes apoptosis of non-small cell lung cancer cells by regulating miR-489-3p and miR-625-5p. Pathol. Res. Pract. 2020, 216, 152823. [Google Scholar] [CrossRef]
- Qi, L.; Liu, F.; Zhang, F.; Zhang, S.; Lv, L.; Bi, Y.; Yu, Y. lncRNA NEAT1 competes against let-7a to contribute to non-small cell lung cancer proliferation and metastasis. Biomed. Pharmacother. 2018, 103, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, Y.; Xia, S. LncRNA NNT-AS1 promotes non-small cell lung cancer progression through regulating miR-22-3p/YAP1 axis. Thorac. Cancer 2020, 11, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Wang, B.; Ma, Y.; Chen, P. LncRNA-PCAT-1 promotes non–small cell lung cancer progression by regulating miR-149-5p/LRIG2 axis. J. Cell. Biochem. 2018, 120, 7725–7733. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Z.; Liu, Z.; Feng, X.; Hua, F.; Hu, X.; Wang, B.; Lu, K.; Nie, F. Long noncoding RNA PCAT6 functions as an oncogene by binding to EZH2 and suppressing LATS2 in non-small-cell lung cancer. EBioMedicine 2018, 37, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liao, Q.; Zou, P. PRKCZ-AS1 promotes the tumorigenesis of lung adenocarcinoma via sponging miR-766-5p to modulate MAPK1. Cancer Boil. Ther. 2020, 21, 364–371. [Google Scholar] [CrossRef]
- Guo, R.; Hu, T.; Liu, Y.; He, Y.; Cao, Y. Long non-coding RNA PRNCR1 modulates non-small cell lung cancer cells proliferation, apoptosis, migration, invasion and EMT through PRNCR1/miR-126-5p/MTDH axis. Biosci. Rep. 2020. [Google Scholar] [CrossRef]
- Wan, L.; Sun, M.; Liu, G.-J.; Wei, C.-C.; Zhang, E.-B.; Kong, R.; Xu, T.; Huang, M.-D.; Wang, Z. Long non-coding RNA PVT1 promotes non-small cell lung cancer cell proliferation through epigenetically regulating LATS2 expression. Mol. Cancer Ther. 2016, 15, 1082–1094. [Google Scholar] [CrossRef]
- Qiu, C.; Li, S.; Sun, D.; Yang, S. lncRNA PVT1 accelerates progression of non-small cell lung cancer via targeting miRNA-526b/EZH2 regulatory loop. Oncol. Lett. 2019, 19, 1267–1272. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, H.; Yin, L.; Wang, T.; Wu, J.; Xu, J.; Tao, H.; Liu, J.; He, X. lncRNA-PVT1 Facilitates Invasion Through Upregulation of MMP9 in Nonsmall Cell Lung Cancer Cell. DNA Cell Boil. 2017, 36, 787–793. [Google Scholar] [CrossRef]
- Xi, Y.; Shen, W.; Jin, C.; Wang, L.; Yu, B. PVT1 Promotes the Proliferation and Migration of Non-Small Cell Lung Cancer via Regulating miR-148/RAB34 Signal Axis. Onco. Targets Ther. 2020, 13, 1819–1832. [Google Scholar] [CrossRef]
- Wei, C.M.; Zhao, X.F.; Qiu, H.B.; Ming, Z.; Liu, K.; Yan, J. The long non-coding RNA PVT1/miR-145-5p/ITGB8 axis regulates cell proliferation, apoptosis, migration and invasion in non-small cell lung cancer cells. Neoplasma 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Li, L. Long non-coding RNA PVT1 contributes to cell growth and metastasis in non-small-cell lung cancer by regulating miR-361-3p/SOX9 axis and activating Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 2020, 126, 110100. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Shan, S.; Li, Y.; Zhu, D.; Jin, W.; Ren, T. Long noncoding RNA SNHG1 promotes non-small cell lung cancer progression by up-regulating MTDH via sponging miR-145–5p. FASEB J. 2018, 32, 3957–3967. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, F.; Zhu, C.; Geng, L.; Tian, T.; Liu, H. Upregulated lncRNA SNHG1 contributes to progression of non-small cell lung cancer through inhibition of miR-101-3p and activation of Wnt/β-catenin signaling pathway. Oncotarget 2017, 8, 17785–17794. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, H. LncRNA SNHG1 influences cell proliferation, migration, invasion, and apoptosis of non-small cell lung cancer cells via the miR-361-3p/FRAT1 axis. Thorac. Cancer 2019, 11, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Jin, H.; Wu, H.-B.; Xu, J.-J.; Li, B. Long non-coding RNA SNHG15 promotes CDK14 expression via miR-486 to accelerate non-small cell lung cancer cells progression and metastasis. J. Cell. Physiol. 2018, 233, 7164–7172. [Google Scholar] [CrossRef]
- Ma, X.-R.; Xu, Y.-L.; Qian, J.; Wang, Y. Long non-coding RNA SNHG15 accelerates the progression of non-small cell lung cancer by absorbing miR-211-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1536–1544. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, H.; Zhao, G. Long Noncoding RNA SNHG6 Promotes Proliferation and Inhibits Apoptosis in Non-small Cell Lung Cancer Cells by Regulating miR-490–3p/RSF1 Axis. Cancer Biother. Radiopharm. 2020, 35, 351–361. [Google Scholar] [CrossRef]
- Geng, H.; Li, S.; Xu, M. Long Noncoding RNA SNHG6 Functions as an Oncogene in Non-Small Cell Lung Cancer via Modulating ETS1 Signaling. OncoTargets Ther. 2020, 13, 921–930. [Google Scholar] [CrossRef]
- Kewei, Z.; Yang, L.; Limei, Q.; Xiaobo, M.; Hongguang, Z.; Ying, T.; Zhang, K.; Qu, L.; Ma, X.-B.; Zhao, H.; et al. Long noncoding RNA Sox2 overlapping transcript (SOX2OT) promotes non-small-cell lung cancer migration and invasion via sponging microRNA 132 (miR-132). OncoTargets Ther. 2018, 11, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mu, Y.; Wang, J.; Zhao, Y. LncRNA TDRG1 promotes the metastasis of NSCLC cell through regulating miR-873–5p/ZEB1 axis. J. Cell Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, X.; Gao, C.; Cui, Y. XIST promote the proliferation and migration of non-small cell lung cancer cells via sponging miR-16 and regulating CDK8 expression. Am. J. Transl. Res. 2019, 11, 6196–6206. [Google Scholar]
- Zhang, Y.-L.; Li, X.-B.; Hou, Y.-X.; Fang, N.-Z.; You, J.-C.; Zhou, Q.-H. The lncRNA XIST exhibits oncogenic properties via regulation of miR-449a and Bcl-2 in human non-small cell lung cancer. Acta. Pharmacol. Sin. 2017, 38, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, H.; Hu, X.; Li, W. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int. J. Biol. Macromol. 2018, 111, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wan, L.; Liu, Z.; Xu, G.; Wang, S.; Su, Z.; Zhang, Y.; Zhang, C.; Liu, X.; Lei, Z.; et al. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018, 418, 185–195. [Google Scholar] [CrossRef]
- Yang, R.; Li, P.; Zhang, G.; Lu, C.; Wang, H.; Zhao, G. Long Non-Coding RNA XLOC_008466 Functions as an Oncogene in Human Non-Small Cell Lung Cancer by Targeting miR-874. Cell. Physiol. Biochem. 2017, 42, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Zhang, C. LncRNA ZEB1-AS1/miR-409-3p/ZEB1 feedback loop is involved in the progression of non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018, 507, 450–456. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, G.; Rao, C.; Hua, G.; Yang, M.; Miao, X.; Ying, J.; Nie, L. Knockdown of lncRNA ZFAS1-suppressed non-small cell lung cancer progression via targeting the miR-150-5p/HMGA2 signaling. J. Cell. Biochem. 2019. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Zhong, W.; Zhao, Z.; Liu, Z. Long non-coding RNA BRE-AS1 represses non-small cell lung cancer cell growth and survival via up-regulating NR4A3. Arch. Biochem. Biophys. 2018, 660, 53–63. [Google Scholar] [CrossRef]
- Kang, M.; Shi, J.; Li, B.; Luo, M.; Xu, S.; Liu, X. LncRNA DGCR5 regulates the non-small cell lung cancer cell growth, migration, and invasion through regulating miR-211-5p/EPHB6 axis. BioFactors 2019, 45, 788–794. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Q.; Zhang, X.; Ding, Z.; Liu, R. LncRNA FENDRR suppresses the progression of NSCLC via regulating miR-761/TIMP2 axis. Biomed. Pharmacother. 2019, 118, 109309. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Huang, Z.; Zengli, Z.; Li, H.; Chen, Q.; Yao, C.; Cai, H.; Xiao, Y.; Xia, H.; Wang, Y. Loss of long noncoding RNA FOXF1-AS1 regulates epithelial-mesenchymal transition, stemness and metastasis of non-small cell lung cancer cells. Oncotarget 2016, 7, 68339–68349. [Google Scholar] [CrossRef]
- Jin, S.; He, J.; Zhou, Y.; Wu, D.; Li, J.; Gao, W. LncRNA FTX activates FOXA2 expression to inhibit non–small-cell lung cancer proliferation and metastasis. J. Cell. Mol. Med. 2020, 24, 4839–4849. [Google Scholar] [CrossRef]
- Shi, X.; Sun, M.; Liu, H.; Yao, Y.; Kong, R.; Chen, F.; Song, Y. A critical role for the long non-coding RNA GAS5 in proliferation and apoptosis in non-small-cell lung cancer. Mol. Carcinog. 2013, 54, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lyu, H.; Liu, H.; Shi, X.; Song, Y.; Liu, B. Downregulation of the long noncoding RNA GAS5-AS1 contributes to tumor metastasis in non-small cell lung cancer. Sci. Rep. 2016, 6, 31093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Li, J.; Yang, Q.; Hao, M.; Bu, L. Long noncoding RNA LINC-PINT inhibits non-small cell lung cancer progression through sponging miR-218-5p/PDCD4. Artif. Cells Nanomedicine Biotechnol. 2019, 47, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, H.; Wang, H.; Zhu, W.; Jiang, S.; Dou, R.; Yan, B. Overexpression of LINC00261 inhibits non–small cell lung cancer cells progression by interacting with miR-522-3p and suppressing Wnt signaling. J. Cell. Biochem. 2019, 120, 18378–18387. [Google Scholar] [CrossRef]
- Yu, W.; Li, D.; Ding, X.; Sun, Y.; Liu, Y.; Cong, J.; Yang, J.; Sun, J.; Ning, X.; Wang, H.; et al. LINC00702 suppresses proliferation and invasion in non-small cell lung cancer through regulating miR-510/PTEN axis. Aging 2019, 11, 1471–1485. [Google Scholar] [CrossRef]
- Peng, W.; He, D.; Shan, B.; Wang, J.; Shi, W.; Zhao, W.; Peng, Z.; Luo, Q.; Duan, M.; Li, B.; et al. LINC81507 act as a competing endogenous RNA of miR-199b-5p to facilitate NSCLC proliferation and metastasis via regulating the CAV1/STAT3 pathway. Cell Death Dis. 2019, 10, 533. [Google Scholar] [CrossRef]
- Hao, X.-Z.; Yang, K. LncRNA MAGI2-AS3 suppresses the proliferation and invasion of non-small cell lung carcinoma through miRNA-23a-3p/PTEN axis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7399–7407. [Google Scholar] [CrossRef]
- Wu, J.-L.; Meng, F.-M.; Li, H.-J. High expression of lncRNA MEG3 participates in non-small cell lung cancer by regulating microRNA-7-5p. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Z.; Shi, S.; Wang, J.; Li, N. Long non-coding RNA MEG3 regulates migration and invasion of lung cancer stem cells via miR-650/SLC34A2 axis. Biomed. Pharmacother. 2019, 120, 109457. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhai, S.; Du, T.; Li, Z. LncRNA MIR503HG Inhibits Non-Small Cell Lung Cancer Cell Proliferation by Inducing Cell Cycle Arrest Through the Downregulation of Cyclin D1. Cancer Manag. Res. 2020, 12, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Li, Y.; Wang, J.; Che, Y.; Sun, S.; Huang, J.; Chen, Z.; He, J. Long non-coding RNA NKILA inhibits migration and invasion of non-small cell lung cancer via NF-κB/Snail pathway. J. Exp. Clin. Cancer Res. 2017, 36, 54. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, X. Long non-coding RNA NKILA inhibits proliferation and migration of lung cancer via IL-11/STAT3 signaling. Int. J. Clin. Exp. Pathol. 2019, 12, 2595–2603. [Google Scholar]
- A Stewart, Z.; Westfall, M.D.; A Pietenpol, J. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef]
- Pauzaite, T.; Thacker, U.; Tollitt, J.; Copeland, N. Emerging Roles for Ciz1 in Cell Cycle Regulation and as a Driver of Tumorigenesis. Biomolecules 2016, 7, 1. [Google Scholar] [CrossRef]
- Yin, D.; Lu, X.; Su, J.; He, X.; De, W.; Yang, J.; Li, W.; Han, L.; Zhang, E. Long noncoding RNA AFAP1-AS1 predicts a poor prognosis and regulates non-small cell lung cancer cell proliferation by epigenetically repressing p21 expression. Mol. Cancer 2018, 17, 92. [Google Scholar] [CrossRef]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Boil. 2000, 10, 415–433. [Google Scholar] [CrossRef]
- Moran, C.A. Importance of Molecular Features of Non–Small Cell Lung Cancer for Choice of Treatment. Am. J. Pathol. 2011, 178, 1940–1948. [Google Scholar] [CrossRef]
- Lu, T.; Wang, Y.; Chen, D.; Liu, J.; Jiao, W. Potential clinical application of lncRNAs in non-small cell lung cancer. OncoTargets Ther. 2018, 11, 8045–8052. [Google Scholar] [CrossRef] [PubMed]
- Bolha, L.; Ravnik-Glavač, M.; Glavač, D. Long Noncoding RNAs as Biomarkers in Cancer. Dis. Markers 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Shi, T.; Gao, G.; Cao, Y. Long Noncoding RNAs as Novel Biomarkers Have a Promising Future in Cancer Diagnostics. Dis. Markers 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lemos, A.E.G.; Matos, A.D.R.; Ferreira, L.B.; Gimba, E.R.P. The long non-coding RNA PCA3: An update of its functions and clinical applications as a biomarker in prostate cancer. Oncotarget 2019, 10, 6589–6603. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.G.; Johnen, G.; Casjens, S.; Bryk, O.; Pesch, B.; Jöckel, K.-H.; Kollmeier, J.; Brüning, T. Evaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancer. BMC Res. Notes 2013, 6, 518. [Google Scholar] [CrossRef] [PubMed]
- Tantai, J.; Hu, D.; Yang, Y.; Geng, J. Combined identification of long non-coding RNA XIST and HIF1A-AS1 in serum as an effective screening for non-small cell lung cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 7887–7895. [Google Scholar]
- Li, Y.; Yang, Z.; Wan, X.; Zhou, J.-G.; Zhang, Y.; Ma, H.; Bai, Y. Clinical Prognostic Value of Metastasis-Associated Lung Adenocarcinoma Transcript 1 in Various Human Cancers: An Updated Meta-Analysis. Int. J. Boil. Markers 2016, 31, 173–182. [Google Scholar] [CrossRef]
- Zhang, C.-G.; Yin, D.-D.; Sun, S.-Y.; Han, L. The use of lncRNA analysis for stratification management of prognostic risk in patients with NSCLC. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 115–119. [Google Scholar]
- Sanchez, Y.; Huarte, M. Long Non-Coding RNAs: Challenges for Diagnosis and Therapies. Nucleic Acid Ther. 2013, 23, 15–20. [Google Scholar] [CrossRef]
- Liu, X.-H.; Liu, Z.-L.; Sun, M.; Liu, J.; Wang, Z.; De, W. The long non-coding RNA HOTAIR indicates a poor prognosis and promotes metastasis in non-small cell lung cancer. BMC Cancer 2013, 13, 464. [Google Scholar] [CrossRef]
- Guo, F.; Cao, Z.; Guo, H.; Li, S. The action mechanism of lncRNA-HOTAIR on the drug resistance of non-small cell lung cancer by regulating Wnt signaling pathway. Exp. Ther. Med. 2018, 15, 4885–4889. [Google Scholar] [CrossRef] [PubMed]
- Chery, J.; Näär, A. RNA therapeutics: RNAi and antisense mechanisms and clinical applications. Postdoc. J. 2016, 4, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Gutschner, T.; Haemmerle, M.; Eissmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Gross, M.; et al. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res. 2012, 73, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, R.P.; Salah-Uddin, S.; Modarresi, F.; Khoury, N.; Wahlestedt, C.; Faghihi, M. Screening for Small-Molecule Modulators of Long Noncoding RNA-Protein Interactions Using AlphaScreen. J. Biomol. Screen. 2015, 20, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, D.; Ma, H.; Li, Y. LncRNA MEG3 enhances cisplatin sensitivity in non-small cell lung cancer by regulating miR-21–5p/SOX7 axis. Onco. Targets Ther. 2017, 10, 5137–5149. [Google Scholar] [CrossRef]
- Lu, K.-H.; Li, W.; Liu, X.-H.; Sun, M.; Zhang, M.-L.; Wu, W.-Q.; Xie, W.; Hou, Y. Long non-coding RNA MEG3 inhibits NSCLC cells proliferation and induces apoptosis by affecting p53 expression. BMC Cancer 2013, 13, 461. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, D.; Sood, A.K.; Dang, C.V.; Zhong, X. Shedding Light on the Dark Cancer Genomes: Long Noncoding RNAs as Novel Biomarkers and Potential Therapeutic Targets for Cancer. Mol. Cancer Ther. 2018, 17, 1816–1823. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.; Spector, D.L. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef]
- Kjems, J.; A Howard, K. Oligonucleotide delivery to the lung: Waiting to inhale. Mol. Ther. Nucleic Acids 2012, 1, e1. [Google Scholar] [CrossRef]
- Aartsma-Rus, A. FDA Approval of Nusinersen for Spinal Muscular Atrophy Makes 2016 the Year of Splice Modulating Oligonucleotides. Nucleic Acid Ther. 2017, 27, 67–69. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Krieg, A.M. FDA Approves Eteplirsen for Duchenne Muscular Dystrophy: The Next Chapter in the Eteplirsen Saga. Nucleic Acid Ther. 2016, 27, 1–3. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).