Noncoding RNA-Mediated Epigenetic Regulation in Hepatic Stellate Cells of Liver Fibrosis
Abstract
1. Introduction
2. NcRNA-Mediated Epigenetic Regulation in HSCs Activation
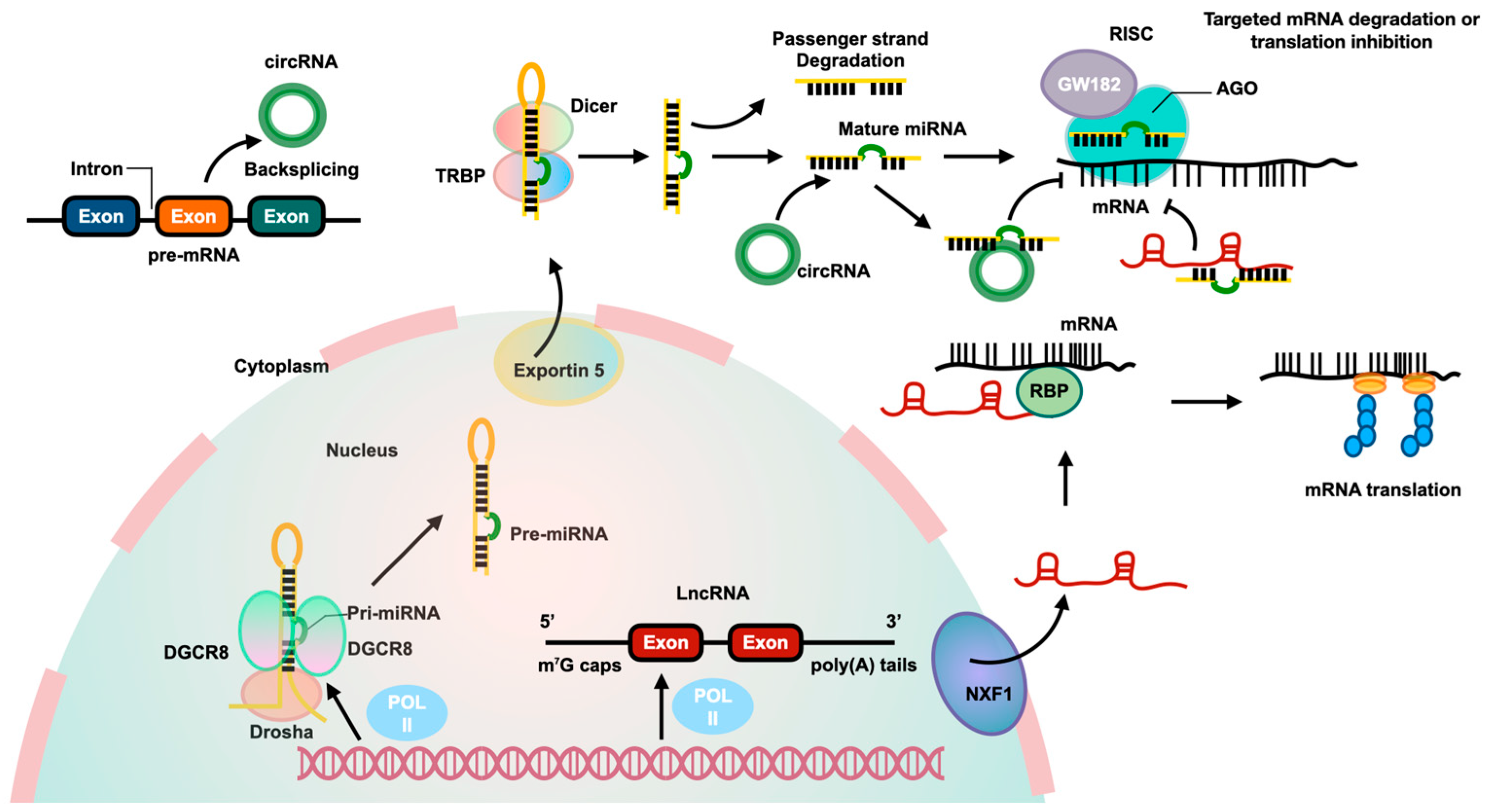
2.1. Identified MiRNAs in HSCs Activation
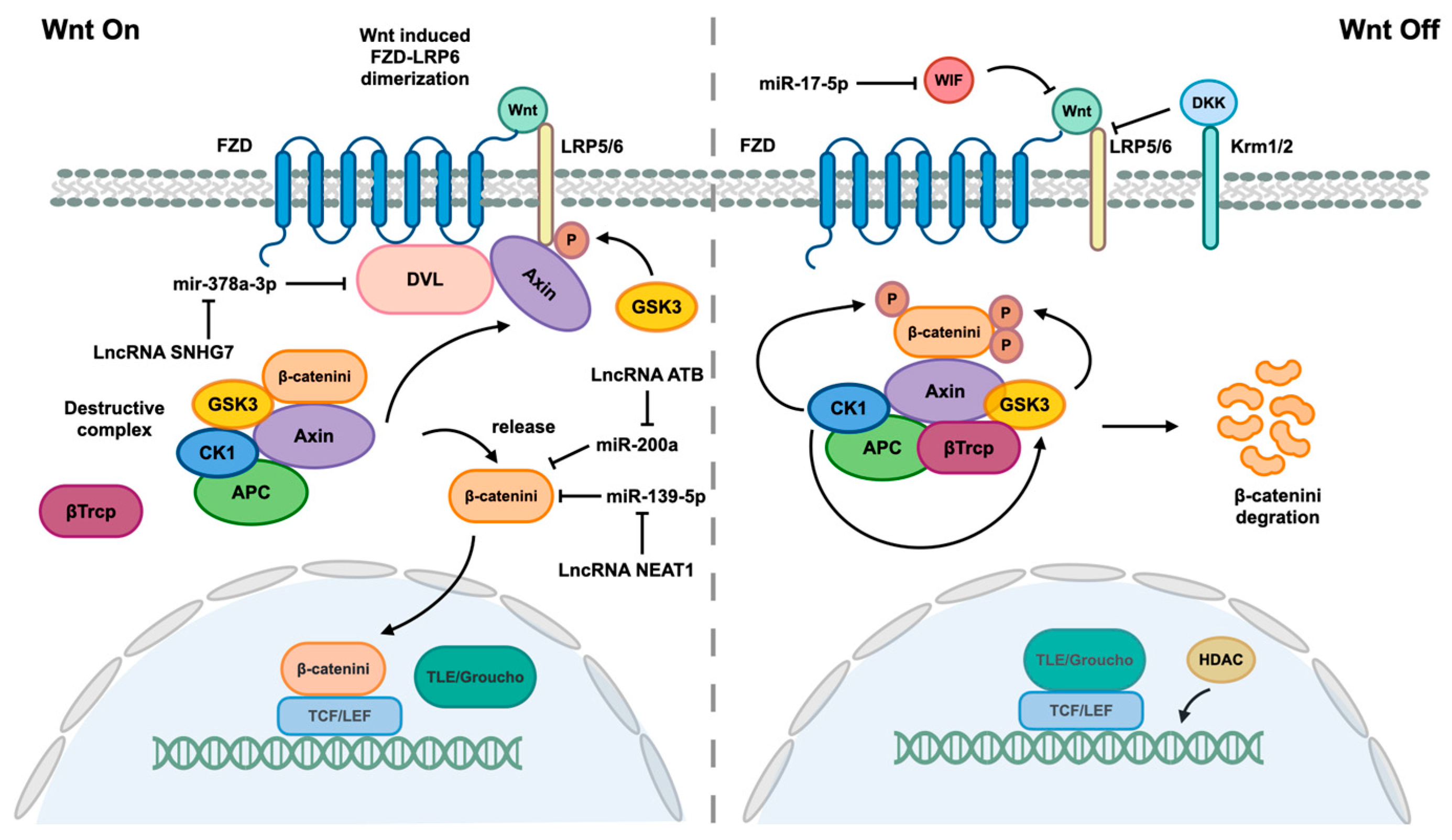
2.2. LncRNAs Involved in HSCs Activation
2.3. CircRNAs Involved in HSCs Activation
3. DNA Modifications Mediated by ncRNAs in HSCs Activation
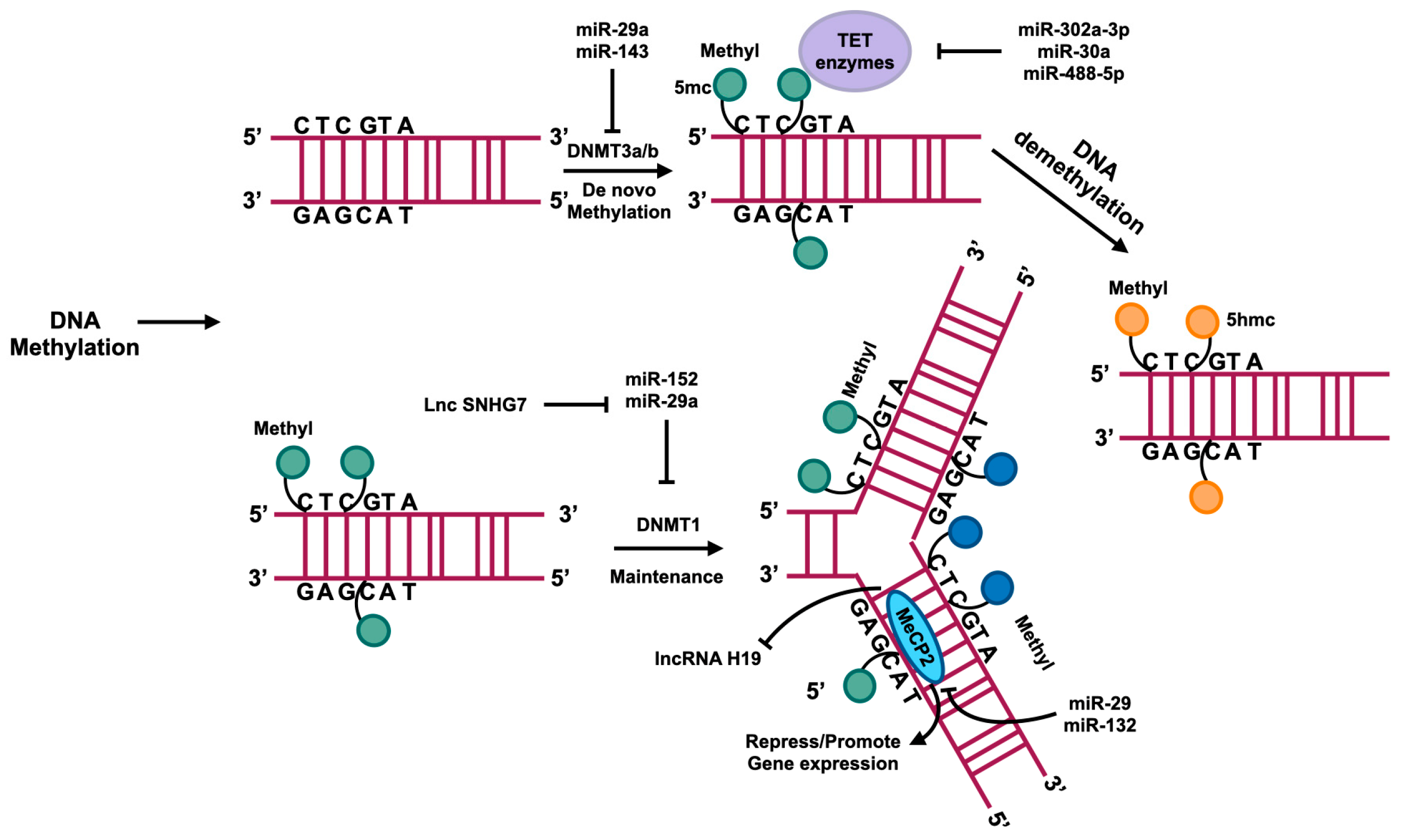
3.1. DNA Methylation in HSCs
3.2. DNA Demethylation in HSCs
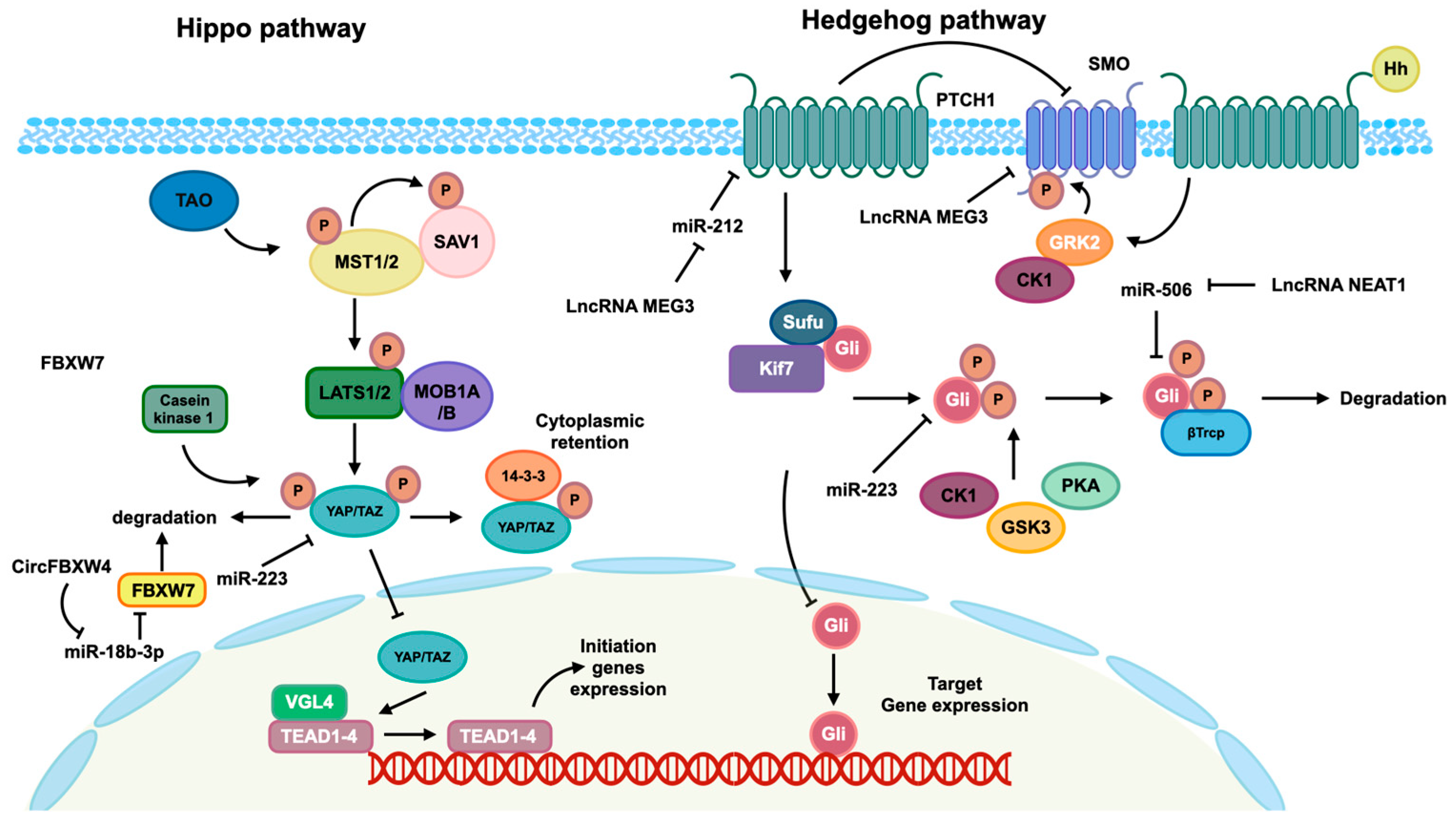
4. Histone Modifications Mediated by ncRNAs in HSCs Activation
4.1. Histone Acetylation in HSCs
4.2. Histone Deacetylation in HSCs
4.3. Histone Methylation and Demethylation
5. N6-Methyladenosine Mediated by ncRNAs in HSCs Activation
6. The Therapeutic Implications of Noncoding RNAs in Liver Fibrosis
7. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Asp. Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, L.; Liang, Y.; Lu, L. Pathology and molecular mechanisms of Schistosoma japonicum-associated liver fibrosis. Front. Cell. Infect. Microbiol. 2022, 12, 1035765. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Radiation-induced liver disease: Current understanding and future perspectives. Exp. Mol. Med. 2017, 49, e359. [Google Scholar] [CrossRef] [PubMed]
- Kamm, D.R.; McCommis, K.S. Hepatic stellate cells in physiology and pathology. J. Physiol. 2022, 600, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Friedman, S.L. Mechanisms of hepatic stellate cell activation. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Tabas, I.; Pajvani, U.B. Mechanisms of Fibrosis Development in Nonalcoholic Steatohepatitis. Gastroenterology 2020, 158, 1913–1928. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, L.; Tacke, F. Hepatic inflammatory responses in liver fibrosis. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-C.; Wu, P.-S.; Lin, H.-C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; Gallego-Duran, R.; Gallego, P.; Grande, L. Genetic and Epigenetic Regulation in Nonalcoholic Fatty Liver Disease (NAFLD). Int. J. Mol. Sci. 2018, 19, 911. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Valenti, L.; Romeo, S. Genetics and epigenetics of NAFLD and NASH: Clinical impact. J. Hepatol. 2018, 68, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans Heterochronic Gene lin-4 Encodes Small RNAs with Antisense Complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Wightman, B.; Burglin, T.R.; Gatto, J.; Arasu, P.; Ruvkun, G. Negative regulatory sequences in the lin-14 3′-untranslated region are necessary to generate a temporal switch during Caenorhabditis elegans development. Genes Dev. 1991, 5, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Yin, W.; Zhang, W.; Zhu, Y.; Ni, H.; Gong, L. MicroRNA-15a inhibits hepatic stellate cell activation and proliferation via targeting SRY-box transcription factor 9. Bioengineered 2022, 13, 13011–13020. [Google Scholar] [CrossRef]
- Yang, A.; Yan, X.; Fan, X.; Shi, Y.; Huang, T.; Li, W.; Chen, W.; Jia, J.; You, H. Hepatic stellate cells-specific LOXL1 deficiency abrogates hepatic inflammation, fibrosis, and corrects lipid metabolic abnormalities in non-obese NASH mice. Hepatol. Int. 2021, 15, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Xiao, E.; Ning, H.; Li, K.; Shang, J.; Kang, Y. MiR-15b and miR-16 suppress TGF-beta1-induced proliferation and fibrogenesis by regulating LOXL1 in hepatic stellate cells. Life Sci. 2021, 270, 119144. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Han, C.Y.; Kim, J.Y.; Cho, S.S.; Kim, Y.S.; Koo, J.H.; Lee, J.M.; Lim, S.C.; Kang, K.W.; Kim, J.S.; et al. Galpha12 overexpression induced by miR-16 dysregulation contributes to liver fibrosis by promoting autophagy in hepatic stellate cells. J. Hepatol. 2018, 68, 493–504. [Google Scholar] [CrossRef]
- Song, L.Y.; Ma, Y.T.; Wu, C.F.; Wang, C.J.; Fang, W.J.; Liu, S.K. MicroRNA-195 Activates Hepatic Stellate Cells In Vitro by Targeting Smad7. Biomed. Res. Int. 2017, 2017, 1945631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.; Yang, H.-M.; Liu, J.-X.; Xu, N.; Li, J.; Shen, L.-P.; Zhang, Y.-Z.; Koda, S.; Zhang, B.-B.; Yu, Q.; et al. MicroRNA-497 induced by Clonorchis sinensis enhances the TGF-β/Smad signaling pathway to promote hepatic fibrosis by targeting Smad7. Parasites Vectors 2021, 14, 472. [Google Scholar] [CrossRef]
- Liu, Q.; Lei, X.; Cao, Z.; Zhang, J.; Yan, L.; Fu, J.; Tong, Q.; Qin, W.; Shao, Y.; Liu, C.; et al. TRPM8 deficiency attenuates liver fibrosis through S100A9-HNF4α signaling. Cell Biosci. 2022, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Watany, M.M.; Hagag, R.Y.; Okda, H.I. Circulating miR-21, miR-210 and miR-146a as potential biomarkers to differentiate acute tubular necrosis from hepatorenal syndrome in patients with liver cirrhosis: A pilot study. Clin. Chem. Lab. Med. 2018, 56, 739–747. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Xue, J.; Zhou, X.; Luo, L.; Ma, Q.; Chen, Y.F.; Zhang, J.; Zhang, S.L.; Zhao, L. Antischistosomiasis Liver Fibrosis Effects of Chlorogenic Acid through IL-13/miR-21/Smad7 Signaling Interactions In Vivo and In Vitro. Antimicrob. Agents Chemother. 2017, 61, e01347-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; McDaniel, K.; Zhou, T.; Ramos-Lorenzo, S.; Wu, C.; Huang, L.; Chen, D.; Annable, T.; Francis, H.; Glaser, S.; et al. Knockout of microRNA-21 attenuates alcoholic hepatitis through the VHL/NF-κappaB signaling pathway in hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G385–G398. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Ye, M.; Wang, F.; Fang, J.; Wang, C.; Luo, J.; Liu, J.; Liu, J.; Liu, L.; Zhao, Q.; et al. MiR-21-3p Promotes Hepatocellular Carcinoma Progression via SMAD7/YAP1 Regulation. Front. Oncol. 2021, 11, 642030. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Xu, C.Z.; Wang, J.T.; Li, X.J.; Wang, M.M.; Gu, Y.H.; Liang, Z.G. miR-21 promotes proliferation and inhibits apoptosis of hepatic stellate cells through targeting PTEN/PI3K/AKT pathway. J. Recept. Signal Transduct. Res. 2018, 38, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Yang, F.; Wang, Y.; Zhou, J.; Qian, H.; Yan, Y. Mesenchymal stem cell-derived exosomal miR-27b-3p alleviates liver fibrosis via downregulating YAP/LOXL2 pathway. J. Nanobiotechnol. 2023, 21, 195. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Peng, H.; Ni, Y.R.; Jiang, X.P.; Wang, J.J.; Zhang, Y.Q.; Ma, L.; Li, R.; Han, L.; Tan, Y.; et al. The miR-23b:27b:24-1 Cluster Inhibits Hepatic Fibrosis by Inactivating Hepatic Stellate Cells. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 1393–1412. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Li, S.; Wang, X.; Si, L.; Ma, R.; Bao, L.; Bo, A. lncRNA GAS5 restrains CCl(4)-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G539–G550. [Google Scholar] [CrossRef] [PubMed]
- Brea, R.; Motino, O.; Frances, D.; Garcia-Monzon, C.; Vargas, J.; Fernandez-Velasco, M.; Bosca, L.; Casado, M.; Martin-Sanz, P.; Agra, N. PGE(2) induces apoptosis of hepatic stellate cells and attenuates liver fibrosis in mice by downregulating miR-23a-5p and miR-28a-5p. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, J.; Xin, X.; Zhang, L.; Zhou, J.; Xia, C.; Zhu, W.; Yu, H. MiR-34c promotes hepatic stellate cell activation and Liver Fibrogenesis by suppressing ACSL1 expression. Int. J. Med. Sci. 2021, 18, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Feili, X.; Wu, S.; Ye, W.; Tu, J.; Lou, L. MicroRNA-34a-5p inhibits liver fibrosis by regulating TGF-β1/Smad3 pathway in hepatic stellate cells. Cell Biol. Int. 2018, 42, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wei, S.; Li, L.; Bu, Q.; Zhou, H.; Su, W.; Liu, Z.; Wang, M.; Lu, L. miR-139-5p sponged by LncRNA NEAT1 regulates liver fibrosis via targeting beta-catenin/SOX9/TGF-beta1 pathway. Cell Death Discov. 2021, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Jiang, Z.; Chen, B.; Dong, P.; Zheng, J. NEAT1 accelerates the progression of liver fibrosis via regulation of microRNA-122 and Kruppel-like factor 6. J. Mol. Med. 2017, 95, 1191–1202. [Google Scholar] [CrossRef]
- Huang, W.; Huang, F.; Zhang, R.; Luo, H. LncRNA Neat1 expedites the progression of liver fibrosis in mice through targeting miR-148a-3p and miR-22-3p to upregulate Cyth3. Cell Cycle 2021, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Huang, T.; Zhang, H.; Zhang, Q.; Ren, J.; Guo, X.; Fan, H.; Liu, L. The lncRNA NEAT1/miR-29b/Atg9a axis regulates IGFBPrP1-induced autophagy and activation of mouse hepatic stellate cells. Life Sci. 2019, 237, 116902. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.S.; Lin, X.F.; Zheng, J.Z.; Wang, Q.; Guan, H.Q. lncRNA NEAT1 regulates fibrosis and inflammatory response induced by nonalcoholic fatty liver by regulating miR-506/GLI3. Eur. Cytokine Netw. 2019, 30, 98–106. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Y.; Lu, Y.; Wang, W.; Du, J.; Chen, S.; Zhou, H.; Cai, W.; Xiao, Y. Conditional depletion of macrophages ameliorates cholestatic liver injury and fibrosis via lncRNA-H19. Cell Death Dis. 2021, 12, 646. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Luo, Z.; Pan, Y.; Zheng, W.; Li, W.; Zhang, Z.; Xiong, P.; Xu, D.; Du, M.; Wang, B.; et al. H19/miR-148a/USP4 axis facilitates liver fibrosis by enhancing TGF-β signaling in both hepatic stellate cells and hepatocytes. J. Cell. Physiol. 2019, 234, 9698–9710. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, X.; Kai, J.; Wang, F.; Wang, Z.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Wang, S.; et al. HIF-1α-upregulated lncRNA-H19 regulates lipid droplet metabolism through the AMPKα pathway in hepatic stellate cells. Life Sci. 2020, 255, 117818. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lin, C.; Jiang, J.; Zhang, X.; Li, F.; Liu, T.; Diao, H. lncRNA-HEIM Facilitated Liver Fibrosis by Up-Regulating TGF-β Expression in Long-Term Outcome of Chronic Hepatitis B. Front. Immunol. 2021, 12, 666370. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Guo, H.; Xu, J.; Wang, J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell. Physiol. 2019, 234, 18169–18179. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Sun, J.; Wang, L.; Wang, P.; Xiao, T.; Wang, S.; Liu, Q. The lncRNA HOTAIR via miR-17-5p is involved in arsenite-induced hepatic fibrosis through regulation of Th17 cell differentiation. J. Hazard. Mater. 2023, 443, 130276. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Hu, S.; Ren, X.; Hu, H.; Deng, X.; Yu, B.; Cobos, I.; Chen, X.; Zhang, W. Whole-Transcriptome Profiling and circRNA-miRNA-mRNA Regulatory Networks in B-Cell Development. Front. Immunol. 2022, 13, 812924. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Dong, R.; Guo, Y.; He, J.; Shao, C.; Yi, P.; Yu, F.; Gu, D.; Zheng, J. CircMTO1 inhibits liver fibrosis via regulation of miR-17-5p and Smad7. J. Cell. Mol. Med. 2019, 23, 5486–5496. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Li, C.; Dong, P.; Huang, J.; Yu, J.; Zheng, J. Circular RNA cMTO1 Promotes PTEN Expression through Sponging miR-181b-5p in Liver Fibrosis. Front. Cell Dev. Biol. 2020, 8, 714. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-J.; Chen, X.; Zhu, S.; Jiang, L.-F.; Ma, W.-X.; Chen, S.-Y.; Meng, X.-M.; Huang, C.; Li, J. Myc-mediated circular RNA circMcph1/miR-370-3p/Irak2 axis is a progressive regulator in hepatic fibrosis. Life Sci. 2023, 312, 121182. [Google Scholar] [CrossRef] [PubMed]
- Argemi, J.; Bataller, R. Identifying New Epigenetic Drivers of Liver Fibrosis. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhan, Y.; Zhang, R.; Tao, Q.; Lang, Z.; Zheng, J. 20(S)-Protopanaxadiol suppresses hepatic stellate cell activation via WIF1 demethylation-mediated inactivation of the Wnt/β-catenin pathway. J. Ginseng Res. 2023, 47, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; Yang, Y.; Meng, H.W.; Li, H.D.; Chen, X.; Huang, H.M.; Bu, F.T.; Yu, H.X.; Wang, Q.; Huang, C.; et al. DNA Methylation of PTGIS Enhances Hepatic Stellate Cells Activation and Liver Fibrogenesis. Front. Pharmacol. 2018, 9, 553. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Habber, D.A.; Li, E. DNA Methyltransferases Dnmt3a and Dnmt3b Are Essential for De Novo Methylation and Mammalian Development. Cell 1990, 99, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Gao, Y.; Su, Y.; Zhou, Y.; Yang, T.; Li, Y.; Wang, Y.; Sun, Y.; Chen, L.; Zhang, F.; et al. Oroxylin A regulates cGAS DNA hypermethylation induced by methionine metabolism to promote HSC senescence. Pharmacol. Res. 2023, 187, 106590. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Y.; You, H.M.; Wang, L.; Bi, Y.H.; Yang, Y.; Meng, H.W.; Meng, X.M.; Ma, T.T.; Huang, C.; Li, J. Methylation of RCAN1.4 mediated by DNMT1 and DNMT3b enhances hepatic stellate cell activation and liver fibrogenesis through Calcineurin/NFAT3 signaling. Theranostics 2019, 9, 4308–4323. [Google Scholar] [CrossRef] [PubMed]
- Barcena-Varela, M.; Paish, H.; Alvarez, L.; Uriarte, I.; Latasa, M.U.; Santamaria, E.; Recalde, M.; Garate, M.; Claveria, A.; Colyn, L.; et al. Epigenetic mechanisms and metabolic reprogramming in fibrogenesis: Dual targeting of G9a and DNMT1 for the inhibition of liver fibrosis. Gut 2021, 70, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Lu, Z.; Chen, B.; Wu, X.; Dong, P.; Zheng, J. Salvianolic acid B-induced microRNA-152 inhibits liver fibrosis by attenuating DNMT1-mediated Patched1 methylation. J. Cell. Mol. Med. 2015, 19, 2617–2632. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; She, Q.; Yang, Y.; Tao, H.; Li, J. DNMT1 controls LncRNA H19/ERK signal pathway in hepatic stellate cell activation and fibrosis. Toxicol. Lett. 2018, 295, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Wang, F.S.; Li, S.C.; Tiao, M.M.; Huang, Y.H. MicroRNA-29a Alleviates Bile Duct Ligation Exacerbation of Hepatic Fibrosis in Mice through Epigenetic Control of Methyltransferases. Int. J. Mol. Sci. 2017, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Chen, B.; Dong, P.; Zheng, J. HOTAIR Epigenetically Modulates PTEN Expression via MicroRNA-29b: A Novel Mechanism in Regulation of Liver Fibrosis. Mol. Ther. 2017, 25, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Wu, Y.; Liu, S.; Lai, Y.; Tang, S. LncRNA-SNHG7/miR-29b/DNMT3A axis affects activation, autophagy and proliferation of hepatic stellate cells in liver fibrosis. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101469. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Yang, Y.; Zhang, C.; Li, J.; Yang, Y. Epigenetic silencing of LncRNA ANRIL enhances liver fibrosis and HSC activation through activating AMPK pathway. J. Cell. Mol. Med. 2020, 24, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Luo, J.; Liu, J.; Chen, T.; Sun, J.; Zhang, Y.; Xi, Q. Exploration of the Effect on Genome-Wide DNA Methylation by miR-143 Knock-Out in Mice Liver. Int. J. Mol. Sci. 2021, 22, 13075. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Liu, D.; Zhou, Y.; Wang, L.; Hou, H.; Chen, H.; Zhang, L.; Chen, W.; Li, X.; Zhao, L. The negative feedback between miR-143 and DNMT3A regulates cisplatin resistance in ovarian cancer. Cell Biol. Int. 2021, 45, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Chen, D.; Cai, C.; Du, Q.; Lin, H.; Pan, T.; Sheng, L.; Xu, Y.; Teng, T.; Tu, J.; et al. microRNA-143-3p attenuated development of hepatic fibrosis in autoimmune hepatitis through regulation of TAK1 phosphorylation. J. Cell. Mol. Med. 2020, 24, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Moran-Salvador, E.; Garcia-Macia, M.; Sivaharan, A.; Sabater, L.; Zaki, M.Y.W.; Oakley, F.; Knox, A.; Page, A.; Luli, S.; Mann, J.; et al. Fibrogenic Activity of MECP2 Is Regulated by Phosphorylation in Hepatic Stellate Cells. Gastroenterology 2019, 157, 1398–1412.e9. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Chu, D.C.; Maxwell, A.; Oakley, F.; Zhu, N.L.; Tsukamoto, H.; Mann, D.A. MeCP2 controls an epigenetic pathway that promotes myofibroblast transdifferentiation and fibrosis. Gastroenterology 2010, 138, 705–714.e4. [Google Scholar] [CrossRef]
- Huang, Y.H.; Kuo, H.C.; Yang, Y.L.; Wang, F.S. MicroRNA-29a is a key regulon that regulates BRD4 and mitigates liver fibrosis in mice by inhibiting hepatic stellate cell activation. Int. J. Med. Sci. 2019, 16, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Liu, L.P.; Tao, H.; Hu, W.; Shi, P.; Deng, Z.Y.; Li, J. MeCP2 silencing of LncRNA H19 controls hepatic stellate cell proliferation by targeting IGF1R. Toxicology 2016, 359-360, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.P.; Wu, W.; Callen, E.; Pavani, R.; Zolnerowich, N.; Kodali, S.; Zong, D.; Wong, N.; Noriega, S.; Nathan, W.J.; et al. Active DNA demethylation promotes cell fate specification and the DNA damage response. Science 2022, 378, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-Methylcytosine to 5-Hydroxymethylcytosine in Mammalian DNA by MLL Partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; D’Alessio, A.C.; Taranova, O.V.; Hong, K.; Sowers, L.C.; Zhang, Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 2010, 466, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gao, Y.; Zhang, W.; Chen, Y.; Jin, M.; Yang, Z. Exosomes derived from induced pluripotent stem cells suppresses M2-type macrophages during pulmonary fibrosis via miR-302a-3p/TET1 axis. Int. Immunopharmacol. 2021, 99, 108075. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Liu, Y.; Zhang, J.; Li, H.; Liu, W.; Cao, G.; Xv, P.; Zhang, J.; Lv, C.; et al. miR-30a as Potential Therapeutics by Targeting TET1 through Regulation of Drp-1 Promoter Hydroxymethylation in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2017, 18, 633. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wu, S.; Wang, P.; Zhou, Y.; Wang, Z.; Sun, Y.; Jiang, C. miR-488-5p mitigates hepatic stellate cell activation and hepatic fibrosis via suppressing TET3 expression. Hepatol. Int. 2022, 17, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Yu, L.R.; Wang, L.; Zhang, Z.; Kasper, L.H.; Lee, J.E.; Wang, C.; Brindle, P.K.; Dent, S.Y.; Ge, K. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 2011, 30, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; McCann, F.E.; Cabrita, M.A.; Layton, T.; Cribbs, A.; Knezevic, B.; Fang, H.; Knight, J.; Zhang, M.; Fischer, R.; et al. Identifying collagen VI as a target of fibrotic diseases regulated by CREBBP/EP300. Proc. Natl. Acad. Sci. USA 2020, 117, 20753–20763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Yang, X.; Lu, W.; Chen, Y.; Lin, Y.; Wang, J.; Lin, S.; Yun, J.P. H3K27 acetylation activated-COL6A1 promotes osteosarcoma lung metastasis by repressing STAT1 and activating pulmonary cancer-associated fibroblasts. Theranostics 2021, 11, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Dou, C.; Liu, Z.; Tu, K.; Zhang, H.; Chen, C.; Yaqoob, U.; Wang, Y.; Wen, J.; van Deursen, J.; Sicard, D.; et al. P300 Acetyltransferase Mediates Stiffness-Induced Activation of Hepatic Stellate Cells Into Tumor-Promoting Myofibroblasts. Gastroenterology 2018, 154, 2209–2221.e14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, K.; Liu, D.; Guo, L.; Chen, Y.; Li, Q.; Maiers, J.L.; Liu, Z.; Shah, V.H.; Dou, C.; et al. p300 Acetyltransferase Is a Cytoplasm-to-Nucleus Shuttle for SMAD2/3 and TAZ Nuclear Transport in Transforming Growth Factor β-Stimulated Hepatic Stellate Cells. Hepatology 2019, 70, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Corcuera, A.; Sehrawat, T.S.; Jalan-Sakrikar, N.; Gibbons, H.R.; Pirius, N.E.; Khanal, S.; Hamdan, F.H.; Aseem, S.O.; Cao, S.; Banales, J.M.; et al. Long non-coding RNA ACTA2-AS1 promotes ductular reaction by interacting with the p300/ELK1 complex. J. Hepatol. 2022, 76, 921–933. [Google Scholar] [CrossRef] [PubMed]
- Aseem, S.O.; Jalan-Sakrikar, N.; Chi, C.; Navarro-Corcuera, A.; De Assuncao, T.M.; Hamdan, F.H.; Chowdhury, S.; Banales, J.M.; Johnsen, S.A.; Shah, V.H.; et al. Epigenomic Evaluation of Cholangiocyte Transforming Growth Factor-beta Signaling Identifies a Selective Role for Histone 3 Lysine 9 Acetylation in Biliary Fibrosis. Gastroenterology 2021, 160, 889–905.e810. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Luedde, M.; Vargas Cardenas, D.; Vucur, M.; Mollnow, T.; Zimmermann, H.W.; Koch, A.; Hellerbrand, C.; Weiskirchen, R.; Frey, N.; et al. miR-133a mediates TGF-beta-dependent derepression of collagen synthesis in hepatic stellate cells during liver fibrosis. J. Hepatol. 2013, 58, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Renaud, L.; Harris, L.G.; Mani, S.K.; Kasiganesan, H.; Chou, J.C.; Baicu, C.F.; Van Laer, A.; Akerman, A.W.; Stroud, R.E.; Jones, J.A.; et al. HDACs Regulate miR-133a Expression in Pressure Overload-Induced Cardiac Fibrosis. Circ. Heart Fail. 2015, 8, 1094–1104. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Wang, L.; Bu, F.; Meng, H.; Pan, X.; Li, J.; Zhang, Y.; Wang, A.; Yin, N.; Huang, C.; et al. The miR-455-3p/HDAC2 axis plays a pivotal role in the progression and reversal of liver fibrosis and is regulated by epigenetics. FASEB J. 2021, 35, e21700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, L.; Ali, T.; Li, L.; Bi, X.; Wang, J.; Lu, G.; Shao, Y.; Vuitton, D.A.; Wen, H.; et al. Hydatid cyst fluid promotes peri-cystic fibrosis in cystic echinococcosis by suppressing miR-19 expression. Parasit. Vectors 2016, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, K.; Mai, X.; Wei, J.; Ma, C. Depleted HDAC3 attenuates hyperuricemia-induced renal interstitial fibrosis via miR-19b-3p/SF3B3 axis. Cell Cycle 2022, 21, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Tiao, M.M.; Huang, L.T.; Chuang, J.H.; Kuo, K.C.; Yang, Y.L.; Wang, F.S. Activation of Mir-29a in Activated Hepatic Stellate Cells Modulates Its Profibrogenic Phenotype through Inhibition of Histone Deacetylases 4. PLoS ONE 2015, 10, e0136453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, J.; Wang, X.; Zhao, X.; Li, Z.; Niu, J.; Steer, C.J.; Zheng, G.; Song, G. MicroRNA-378 promotes hepatic inflammation and fibrosis via modulation of the NF-kappaB-TNFalpha pathway. J. Hepatol. 2019, 70, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zeng, Z.; Guan, L.; Ao, R. GRHL2 induces liver fibrosis and intestinal mucosal barrier dysfunction in non-alcoholic fatty liver disease via microRNA-200 and the MAPK pathway. J. Cell. Mol. Med. 2020, 24, 6107–6119. [Google Scholar] [CrossRef] [PubMed]
- Sheen-Chen, S.M.; Lin, C.R.; Chen, K.H.; Yang, C.H.; Lee, C.T.; Huang, H.W.; Huang, C.Y. Epigenetic histone methylation regulates transforming growth factor beta-1 expression following bile duct ligation in rats. J. Gastroenterol. 2014, 49, 1285–1297. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Zhang, Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 2004, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Liu, M.; Wang, Z.; Lin, Z.; Feng, Y.; Tian, D.; Xia, L. EZH2-mediated inhibition of KLF14 expression promotes HSCs activation and liver fibrosis by downregulating PPARgamma. Cell Prolif. 2021, 54, e13072. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xiang, C.; Zhong, F.; Zhang, Y.; Wang, L.; Zhao, Y.; Wang, J.; Ding, C.; Jin, L.; He, F.; et al. Histone H3K27 methyltransferase EZH2 and demethylase JMJD3 regulate hepatic stellate cells activation and liver fibrosis. Theranostics 2021, 11, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Martin-Mateos, R.; De Assuncao, T.M.; Arab, J.P.; Jalan-Sakrikar, N.; Yaqoob, U.; Greuter, T.; Verma, V.K.; Mathison, A.J.; Cao, S.; Lomberk, G.; et al. Enhancer of Zeste Homologue 2 Inhibition Attenuates TGF-beta Dependent Hepatic Stellate Cell Activation and Liver Fibrosis. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, X.X.; Li, W.X.; Wu, X.Q.; Huang, C.; Xie, J.; Zhao, Y.X.; Meng, X.M.; Li, J. EZH2-mediated repression of Dkk1 promotes hepatic stellate cell activation and hepatic fibrosis. J. Cell. Mol. Med. 2017, 21, 2317–2328. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Zhou, F.; Li, Y.J.; Xue, X.Y.; Qu, J.R.; Fan, G.F.; Liu, J.; Sun, R.; Wu, J.Z.; Zheng, Q.; et al. LncRNA H19-EZH2 interaction promotes liver fibrosis via reprogramming H3K27me3 profiles. Acta Pharmacol. Sin. 2023, 44, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Bian, E.B.; Wang, Y.Y.; Yang, Y.; Wu, B.M.; Xu, T.; Meng, X.M.; Huang, C.; Zhang, L.; Lv, X.W.; Xiong, Z.G.; et al. Hotair facilitates hepatic stellate cells activation and fibrogenesis in the liver. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Zheng, J.; Li, N.; Cheng, Q.; Zhu, M.; Wang, Y.; Zhou, X.; Zhang, Z.; Shi, G. DZNep, an inhibitor of the histone methyltransferase EZH2, suppresses hepatic fibrosis through regulating miR-199a-5p/SOCS7 pathway. PeerJ 2021, 9, e11374. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Lv, L.; Zhan, Y.; Ma, Y.; Feng, J.; He, Y.; Wen, Y.; Zhang, Y.; Pu, Q.; Ji, F.; et al. METTL3/N6-methyladenosine/ miR-21-5p promotes obstructive renal fibrosis by regulating inflammation through SPRY1/ERK/NF-κB pathway activation. J. Cell. Mol. Med. 2021, 25, 7660–7674. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Pan, X.; Du, N.; Li, K.; Hu, Y.; Wang, L.; Zhang, J.; Liu, Y.; Zuo, L.; Meng, X.; et al. ASIC1a regulates miR-350/SPRY2 by N6-methyladenosine to promote liver fibrosis. FASEB J. 2020, 34, 14371–14388. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, S.; Li, H.D.; Wang, J.N.; Sun, L.J.; Xu, J.J.; Hui, Y.R.; Li, X.F.; Li, L.Y.; Zhao, Y.X.; et al. N6-methyladenosine-modified circIRF2, identified by YTHDF2, suppresses liver fibrosis via facilitating FOXO3 nuclear translocation. Int. J. Biol. Macromol. 2023, 248, 125811. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, S.; Scamporrino, A.; Petta, S.; Urbano, F.; Filippello, A.; Ragusa, M.; Di Martino, M.T.; Scionti, F.; Grimaudo, S.; Pipitone, R.M.; et al. Serum coding and non-coding RNAs as biomarkers of NAFLD and fibrosis severity. Liver Int. 2019, 39, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cheng, L.; Xu, M.; Wang, W.; Wan, Z.; Xiong, H.; Guo, W.; Cai, M.; Xu, F. SGLT2 inhibitor empagliflozin downregulates miRNA-34a-5p and targets GREM2 to inactivate hepatic stellate cells and ameliorate non-alcoholic fatty liver disease-associated fibrosis. Metabolism 2023, 146, 155657. [Google Scholar] [CrossRef] [PubMed]
- Zaafan, M.A.; Abdelhamid, A.M. Dasatinib ameliorates thioacetamide-induced liver fibrosis: Modulation of miR-378 and miR-17 and their linked Wnt/β-catenin and TGF-β/smads pathways. J. Enzyme Inhib. Med. Chem. 2022, 37, 118–124. [Google Scholar] [CrossRef]
- Li, M.; Zhou, Y.; Zhu, H.; Xu, L.M.; Ping, J. Danhongqing formula alleviates cholestatic liver fibrosis by downregulating long non-coding RNA H19 derived from cholangiocytes and inhibiting hepatic stellate cell activation. J. Integr. Med. 2024, 22, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, S.; Chen, P.; Yue, X.; Wang, S.; Gu, Y.; Yuan, Y. Salvianolic acid B suppresses hepatic stellate cell activation and liver fibrosis by inhibiting the NF-κB signaling pathway via miR-6499-3p/LncRNA-ROR. Phytomedicine 2022, 107, 154435. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yan, M.; Bai, Z.; Xie, Y.; Ren, L.; Wei, J.; Zhu, D.; Wang, H.; Liu, Y.; Luo, J.; et al. Huc-MSC-derived exosomes modified with the targeting peptide of aHSCs for liver fibrosis therapy. J. Nanobiotechnol. 2022, 20, 432. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wei, J.; Zeng, Y.; Liu, J.; Xiao, E.; Kang, Y.; Kang, Y. Mesenchymal stem cell-originated exosomal circDIDO1 suppresses hepatic stellate cell activation by miR-141-3p/PTEN/AKT pathway in human liver fibrosis. Drug Deliv. 2022, 29, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zeng, J.; Xing, L.; Li, C. Extra- and Intra-Cellular Mechanisms of Hepatic Stellate Cell Activation. Biomedicines 2021, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Riaz, F.; Chen, Q.; Lu, K.; Osoro, E.K.; Wu, L.; Feng, L.; Zhao, R.; Yang, L.; Zhou, Y.; He, Y.; et al. Inhibition of miR-188-5p alleviates hepatic fibrosis by significantly reducing the activation and proliferation of HSCs through PTEN/PI3K/AKT pathway. J. Cell. Mol. Med. 2021, 25, 4073–4087. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, Q.; Zhou, H.; Qiu, J.; Li, C.; Shi, C.; Zhou, S.; Liu, R.; Lu, L. miR-455-3p Alleviates Hepatic Stellate Cell Activation and Liver Fibrosis by Suppressing HSF1 Expression. Mol. Ther. Nucleic Acids 2019, 16, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Dou, C.-Y.; Zhou, Y.; Zhou, Q.; Tang, H.-B. MicroRNA-503 Targets Mothers against Decapentaplegic Homolog 7 Enhancing Hepatic Stellate Cell Activation and Hepatic Fibrosis. Dig. Dis. Sci. 2021, 66, 1928–1939. [Google Scholar] [CrossRef]
- Yu, F.; Guo, Y.; Chen, B.; Dong, P.; Zheng, J. MicroRNA-17-5p activates hepatic stellate cells through targeting of Smad7. Lab. Investig. 2015, 95, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Li, C.; Zhan, Y.; Zhang, R.; Lv, B.; Geng, W.; Zheng, J. Pinostilbene hydrate suppresses hepatic stellate cell activation via inhibition of miR-17-5p-mediated Wnt/β-catenin pathway. Phytomedicine 2020, 79, 153321. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, X.; Wei, R.; Ye, T.; Zhou, J.-K.; Wen, M.; Men, R.; Li, P.; Dong, B.; Liu, L.; et al. MicroRNA-214 promotes hepatic stellate cell activation and liver fibrosis by suppressing Sufu expression. Cell Death Dis. 2018, 9, 718. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Z.; Zhang, Y.; Li, W.; Zheng, W.; Yu, J.; Wang, B.; Chen, L.; Zhuo, Q.; Chen, L.; et al. MicroRNA-212 activates hepatic stellate cells and promotes liver fibrosis via targeting SMAD7. Biochem. Biophys. Res. Commun. 2018, 496, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sethi, B.; Staller, D.W.; Xin, X.; Ma, J.; Dong, Y.; Talmon, G.A.; Mahato, R.I. Anti-miR-96 and Hh pathway inhibitor MDB5 synergistically ameliorate alcohol-associated liver injury in mice. Biomaterials 2023, 295, 122049. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Wang, K.; Zhang, Y.; Mao, X.-L.; Huang, Q.; Wang, J.; Ye, L.; Li, Y. MiR-542-3p controls hepatic stellate cell activation and fibrosis via targeting BMP-7. J. Cell. Biochem. 2019, 120, 4573–4581. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Pan, L.; Li, L.; Hu, S.; Zhou, H.; Yang, C.; Yang, J.; Li, H.; Liu, Y.; Meng, X.; et al. MicroRNA-708 modulates Hepatic Stellate Cells activation and enhances extracellular matrix accumulation via direct targeting TMEM88. J. Cell. Mol. Med. 2020, 24, 7127–7140. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ghazwani, M.; Zhang, Y.; Lu, J.; Li, J.; Fan, J.; Gandhi, C.R.; Li, S. miR-122 regulates collagen production via targeting hepatic stellate cells and suppressing P4HA1 expression. J. Hepatol. 2013, 58, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Nozari, E.; Moradi, A.; Samadi, M. Effect of Atorvastatin, Curcumin, and Quercetin on miR-21 and miR-122 and their correlation with TGFβ1 expression in experimental liver fibrosis. Life Sci. 2020, 259, 118293. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Shi, C.; Duan, X.; Zhang, Y.; Wang, B. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4. Bioengineered 2022, 13, 5915–5927. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, H.; Zhang, S.; Zhang, R.; Li, J.; Wei, Y.; Yang, C.; Zhang, F.; Zhou, H. Protective effect of Idelalisib on carbon tetrachloride-induced liver fibrosis via microRNA-124-3P/phosphatidylinositol-3-hydroxykinase signalling pathway. J. Cell. Mol. Med. 2021, 25, 11185–11197. [Google Scholar] [CrossRef] [PubMed]
- Genz, B.; Coleman, M.A.; Irvine, K.M.; Kutasovic, J.R.; Miranda, M.; Gratte, F.D.; Tirnitz-Parker, J.E.E.; Olynyk, J.K.; Calvopina, D.A.; Weis, A.; et al. Overexpression of miRNA-25-3p inhibits Notch1 signaling and TGF-β-induced collagen expression in hepatic stellate cells. Sci. Rep. 2019, 9, 8541. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhi, F.; Lun, W.; Deng, Q.; Zhang, W. Baicalin inhibits PDGF-BB-induced hepatic stellate cell proliferation, apoptosis, invasion, migration and activation via the miR-3595/ACSL4 axis. Int. J. Mol. Med. 2018, 41, 1992–2002. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Li, C.; Yang, G.; Sun, Y.; Zhuang, L.; Ou, Y.; Li, H.; Wang, G.; Kisseleva, T.; Brenner, D.; et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology 2018, 68, 1070–1086. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shu, B.; Zhou, Y.; Li, Z.; He, C. Ppic modulates CCl4-induced liver fibrosis and TGF-β-caused mouse hepatic stellate cell activation and regulated by miR-137-3p. Toxicol. Lett. 2021, 350, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, P.; Wang, Y.-S.; Zhang, Y.-N.; Li, C.; Yang, Z.-Y.; Liu, Z.-H.; Zhan, T.-Z.; Xu, J.; Xia, C.-M. MiR-130a-3p Alleviates Liver Fibrosis by Suppressing HSCs Activation and Skewing Macrophage to Ly6Clo Phenotype. Front. Immunol. 2021, 12, 696069. [Google Scholar] [CrossRef]
- Yang, L.; Yue, W.; Zhang, H.; Zhang, Z.; Xue, R.; Dong, C.; Liu, F.; Chang, N.; Yang, L.; Li, L. Dual Targeting of Angipoietin-1 and von Willebrand Factor by microRNA-671-5p Attenuates Liver Angiogenesis and Fibrosis. Hepatol. Commun. 2022, 6, 1425–1442. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-C.; Chen, R.; Luo, X.; Li, Z.-H.; Luo, S.-Z.; Xu, M.-Y. MicroRNA-194 inactivates hepatic stellate cells and alleviates liver fibrosis by inhibiting AKT2. World J. Gastroenterol. 2019, 25, 4468–4480. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Fang, Y.; Qiu, J.; Zhou, Y.; Wang, Z.; Jiang, C. miR-345-5p curbs hepatic stellate cell activation and liver fibrosis progression by suppressing hypoxia-inducible factor-1alpha expression. Toxicol. Lett. 2022, 370, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Nie, Y.; Yang, X.; Wang, X.; Li, F.; Wang, M.; Wang, C.; Zhang, H. miR-193a/b-3p relieves hepatic fibrosis and restrains proliferation and activation of hepatic stellate cells. J. Cell. Mol. Med. 2019, 23, 3824–3832. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.; Wang, S.; Kim, J.; Rao, K.M.; Park, S.Y.; Chung, I.; Ha, C.-S.; Kim, S.-W.; Yun, Y.H.; Jung, Y. MicroRNA-378 limits activation of hepatic stellate cells and liver fibrosis by suppressing Gli3 expression. Nat. Commun. 2016, 7, 10993. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dong, S.; Ye, M.; Peng, G.; Luo, J.; Wang, C.; Wang, J.; Zhao, Q.; Chang, Y.; Wang, H. MicroRNA-489-3p Represses Hepatic Stellate Cells Activation by Negatively Regulating the JAG1/Notch3 Signaling Pathway. Dig. Dis. Sci. 2021, 66, 143–150. [Google Scholar] [CrossRef]
- Dai, W.; Zhao, J.; Tang, N.; Zeng, X.; Wu, K.; Ye, C.; Shi, J.; Lu, C.; Ning, B.; Zhang, J.; et al. MicroRNA-155 attenuates activation of hepatic stellate cell by simultaneously preventing EMT process and ERK1 signalling pathway. Liver Int. 2015, 35, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Shu, B.; Zhou, Y.; Zhang, R.; Yang, X. The miR-139-5p/peripheral myelin protein 22 axis modulates TGF-β-induced hepatic stellate cell activation and CCl4-induced hepatic fibrosis in mice. Life Sci. 2021, 276, 119294. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tao, Q.; Zhou, Y.; Chen, Q.; Li, L.; Hu, S.; Liu, Y.; Zhang, Y.; Shu, J.; Zhang, X.; et al. MicroRNA-708 represses hepatic stellate cells activation and proliferation by targeting ZEB1 through Wnt/β-catenin pathway. Eur. J. Pharmacol. 2020, 871, 172927. [Google Scholar] [CrossRef]
- Zhou, Q.; Rong, C.; Gu, T.; Li, H.; Wu, L.; Zhuansun, X.; Zhao, X.; Xiao, Z.; Kuang, Y.; Xu, S.; et al. Mesenchymal stem cells improve liver fibrosis and protect hepatocytes by promoting microRNA-148a-5p-mediated inhibition of Notch signaling pathway. Stem Cell Res. Ther. 2022, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Du, Y.; Liu, X.; Ren, Y.; Dong, Y.; Xu, H.-Y.; Shi, J.-S.; Jiang, D.; Xu, X.; Li, L.; et al. Targeting Follistatin like 1 ameliorates liver fibrosis induced by carbon tetrachloride through TGF-β1-miR29a in mice. Cell Commun. Signal. 2020, 18, 151. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Dong, P.; Mao, Y.; Zhao, B.; Huang, Z.; Zheng, J. Loss of lncRNA-SNHG7 Promotes the Suppression of Hepatic Stellate Cell Activation via miR-378a-3p and DVL2. Mol. Ther. Nucleic Acids 2019, 17, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lu, P.; Sun, T.; Ding, A. Long non-coding RNA HOX transcript antisense intergenic RNA depletion protects against alcoholic hepatitis through the microRNA-148a-3p/sphingosine 1-phosphate receptor 1 axis. Cell Tissue Res. 2023, 394, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Zhao, S.X.; Kong, L.B.; Du, J.H.; Ren, W.G.; Han, F.; Zhang, Q.S.; Li, W.C.; Cui, P.; Wang, R.Q.; et al. LncRNA-ATB/microRNA-200a/β-catenin regulatory axis involved in the progression of HCV-related hepatic fibrosis. Gene 2017, 618, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Bai, W.D.; Li, C.; Zheng, Z.; Guan, H.; Liu, J.Q.; Yang, X.K.; Han, S.C.; Gao, J.X.; Wang, H.T.; et al. Knockdown of lncRNA-ATB suppresses autocrine secretion of TGF-β2 by targeting ZNF217 via miR-200c in keloid fibroblasts. Sci. Rep. 2016, 6, 24728. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Z.; Yuan, Q.; Luo, L.; Hu, X. The mouse Anxa6/miR-9-5p/Anxa2 axis modulates TGF-β1-induced mouse hepatic stellate cell (mHSC) activation and CCl(4)-caused liver fibrosis. Toxicol. Lett. 2022, 362, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, X.; Ma, H.; Lin, S.; Zhang, D.; Wu, W.; Liao, Z.; Chen, M.; Ye, H.; Li, Q.; et al. LncRNA CCAT2, involving miR-34a/TGF-β1/Smad4 signaling, regulate hepatic stellate cells proliferation. Sci. Rep. 2022, 12, 21199. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhang, Z.; Yuan, Q.; Liu, Q.; Kuang, J.; Fang, Y.; Hu, X. A lncRNA Gpr137b-ps/miR-200a-3p/CXCL14 axis modulates hepatic stellate cell (HSC) activation. Toxicol. Lett. 2021, 336, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Han, X.; Zhang, Z.; Zheng, L.; Hu, Z.; Yao, Q.; Cui, H.; Shu, G.; Si, M.; Li, C.; et al. The liver-enriched lnc-LFAR1 promotes liver fibrosis by activating TGFβ and Notch pathways. Nat. Commun. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Xuan, J.; Zhu, D.; Cheng, Z.; Qiu, Y.; Shao, M.; Yang, Y.; Zhai, Q.; Wang, F.; Qin, F. Crocin inhibits the activation of mouse hepatic stellate cells via the lnc-LFAR1/MTF-1/GDNF pathway. Cell Cycle 2020, 19, 3480–3490. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Geng, W.; Dong, P.; Huang, Z.; Zheng, J. LncRNA-MEG3 inhibits activation of hepatic stellate cells through SMO protein and miR-212. Cell Death Dis. 2018, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Dong, B.; Dong, P.; He, Y.; Zheng, J.; Xu, P. Hypoxia induces the activation of hepatic stellate cells through the PVT1-miR-152-ATG14 signaling pathway. Mol. Cell. Biochem. 2020, 465, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, X.L.; Xu, D.H.; Li, S.; Yang, Q.; Feng, X.; Wei, Y.G.; Li, H.; Yang, L.; Zhang, Y.J.; et al. NPM promotes hepatotoxin-induced fibrosis by inhibiting ROS-induced apoptosis of hepatic stellate cells and upregulating lncMIAT-induced TGF-β2. Cell Death Dis. 2023, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mou, Q.; Zhu, Z.; Zhao, L.; Zhu, L. MALAT1 promotes liver fibrosis by sponging miR-181a and activating TLR4-NF-κB signaling. Int. J. Mol. Med. 2021, 48. [Google Scholar] [CrossRef]
- Han, X.; Guo, B.; Zhao, S.; Li, Y.; Zhu, J.; He, Y.; Wang, J.; Yao, Q.; Shao, S.; Zheng, L.; et al. lncRNA Helf promotes hepatic inflammation and fibrosis by interacting with PTBP1 to facilitate PIK3R5 mRNA stabilization. Cell. Mol. Biol. Lett. 2023, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Wang, Z.; Liu, M.; Zhu, Y.J.; Zheng, L.; Wang, L.L.; Cheng, J.L.; Liu, T.T.; Zhang, G.D.; Yang, T.Y.; et al. LincRNA-ROR/miR-145/ZEB2 regulates liver fibrosis by modulating HERC5-mediated p53 ISGylation. FASEB J. 2023, 37, e22936. [Google Scholar] [CrossRef] [PubMed]
- Bu, F.T.; Zhu, Y.; Chen, X.; Wang, A.; Zhang, Y.F.; You, H.M.; Yang, Y.; Yang, Y.R.; Huang, C.; Li, J. Circular RNA circPSD3 alleviates hepatic fibrogenesis by regulating the miR-92b-3p/Smad7 axis. Mol. Ther. Nucleic Acids 2021, 23, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H.D.; Bu, F.T.; Li, X.F.; Chen, Y.; Zhu, S.; Wang, J.N.; Chen, S.Y.; Sun, Y.Y.; Pan, X.Y.; et al. Circular RNA circFBXW4 suppresses hepatic fibrosis via targeting the miR-18b-3p/FBXW7 axis. Theranostics 2020, 10, 4851–4870. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Hu, S.; Bu, F.T.; Li, H.; Huang, C.; Meng, X.M.; Zhang, L.; Lv, X.W.; Li, J. Circular RNA CREBBP Suppresses Hepatic Fibrosis Via Targeting the hsa-miR-1291/LEFTY2 Axis. Front. Pharmacol. 2021, 12, 741151. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Feng, R.; Li, X.; Li, D.; Zhai, W. TGF-β- and lipopolysaccharide-induced upregulation of circular RNA PWWP2A promotes hepatic fibrosis via sponging miR-203 and miR-223. Aging 2019, 11, 9569–9580. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Zhang, L.; Wang, B.; Zhang, G.C.; Liu, J.; Wu, Z.F.; Du, S.S.; Zeng, Z.C. CircTUBD1 Regulates Radiation-induced Liver Fibrosis Response via a circTUBD1/micro-203a-3p/Smad3 Positive Feedback Loop. J. Clin. Transl. Hepatol. 2022, 10, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, B.; Chen, G.; Zhang, L.; Zhuang, Y.; Niu, H.; Zeng, Z. Circular RNA RSF1 promotes inflammatory and fibrotic phenotypes of irradiated hepatic stellate cell by modulating miR-146a-5p. J. Cell. Physiol. 2020, 235, 8270–8282. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting Mitochondria-Located circRNA SCAR Alleviates NASH via Reducing mROS Output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, X.; Wang, J.N.; Xu, J.J.; Wang, A.; Li, J.J.; Wu, S.; Wu, Y.Y.; Li, X.F.; Huang, C.; et al. Circular RNA circUbe2k promotes hepatic fibrosis via sponging miR-149-5p/TGF-β2 axis. FASEB J. 2021, 35, e21622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yuan, B.; Wu, Z.; Dong, Y.; Zhang, L.; Zeng, Z. Microarray profiling of circular RNAs and the potential regulatory role of hsa_circ_0071410 in the activated human hepatic stellate cell induced by irradiation. Gene 2017, 629, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Jiang, L.; Jia, P.; Niu, R.; Bu, F.; Zhu, Y.; Pan, X.; Li, J.; Liu, J.; Zhang, Y.; et al. Inhibition of circular RNA ASPH reduces the proliferation and promotes the apoptosis of hepatic stellate cells in hepatic fibrosis. Biochem. Pharmacol. 2023, 210, 115451. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.X.; Li, J.Z.; Li, Q.; Xu, M.Y.; Li, H.Y. CircRNA608-microRNA222-PINK1 axis regulates the mitophagy of hepatic stellate cells in NASH related fibrosis. Biochem. Biophys. Res. Commun. 2022, 610, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, L.; Fan, H.; Yu, Y.; Luo, Y.; Gu, F.; Yu, H.; Liao, X. Anthocyanins improve liver fibrosis in mice by regulating the autophagic flux level of hepatic stellate cells by mmu_circ_0000623. Food Sci. Nutr. 2023, 11, 3002–3018. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Chen, G.F.; Wang, J.C.; Ji, S.H.; Wu, X.W.; Lu, X.J.; Chen, J.L.; Li, J.T. Hsa_circ_0070963 inhibits liver fibrosis via regulation of miR-223-3p and LEMD3. Aging 2020, 12, 1643–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, R.; Li, X.; Zhang, W.; Zhan, Y.; Lang, Z.; Tao, Q.; Yu, J.; Yu, S.; Yu, Z.; et al. Circular RNA cVIM promotes hepatic stellate cell activation in liver fibrosis via miR-122-5p/miR-9-5p-mediated TGF-β signaling cascade. Commun. Biol. 2024, 7, 113. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, R.; Mao, J. Noncoding RNA-Mediated Epigenetic Regulation in Hepatic Stellate Cells of Liver Fibrosis. Non-Coding RNA 2024, 10, 44. https://doi.org/10.3390/ncrna10040044
Gao R, Mao J. Noncoding RNA-Mediated Epigenetic Regulation in Hepatic Stellate Cells of Liver Fibrosis. Non-Coding RNA. 2024; 10(4):44. https://doi.org/10.3390/ncrna10040044
Chicago/Turabian StyleGao, Ruoyu, and Jingwei Mao. 2024. "Noncoding RNA-Mediated Epigenetic Regulation in Hepatic Stellate Cells of Liver Fibrosis" Non-Coding RNA 10, no. 4: 44. https://doi.org/10.3390/ncrna10040044
APA StyleGao, R., & Mao, J. (2024). Noncoding RNA-Mediated Epigenetic Regulation in Hepatic Stellate Cells of Liver Fibrosis. Non-Coding RNA, 10(4), 44. https://doi.org/10.3390/ncrna10040044






