-
 Direct circRNA-mRNA Binding Controls mRNA Fate: A New Mechanism for circRNAs
Direct circRNA-mRNA Binding Controls mRNA Fate: A New Mechanism for circRNAs -
 Human Papillomavirus-Encoded microRNAs as Regulators of Human Gene Expression in Anal Squamous Cell Carcinoma: A Meta-Transcriptomics Study
Human Papillomavirus-Encoded microRNAs as Regulators of Human Gene Expression in Anal Squamous Cell Carcinoma: A Meta-Transcriptomics Study -
 Knockdown of the snoRNA-Jouvence Blocks the Proliferation and Leads to the Death of Human Primary Glioblastoma Cells
Knockdown of the snoRNA-Jouvence Blocks the Proliferation and Leads to the Death of Human Primary Glioblastoma Cells
Journal Description
Non-Coding RNA
Non-Coding RNA
is an international, peer-reviewed, open access journal on non-coding RNA research dealing with elucidating the structure, function and biology of regulatory non-coding RNAs. Non-Coding RNA is published bimonthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Genetics and Heredity) / CiteScore - Q1 (Genetics)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 23.4 days after submission; acceptance to publication is undertaken in 4.7 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.0 (2024);
5-Year Impact Factor:
4.2 (2024)
Latest Articles
Human-Specific Suppression of Hepatic Fatty Acid Catabolism by RNA-Binding Protein HuR
Non-Coding RNA 2025, 11(5), 65; https://doi.org/10.3390/ncrna11050065 - 1 Sep 2025
Abstract
►
Show Figures
RNA-binding proteins (RBPs) play essential roles in all major steps of RNA processing. Genetic studies in human and mouse models support that many RBPs are crucial for maintaining homeostasis in key tissues/organs, but to what extent the function of RBPs is conserved between
[...] Read more.
RNA-binding proteins (RBPs) play essential roles in all major steps of RNA processing. Genetic studies in human and mouse models support that many RBPs are crucial for maintaining homeostasis in key tissues/organs, but to what extent the function of RBPs is conserved between humans and mice is not clear. Our recent study using a chimeric humanized liver mouse model found that knocking down human HuR in human hepatocytes resulted in a broad upregulation of human genes involved in fatty acid catabolism. This regulation is human-specific, as the knocking down of mouse HuR in the liver of traditional mouse models did not show these effects. To further study this human-specific role of HuR, we co-overexpressed HuR with PPARα, a master transcription factor that promotes fatty acid catabolism, in cultured cells. We found that HuR suppressed the expression of PPARα-induced fatty acid catabolism genes in human cells but not in mouse cells. We provide evidence supporting that the human-specific suppressive effect of HuR is independent of PPARα expression or location. The regulatory effects of HuR are also independent of its role in regulating mRNA stability. Using the human HMGCS2 gene as an example, we found that the suppressive effect of HuR cannot be explained by decreased promoter activity. We further provide evidence supporting that HuR suppresses the pre-mRNA processing of HMGCS2 gene, leading to accumulated intron/pre-mRNA expression of HMGCS2 gene. Furthermore, overexpression of HuR blocked and knocking down of HuR sensitized PPARα agonist-induced gene expression. By analyzing published RNA-seq data, we found compromised pre-mRNA processing for fatty acid catabolism genes in patients with fatty liver diseases, which was not observed in mouse fatty liver disease models. Our study supports the model that HuR suppresses the expression of fatty acid catabolism genes by blocking their pre-mRNA processing, which may partially explain the mild effects of PPARα agonists in treating fatty liver diseases in humans as compared with studies in mice.
Full article
Open AccessReview
Navigating the Landscape of Exosomal microRNAs: Charting Their Pivotal Role as Biomarkers in Hematological Malignancies
by
Manlio Fazio, Fabio Stagno, Giuseppa Penna, Giuseppe Mirabile and Alessandro Allegra
Non-Coding RNA 2025, 11(5), 64; https://doi.org/10.3390/ncrna11050064 - 31 Aug 2025
Abstract
►▼
Show Figures
Under physiological and pathological conditions, all cells release extracellular vesicles named exosomes, which act as transporters of lipidic, protein, and genetic material from parent to recipient cells. Neoplastic cells can secrete higher number of exosomes to exert pro-tumoral effects such as microenvironmental changes,
[...] Read more.
Under physiological and pathological conditions, all cells release extracellular vesicles named exosomes, which act as transporters of lipidic, protein, and genetic material from parent to recipient cells. Neoplastic cells can secrete higher number of exosomes to exert pro-tumoral effects such as microenvironmental changes, disease progression, immunosuppression and drug-resistance. This holds true for both organ-specific cancers and hematologic malignancies. One of the most important components of exosomal cargo are microRNAs which can mediate all the abovementioned effects. More specifically, microRNAs are small non-coding RNAs, routinely detected through quantitative real-time PCR, which act as translational suppressors by regulating protein-coding genes. Considering their high stability in all body fluids and viability in circulation, research is currently focusing on this type of RNAs for the so called “liquid biopsy”, a non-invasive tool for disease diagnosis and longitudinal monitoring. However, several issues remain to be solved including the lack of standardized protocols for exosome isolation and miRNA detection. Starting with this premise, our review aims to provide a wide description of the known microRNA panels employed in the prominent hematological malignancies, which will hopefully redefine the approach to these very challenging diseases in the near future.
Full article

Figure 1
Open AccessArticle
Same Fragments, Different Diseases: Analysis of Identical tRNA Fragments Across Diseases Utilizing Functional and Abundance-Based Databases
by
Adesupo Adetowubo, Sathyanarayanan Vaidhyanathan and Andrey Grigoriev
Non-Coding RNA 2025, 11(5), 63; https://doi.org/10.3390/ncrna11050063 - 29 Aug 2025
Abstract
Background/Objectives: Transfer RNA-derived fragments (tRFs) are small non-coding RNAs increasingly implicated in gene regulation and disease, yet their target specificity and disease relevance remain poorly understood. This is an exploratory study that investigates the phenomenon of identical tRF sequences reported in distinct disease
[...] Read more.
Background/Objectives: Transfer RNA-derived fragments (tRFs) are small non-coding RNAs increasingly implicated in gene regulation and disease, yet their target specificity and disease relevance remain poorly understood. This is an exploratory study that investigates the phenomenon of identical tRF sequences reported in distinct disease contexts and evaluates the consistency between experimental findings and predictions from both target-based and abundance-based tRF databases. Methods: Five tRFs with identical sequences across at least two peer-reviewed disease studies were selected from a recent systematic review. Their validated targets and disease associations were extracted from the literature. Motifs and predicted targets were cross-referenced using three target-oriented databases: tatDB, tRFTar, and tsRFun. In parallel, the abundance enrichment of cancer-associated tRFs was assessed in OncotRF and MINTbase using TCGA-based abundance data. Results: Among the five tRFs, only LeuAAG-001-N-3p-68-85 showed complete alignment between experimental data and both tatDB and tRFTar predictions. Most of the other four displayed at least partial overlaps in motif/binding regions with some of validated targets. tRF abundance data from MINTbase and OncotRF showed inconsistent enrichment, with only AlaAGC-002-N-3p-58-75 exhibiting concordance with its experimentally validated cancer type. Most functionally relevant tRFs were not strongly represented in abundance-only databases. Conclusions: Given the limited number of tRFs analyzed, this study serves primarily as a pilot analysis designed to generate hypotheses and guide future in-depth research, rather than offering comprehensive conclusions. We did, however, illustrate how the analysis of tRFs can benefit from utilizing currently available databases. Target-based databases more closely reflected experimental evidence for mechanistic details when a tRF or a motif match is found. Yet all database types are incomplete, including the abundance-focused tools, which often fail to capture disease-specific regulatory roles of tRFs. These findings underscore the importance of using integrated data sources for tRF annotation. As a pilot analysis, the study provides insights into how identical tRF sequences might function differently across disease contexts, highlighting areas for further investigation while pointing out the limitations of relying on expression data alone to infer functional relevance.
Full article
(This article belongs to the Section Small Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessReview
Partners in Silencing: Decoding the Mammalian Argonaute Interactome
by
Srinaath Narasimhan and Stefan J. Erkeland
Non-Coding RNA 2025, 11(4), 62; https://doi.org/10.3390/ncrna11040062 - 19 Aug 2025
Abstract
►▼
Show Figures
MicroRNAs (miRNAs) are key post-transcriptional regulators controlling gene expression across several cellular processes, including development, proliferation, and apoptosis. Their biogenesis involves a multi-step pathway, including the processing of primary transcripts and the assembly of the RNA-Induced Silencing Complex (RISC) with Argonaute (AGO) proteins
[...] Read more.
MicroRNAs (miRNAs) are key post-transcriptional regulators controlling gene expression across several cellular processes, including development, proliferation, and apoptosis. Their biogenesis involves a multi-step pathway, including the processing of primary transcripts and the assembly of the RNA-Induced Silencing Complex (RISC) with Argonaute (AGO) proteins at its core. This review provides a comprehensive overview of the molecular dynamics of miRNA-loaded RISC (miRISC), focusing on the post-translational modifications, the interactors of AGOs and the mechanisms that fine-tune and coordinate miRISC activity. The composition of miRISC influences AGO stability, localization, and silencing efficiency, thereby maintaining cellular homeostasis and development and mediating the response to various types of cellular stress. Uncommon regulatory mechanisms, including AGO modifications during, e.g., hypoxia or Type 2 T cell responses and miRISC functionality, with myriad RNA-binding proteins (RBPs), will be discussed. This review aims at highlighting the recent advances in the understanding of the intricate regulation of miRISC-driven gene silencing.
Full article

Figure 1
Open AccessReview
The Role of Non-Coding RNAs in the Regulation of Oncogenic Pathways in Breast and Gynaecological Cancers
by
Ammar Ansari, Aleksandra Szczesnowska, Natalia Haddad, Ahmed Elbediwy and Nadine Wehida
Non-Coding RNA 2025, 11(4), 61; https://doi.org/10.3390/ncrna11040061 - 6 Aug 2025
Abstract
►▼
Show Figures
Female cancers such as breast and gynaecological cancers contribute to a significant global health burden and are a leading cause of fatality among women. With current treatment options often limited by resistance to cytotoxic drugs, side effects and lack of specificity to the
[...] Read more.
Female cancers such as breast and gynaecological cancers contribute to a significant global health burden and are a leading cause of fatality among women. With current treatment options often limited by resistance to cytotoxic drugs, side effects and lack of specificity to the cancer, there is a pressing need for alternative treatments. Recent research has highlighted the promising role of non-coding RNAs (ncRNA) in regulating these issues and providing more targeted approaches to suppressing key cancer pathways. This review explores the involvement of the various types of non-coding RNAs in regulating key oncogenic pathways, namely, the MAPK, PI3K/Akt/mTOR, Wnt/β-catenin and p53 pathways, in a range of female cancers such as breast, cervical, ovarian and endometrial cancers. Evidence from a multitude of studies suggests that non-coding RNAs function as double-edged swords, serving as both oncogenes and tumour suppressors, depending on their expression and cellular interactions. By mapping and investigating these regulatory interactions, this review demonstrates the complexity and dual functionality of ncRNAs in cancer. Understanding these complex mechanisms is essential for the development of new and effective ncRNA-based diagnostic methods and targeted therapies in female cancer treatment.
Full article

Figure 1
Open AccessArticle
MALAT1 Expression Is Deregulated in miR-34a Knockout Cell Lines
by
Andrea Corsi, Tonia De Simone, Angela Valentino, Elisa Orlandi, Chiara Stefani, Cristina Patuzzo, Stefania Fochi, Maria Giusy Bruno, Elisabetta Trabetti, John Charles Rotondo, Chiara Mazziotta, Maria Teresa Valenti, Alessandra Ruggiero, Donato Zipeto, Cristina Bombieri and Maria Grazia Romanelli
Non-Coding RNA 2025, 11(4), 60; https://doi.org/10.3390/ncrna11040060 - 5 Aug 2025
Abstract
Background/Objectives: Non-coding microRNA-34a (miR-34a) regulates the expression of key factors involved in several cellular processes, such as differentiation, apoptosis, proliferation, cell cycle, and senescence. Deregulation of the expression of these factors is implicated in the onset and progression of several human diseases, including
[...] Read more.
Background/Objectives: Non-coding microRNA-34a (miR-34a) regulates the expression of key factors involved in several cellular processes, such as differentiation, apoptosis, proliferation, cell cycle, and senescence. Deregulation of the expression of these factors is implicated in the onset and progression of several human diseases, including cancer, neurodegenerative disorders, and pathologies associated with viral infections and inflammation. Despite numerous studies, the molecular mechanisms regulated by miR-34a remain to be fully understood. The present study aimed to generate miR-34a knockout cell lines to identify novel genes potentially regulated by its expression. Methods: We employed the CRISPR-Cas9 gene editing system to knock out the hsa-miR-34a gene in HeLa and 293T cell lines, two widely used models for studying molecular and cellular mechanisms. We compared proliferation rates and gene expression profiles via RNA-seq and qPCR analyses between the wild-type and miR-34a KO cell lines. Results: Knockout of miR-34a resulted in a decreased proliferation rate in both cell lines. Noteworthy, the ablation of miR-34a resulted in increased expression of the long non-coding RNA MALAT1. Additionally, miR-34a-5p silencing in the A375 melanoma cell line led to MALAT1 overexpression. Conclusions: Our findings support the role of the miR-34a/MALAT1 axis in regulating proliferation processes.
Full article
(This article belongs to the Section Long Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessCommunication
DEAD-Box Helicase 3 Modulates the Non-Coding RNA Pool in Ribonucleoprotein Condensates During Stress Granule Formation
by
Elizaveta Korunova, B. Celia Cui, Hao Ji, Aliaksandra Sikirzhytskaya, Srestha Samaddar, Mengqian Chen, Vitali Sikirzhytski and Michael Shtutman
Non-Coding RNA 2025, 11(4), 59; https://doi.org/10.3390/ncrna11040059 - 1 Aug 2025
Abstract
►▼
Show Figures
Stress granule formation is a type of liquid–liquid phase separation in the cytoplasm, leading to RNA–protein condensates that are associated with various cellular stress responses and implicated in numerous pathologies, including cancer, neurodegeneration, inflammation, and cellular senescence. One of the key components of
[...] Read more.
Stress granule formation is a type of liquid–liquid phase separation in the cytoplasm, leading to RNA–protein condensates that are associated with various cellular stress responses and implicated in numerous pathologies, including cancer, neurodegeneration, inflammation, and cellular senescence. One of the key components of mammalian stress granules is the DEAD-box RNA helicase DDX3, which unwinds RNA in an ATP-dependent manner. DDX3 is involved in multiple steps of RNA metabolism, facilitating gene transcription, splicing, and nuclear export and regulating cytoplasmic translation. In this study, we investigate the role of the RNA helicase DDX3’s enzymatic activity in shaping the RNA content of ribonucleoprotein (RNP) condensates formed during arsenite-induced stress by inhibiting DDX3 activity with RK-33, a small molecule previously shown to be effective in cancer clinical studies. Using the human osteosarcoma U2OS cell line, we purified the RNP granule fraction and performed RNA sequencing to assess changes in the RNA pool. Our results reveal that RK-33 treatment alters the composition of non-coding RNAs within the RNP granule fraction. We observed a DDX3-dependent increase in circular RNA (circRNA) content and alterations in the granule-associated intronic RNAs, suggesting a novel role for DDX3 in regulating the cytoplasmic redistribution of non-coding RNAs.
Full article

Figure 1
Open AccessArticle
The Good, the Bad, or Both? Unveiling the Molecular Functions of LINC01133 in Tumors
by
Leandro Teodoro Júnior and Mari Cleide Sogayar
Non-Coding RNA 2025, 11(4), 58; https://doi.org/10.3390/ncrna11040058 - 30 Jul 2025
Abstract
Background/Objectives: Increasing evidence suggests that lncRNAs are core regulators in the field of tumor progression, with context-specific functions in oncogenic tumorigenesis. LINC01133, a lncRNA that has been identified as both an oncogene and a tumor suppressor, remains largely unexplored in terms of its
[...] Read more.
Background/Objectives: Increasing evidence suggests that lncRNAs are core regulators in the field of tumor progression, with context-specific functions in oncogenic tumorigenesis. LINC01133, a lncRNA that has been identified as both an oncogene and a tumor suppressor, remains largely unexplored in terms of its molecular mechanisms. The purpose of this study was to conduct an in silico analysis, incorporating literature research on various cancer types, to investigate the structural and functional duality of LINC01133. This analysis aimed to identify pathways influenced by LINC01133 and evaluate its mechanism of action as a potential therapeutic target and diagnostic biomarker. Methods: In silico analyses and a narrative review of the literature were performed to predict conserved structural elements, functional internal loops, and overall conservation of the LINC01133 sequence among different vertebrate organisms, summarizing the empirical evidence regarding its roles as a tumor suppressor and tumor-promoting roles in various types of tumors. Results: LINC01133 harbors the evolutionarily conserved structural regions that might allow for binding to relevant driver signaling pathways, substantiating its specific functionality. Its action extends beyond classical tumor mechanisms, affecting proliferation, migration, invasion, and epigenetic pathways in various types of tumors, as indicated by the in silico results and narrative review of the literature we present here. Clinical outcome associations pointed to its potential as a biomarker. Conclusions: The dual character of LINC01133 in tumor biology further demonstrates its prospective therapeutic value, but complete elucidation of its mechanisms of action requires further investigation. This study establishes LINC01133 as a multifaceted lncRNA, supporting context-specific strategies in targeting its pathways, and calls for expanded research to harness its full potential in oncology.
Full article
(This article belongs to the Special Issue Non-coding RNA as Biomarker in Cancer)
►▼
Show Figures

Figure 1
Open AccessCorrection
Correction: Garmaa et al. A Systematic Review and Meta-Analysis of microRNA Profiling Studies in Chronic Kidney Diseases. Non-Coding RNA 2024, 10, 30
by
Gantsetseg Garmaa, Stefania Bunduc, Tamás Kói, Péter Hegyi, Dezső Csupor, Dariimaa Ganbat, Fanni Dembrovszky, Fanni Adél Meznerics, Ailar Nasirzadeh, Cristina Barbagallo and Gábor Kökény
Non-Coding RNA 2025, 11(4), 57; https://doi.org/10.3390/ncrna11040057 - 30 Jul 2025
Abstract
Text Correction [...]
Full article
Open AccessReview
circRNA/miRNA Networks Regulate KLF4 in Tumor Development
by
Raffaele Frazzi, Enrico Farnetti and Davide Nicoli
Non-Coding RNA 2025, 11(4), 56; https://doi.org/10.3390/ncrna11040056 - 29 Jul 2025
Abstract
Background/Objectives: Krüppel-like factor 4 (KLF4) emerged as an epigenetically regulated gene in a variety of settings, including cell reprogramming and malignant cell proliferation. The aim of the present manuscript is to explore the relationship described in recent years between circular
[...] Read more.
Background/Objectives: Krüppel-like factor 4 (KLF4) emerged as an epigenetically regulated gene in a variety of settings, including cell reprogramming and malignant cell proliferation. The aim of the present manuscript is to explore the relationship described in recent years between circular RNAs, miRNAs, and KLF4. These have been shown to be involved in cancers having diverse histological origins, including some of the most prevalent and deadly tumors for the human population. Expression and protein levels of this transcription factor correlate with invasiveness and prognosis in a context- and tissue-specific fashion. Methods: The literature was obtained through two main PubMed queries. The first is “miRNA and KLF4 and cancer” and is limited to the last 5 years. The second is “circRNA and KLF4”, which yielded publications between 2013 and 2024. The oncological publications were selected. Results: A number of circRNA/miRNA axes that regulate the downstream transcription factor KLF4 emerged in the last few years. circRNAs act as sponges for miRNAs and synergize with KLF4, which can function as either a tumor promoter or suppressor in different tumors. Conclusions: The axes represented by circRNA/miRNA/KLF4 emerged as a new layer of epigenetic regulation. These RNA-based modulators explain the complex regulation of this transcription factor and open the way to new therapeutic targeting possibilities.
Full article
(This article belongs to the Section Detection and Biomarkers of Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessArticle
Small Nucleolar RNA from S. cerevisiae Binds to Phosphatidylinositol 4,5-Bisphosphate
by
Irma A. Jiménez-Ramírez, Miguel A. Uc-Chuc, Luis Carlos Rodríguez Zapata and Enrique Castaño
Non-Coding RNA 2025, 11(4), 55; https://doi.org/10.3390/ncrna11040055 - 28 Jul 2025
Cited by 1
Abstract
Background: snoRNAs have traditionally been known for their role as guides in post-transcriptional rRNA modifications. Previously, our research group identified several RNAs that may bind to PIP2 with LIPRNA-seq. Among them, snR191 stood out due to its potential specific interaction with this
[...] Read more.
Background: snoRNAs have traditionally been known for their role as guides in post-transcriptional rRNA modifications. Previously, our research group identified several RNAs that may bind to PIP2 with LIPRNA-seq. Among them, snR191 stood out due to its potential specific interaction with this lipid, distinguishing itself from other snoRNAs. However, a detailed study is needed to define the molecular interactions between RNA and lipids, which remain unknown but may serve as a mechanism for transport or liquid–liquid phase separation. This study aimed to determine the interaction between a snoRNA called snR191 and PIP2. Method: A novel methodology for RNA-PIP2 interaction was carried out. Total RNA from Saccharomyces cerevisiae was incubated with PIP2-bound nitrocellulose membranes and RT-PCR reactions. We performed the prediction of snR191-PIP2 interaction by molecular docking and in silico mutations of snoR191. Results: From LIPRNA-seq analysis, we identified that PIP2-bound RNAs were significantly enriched in diverse biological processes, including transmembrane transport and redox functions. Our RNA-PIP2 interaction approach was successful. We demonstrated that snR191 specifically interacts with PIP2 in vitro. The elimination of DNA ensured that the interaction assay was RNA-specific, strengthening the robustness of the experiment. PIP2 was docked to snR191 in a stem–loop–stem motif. Six hydrogen bonds across four nucleotides mediated the PIP2-snR191 interaction. Finally, mutations in snR191 affected the structural folding. Conclusions: In this study, we demonstrate the effectiveness of a new methodology for determining RNA–lipid interactions, providing strong evidence for the specific interaction between snR191 and PIP2. Integrating biochemical and computational approaches has allowed us to understand the binding of these biomolecules. Therefore, this work significantly broadens our understanding of snR191-PIP2 interactions and opens new perspectives for further research.
Full article
(This article belongs to the Section Long Non-Coding RNA)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Knockdown of the snoRNA-Jouvence Blocks the Proliferation and Leads to the Death of Human Primary Glioblastoma Cells
by
Lola Jaque-Cabrera, Julia Buggiani, Jérôme Bignon, Patricia Daira, Nathalie Bernoud-Hubac and Jean-René Martin
Non-Coding RNA 2025, 11(4), 54; https://doi.org/10.3390/ncrna11040054 - 18 Jul 2025
Abstract
Background/Objectives: Cancer research aims to understand the cellular and molecular mechanisms involved, in order to identify new therapeutic targets and provide patients with more effective therapies that generate fewer side undesirable and toxic effects. Previous studies have demonstrated the role of small
[...] Read more.
Background/Objectives: Cancer research aims to understand the cellular and molecular mechanisms involved, in order to identify new therapeutic targets and provide patients with more effective therapies that generate fewer side undesirable and toxic effects. Previous studies have demonstrated the role of small nucleolar RNAs (snoRNAs) in many physiological and pathological cellular processes, including cancers. SnoRNAs are a group of non-coding RNAs involved in different post-transcriptional modifications of ribosomal RNAs. Recently, we identified a new snoRNA (jouvence), first in Drosophila, and thereafter, by homology, in humans. Methods: Here, we characterize the effect of the knockdown of jouvence by a sh-lentivirus on human primary patient-derived glioblastoma cells. Results: The sh-lentivirus anti-jouvence induces a significant decrease in cell proliferation and leads to cell death. EdU staining confirmed this decrease, while TUNEL also showed the presence of apoptotic cells. An RNA-Seq analysis revealed a decrease, in particular, in the level of BAALC, a gene known to potentiate the oncogenic ERK pathway and deregulating p21, leading to cell cycle blockage. Conclusions: Altogether, these results allow the hypothesis that the knockdown of jouvence could potentially be used as a new anti-cancer treatment (sno-Therapy), especially against glioblastoma and also, potentially, against acute myeloid leukemia (AML) due to the BAALC deregulation.
Full article
(This article belongs to the Section Small Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessFeature PaperReview
Direct circRNA-mRNA Binding Controls mRNA Fate: A New Mechanism for circRNAs
by
Raffaele Garraffo and Manuel Beltran Nebot
Non-Coding RNA 2025, 11(4), 53; https://doi.org/10.3390/ncrna11040053 - 18 Jul 2025
Abstract
►▼
Show Figures
Circular RNAs (circRNAs) are covalently closed RNA molecules generated through a non-canonical splicing event known as back-splicing. This particular class of non-coding RNAs has attracted growing interest due to its evolutionary conservation across eukaryotes, high expression in the central nervous system, and frequent
[...] Read more.
Circular RNAs (circRNAs) are covalently closed RNA molecules generated through a non-canonical splicing event known as back-splicing. This particular class of non-coding RNAs has attracted growing interest due to its evolutionary conservation across eukaryotes, high expression in the central nervous system, and frequent dysregulation in various pathological conditions, including cancer. Traditionally, circRNAs have been characterised by their ability to function as microRNA (miRNA) and protein sponges. However, recent discoveries from multiple research groups have uncovered a novel and potentially transformative mechanism of action: the direct interaction of circRNAs with messenger RNAs (mRNAs) to regulate their fate. These interactions can influence mRNA stability and translation, revealing a new layer of post-transcriptional gene regulation. In this review, we present and analyse the latest evidence supporting the emerging role of circRNAs in diverse biological contexts. We highlight the growing body of research demonstrating circRNA-mRNA interactions as a functional regulatory mechanism and explore their involvement in key physiological and pathophysiological processes. Understanding this novel mechanism expands our knowledge of RNA-based regulation and opens new opportunities for therapeutic strategies targeting circRNA-mRNA networks in human disease.
Full article

Figure 1
Open AccessArticle
Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice
by
Beshoy Armanios, Jing Jin, Holly Kolmel, Ankit P. Laddha, Neha Mishra, Jose E. Manautou and Xiao-Bo Zhong
Non-Coding RNA 2025, 11(4), 52; https://doi.org/10.3390/ncrna11040052 - 4 Jul 2025
Abstract
Background/Objectives: Long non-coding RNAs (lncRNAs) play significant roles in tissue development and disease progression and have emerged as crucial regulators of gene expression. The hepatocyte nuclear factor alpha antisense RNA 1 (HNF1A-AS1) lncRNA is a particularly intriguing regulatory molecule in liver biology that
[...] Read more.
Background/Objectives: Long non-coding RNAs (lncRNAs) play significant roles in tissue development and disease progression and have emerged as crucial regulators of gene expression. The hepatocyte nuclear factor alpha antisense RNA 1 (HNF1A-AS1) lncRNA is a particularly intriguing regulatory molecule in liver biology that is involved in the regulation of cytochrome P450 enzymes via epigenetic mechanisms. Despite the growing recognition of lncRNAs in liver disease, the comprehensive role of HNF1A-AS1 in liver function remains unclear. This study aimed to investigate the roles of the mouse homolog of the human HNF1A-AS1 lncRNA HNF1A opposite strand 1 (Hnf1aos1) in liver function, gene expression, and cellular processes using a mouse model to identify potential therapeutic targets for liver disorders. Methods: The knockdown of Hnf1aos1 was performed in in vitro mouse liver cell lines using siRNA and in vivo livers of AAV-shRNA complexes. Changes in the global expression landscapes of mRNA and proteins were revealed using RNA-seq and proteomics, respectively. Changes in the selected genes were further validated via real-time quantitative polymerase chain reaction (RT-qPCR). Phenotypic changes were assessed via histological and absorbance-based assays. Results: After the knockdown of Hnf1aos1, RNA-seq and proteomics analysis revealed the differential gene expression of the mRNAs and proteins involved in the processes of molecular transport, liver regeneration, and immune signaling pathways. The downregulation of ABCA1 and SREBF1 indicates their role in cholesterol transport and fatty acid and triglyceride synthesis. Additionally, significant reductions in hepatic triglyceride levels were observed in the Hnf1aos1-knockdown group, underscoring the impact on lipid regulation. Notably, the knockdown of Hnf1aos1 also led to an almost complete depletion of CYP7A1, the rate-limiting enzyme in bile acid synthesis, highlighting its role in cholesterol homeostasis and hepatotoxicity. Histological assessments confirmed these molecular findings, with increased hepatic inflammation, hepatocyte swelling, and disrupted liver architecture observed in the Hnf1aos1-knockdown mice. Conclusions: This study illustrated that Hnf1aos1 is a critical regulator of liver health, influencing both lipid metabolism and immune pathways. It maintains hepatic lipid homeostasis, modulates lipid-induced inflammatory responses, and contributes to viral immunity, indirectly affecting glucose and lipid metabolic balance.
Full article
(This article belongs to the Section Long Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessReview
Role of ncRNAs in the Development of Chronic Pain
by
Mario García-Domínguez
Non-Coding RNA 2025, 11(4), 51; https://doi.org/10.3390/ncrna11040051 - 3 Jul 2025
Abstract
►▼
Show Figures
Chronic pain is a multifactorial and complex condition that significantly affects individuals’ quality of life. The underlying mechanisms of chronic pain involve complex alterations in neural circuits, gene expression, and cellular signaling pathways. Recently, ncRNAs, such as miRNAs, lncRNAs, circRNAs, and siRNAs, have
[...] Read more.
Chronic pain is a multifactorial and complex condition that significantly affects individuals’ quality of life. The underlying mechanisms of chronic pain involve complex alterations in neural circuits, gene expression, and cellular signaling pathways. Recently, ncRNAs, such as miRNAs, lncRNAs, circRNAs, and siRNAs, have been identified as crucial regulators in the pathophysiology of chronic pain. These ncRNAs modulate gene expression at both the transcriptional and post-transcriptional levels, affecting pain-related pathways like inflammation, neuronal plasticity, and sensory processing. miRNAs have been shown to control genes involved in pain perception and nociceptive signaling, while lncRNAs interact with chromatin remodeling factors and transcription factors to modify pain-related gene expression. CircRNAs act as sponges for miRNAs, thereby influencing pain mechanisms. siRNAs, recognized for their gene-silencing capabilities, also participate in regulating the expression of pain-related genes. This review examines the diverse roles of ncRNAs in chronic pain, emphasizing their potential as biomarkers for pain assessment and as targets for novel therapeutic strategies. A profound understanding of the ncRNA-mediated regulatory networks involved in chronic pain could result in more effective and personalized pain management solutions.
Full article
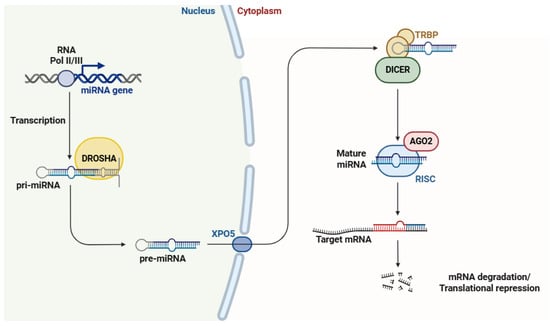
Figure 1
Open AccessCorrection
Correction: Piergentili et al. miR-125 in Breast Cancer Etiopathogenesis: An Emerging Role as a Biomarker in Differential Diagnosis, Regenerative Medicine, and the Challenges of Personalized Medicine. Non-Coding RNA 2024, 10, 16
by
Roberto Piergentili, Enrico Marinelli, Gaspare Cucinella, Alessandra Lopez, Gabriele Napoletano, Giuseppe Gullo and Simona Zaami
Non-Coding RNA 2025, 11(4), 50; https://doi.org/10.3390/ncrna11040050 - 25 Jun 2025
Abstract
There was an error in the original publication [...]
Full article
Open AccessArticle
LncRNA Subcellular Localization Across Diverse Cell Lines: An Exploration Using Deep Learning with Inexact q-mers
by
Weijun Yi, Jason R. Miller, Gangqing Hu and Donald A. Adjeroh
Non-Coding RNA 2025, 11(4), 49; https://doi.org/10.3390/ncrna11040049 - 25 Jun 2025
Abstract
Background: Long non-coding Ribonucleic Acids (lncRNAs) can be localized to different cellular compartments, such as the nuclear and the cytoplasmic regions. Their biological functions are influenced by the region of the cell where they are located. Compared to the vast number of lncRNAs,
[...] Read more.
Background: Long non-coding Ribonucleic Acids (lncRNAs) can be localized to different cellular compartments, such as the nuclear and the cytoplasmic regions. Their biological functions are influenced by the region of the cell where they are located. Compared to the vast number of lncRNAs, only a relatively small proportion have annotations regarding their subcellular localization. It would be helpful if those few annotated lncRNAs could be leveraged to develop predictive models for localization of other lncRNAs. Methods: Conventional computational methods use q-mer profiles from lncRNA sequences and train machine learning models such as support vector machines and logistic regression with the profiles. These methods focus on the exact q-mer. Given possible sequence mutations and other uncertainties in genomic sequences and their role in biological function, a consideration of these variabilities might improve our ability to model lncRNAs and their localization. Thus, we build on inexact q-mers and use machine learning/deep learning techniques to study three specific problems in lncRNA subcellular localization, namely, prediction of lncRNA localization using inexact q-mers, the issue of whether lncRNA localization is cell-type-specific, and the notion of switching (lncRNA) genes. Results: We performed our analysis using data on lncRNA localization across 15 cell lines. Our results showed that using inexact q-mers (with q = 6) can improve the lncRNA localization prediction performance compared to using exact q-mers. Further, we showed that lncRNA localization, in general, is not cell-line-specific. We also identified a category of LncRNAs which switch cellular compartments between different cell lines (we call them switching lncRNAs). These switching lncRNAs complicate the problem of predicting lncRNA localization using machine learning models, showing that lncRNA localization is still a major challenge.
Full article
(This article belongs to the Section Long Non-Coding RNA)
►▼
Show Figures

Graphical abstract
Open AccessBrief Report
Insights into miRNAs of the Stingless Bee Melipona quadrifasciata
by
Dalliane Oliveira Soares, Lucas Yago Melo Ferreira, Gabriel Victor Pina Rodrigues, João Pedro Nunes Santos, Ícaro Santos Lopes, Lucas Barbosa de Amorim Conceição, Tatyana Chagas Moura, Isaque João da Silva de Faria, Roenick Proveti Olmo, Weyder Cristiano Santana, Marco Antônio Costa and Eric Roberto Guimarães Rocha Aguiar
Non-Coding RNA 2025, 11(3), 48; https://doi.org/10.3390/ncrna11030048 - 19 Jun 2025
Abstract
MicroRNAs (miRNAs) are key post-transcriptional regulators involved in a wide range of biological processes in insects, yet little is known about their roles in stingless bees. Here, we present the first characterization of miRNAs in Melipona quadrifasciata using small RNAs (sRNAs) deep sequencing.
[...] Read more.
MicroRNAs (miRNAs) are key post-transcriptional regulators involved in a wide range of biological processes in insects, yet little is known about their roles in stingless bees. Here, we present the first characterization of miRNAs in Melipona quadrifasciata using small RNAs (sRNAs) deep sequencing. A total of 193 high-confidence mature miRNAs were identified, including 106 M. quadrifasciata-exclusive sequences. Expression profiling revealed that mqu-miR-1 and mqu-miR-276 together accounted for over 70% of all miRNA reads, suggesting their central roles in development and reproduction. Comparative analyses showed a higher conservation of M. quadrifasciata miRNAs with other Hymenopterans, especially Apis mellifera and Bombus spp. Putative target genes were predicted using a consensus approach, and functional annotation indicated their involvement in diverse biological regulatory pathways. This work represents the first comprehensive identification of the miRNA repertoire in stingless bees using sRNAs and provides a valuable foundation for understanding miRNA-mediated gene regulation in this ecologically and economically important pollinator.
Full article
(This article belongs to the Section Small Non-Coding RNA)
►▼
Show Figures

Figure 1
Open AccessArticle
Diagnostic Potential of Exosomal and Non-Exosomal Biomarkers in Lung Cancer: A Comparative Analysis Using a Rat Model of Lung Carcinogenesis
by
Sherien M. El-Daly, Sahar S. Abdelrahman, Amira Mohamed Abd El-Jawad, Mahmoud A. Abdel-Monem and Gamila S. M. El-Saeed
Non-Coding RNA 2025, 11(3), 47; https://doi.org/10.3390/ncrna11030047 - 16 Jun 2025
Abstract
Background: Identifying liquid biopsy biomarkers with high efficacy is crucial for cancer diagnosis. Exosomal cargo, including miRNAs and proteins, offers enhanced stability in biofluids compared with their free circulating forms, but direct comparisons of their diagnostic performance remain limited. This study evaluates and
[...] Read more.
Background: Identifying liquid biopsy biomarkers with high efficacy is crucial for cancer diagnosis. Exosomal cargo, including miRNAs and proteins, offers enhanced stability in biofluids compared with their free circulating forms, but direct comparisons of their diagnostic performance remain limited. This study evaluates and compares the diagnostic value of selected miRNAs and protein markers in exosomal versus non-exosomal fractions across stages of lung carcinogenesis in a rat model. Methods: Lung cancer was induced in rats, and blood and lung tissue samples were collected at consecutive stages of tumor induction. We investigated the expression patterns of key miRNAs (miR-19b, miR-21, and miR-145) in exosomes, serum, and tissue and quantified levels of tumor biomarkers CEA and CYFRA 21-1 in exosomal and serum fractions. Results: Our results revealed distinct expression patterns of the evaluated miRNAs across exosomes, serum, and tissue, throughout different stages of tumor induction. The expression of exosomal miRNAs dynamically changed in parallel with the tumor induction process, demonstrating high diagnostic efficacy. Specifically, exosomal miR-19b and miR-21 were significantly upregulated from an early induction stage, whereas their serum and tissue forms increased only during the late stages of induction. On the other hand, miR-145 was consistently downregulated across all fractions at every stage. Both exosomal and serum CEA levels increased significantly during tumor induction, while serum CYFRA 21-1 outperformed its exosomal counterpart. Strong positive correlations linked exosomal miR-19b and miR-145 with their non-exosomal counterparts, while moderate correlations were seen for miR-21 and the protein markers. Conclusions: Our findings underscore the value of integrating exosomal biomarkers in liquid biopsies, highlighting their potential to improve early detection and monitoring of lung cancer development.
Full article
(This article belongs to the Special Issue Non-coding RNA as Biomarker in Cancer)
►▼
Show Figures
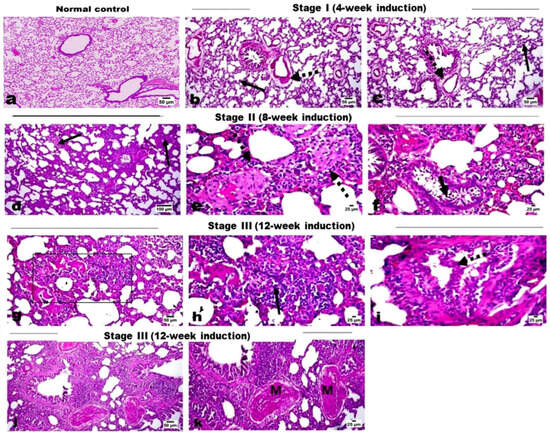
Figure 1
Open AccessArticle
Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation?
by
Caroline Eva Riedel, Javier Ibáñez, Annunziata Fragasso, Angelika Schmitt, Manuel Widmann, Felipe Mattioni Maturana, Andreas M. Niess and Barbara Munz
Non-Coding RNA 2025, 11(3), 46; https://doi.org/10.3390/ncrna11030046 - 16 Jun 2025
Abstract
Background: Physical activity induces a range of physiological and molecular adaptations, particularly affecting skeletal muscle and the cardiovascular system, regulating both tissue architecture and metabolic pathways. Emerging evidence suggests that PIWI-interacting RNAs (piRNAs) may serve as potential biomarkers for these adaptations. Here, we
[...] Read more.
Background: Physical activity induces a range of physiological and molecular adaptations, particularly affecting skeletal muscle and the cardiovascular system, regulating both tissue architecture and metabolic pathways. Emerging evidence suggests that PIWI-interacting RNAs (piRNAs) may serve as potential biomarkers for these adaptations. Here, we analyzed piRNA patterns in the context of exercise. Methods: This study selected eight participants of the iReAct study (DRKS00017446) for piRNA analysis. Baseline assessments included demographic profiling and fitness evaluation, particularly maximal oxygen uptake (V̇O2max) assessment. In addition, blood samples were collected pre- and (for six of the eight participants) post- standard reference training sessions. Subsequently, subjects underwent 6-week training protocols, employing standardized high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT) regimens. Next, RNA sequencing was conducted to identify differentially expressed piRNAs, and correlation analyses were performed between piRNA expression patterns and training-associated changes in V̇O2max. Finally, to identify piRNAs potentially of interest in the context of exercise, different screening procedures were applied. Results: There were unique and specific changes in individual piRNA expression levels in response to exercise. In addition, we could define correlations of piRNA expression patterns, namely of piR-32886, piR-33151, piR-12547, and piR-33074, with changes in V̇O2max. These correlations did not reach significance in the small sample size of this pilot study, but might be verified in larger, confirming studies. Conclusions: This hypothesis-generating study identifies characteristic piRNA patterns in the context of exercise. Their significance as biomarkers is yet to be determined.
Full article
(This article belongs to the Section Detection and Biomarkers of Non-Coding RNA)
►▼
Show Figures
Figure 1

Journal Menu
► ▼ Journal Menu-
- ncRNA Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
- 10th Anniversary
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
3 September 2025
Join Us at the MDPI at the University of Toronto Career Fair, 23 September 2025, Toronto, ON, Canada
Join Us at the MDPI at the University of Toronto Career Fair, 23 September 2025, Toronto, ON, Canada

1 September 2025
MDPI INSIGHTS: The CEO’s Letter #26 – CUJS, Head of Ethics, Open Peer Review, AIS 2025, Reviewer Recognition
MDPI INSIGHTS: The CEO’s Letter #26 – CUJS, Head of Ethics, Open Peer Review, AIS 2025, Reviewer Recognition
Topics

Conferences
Special Issues
Special Issue in
ncRNA
Evolution of Regulatory ncRNAs and ncRNA Genes
Guest Editor: Nicholas DelihasDeadline: 20 September 2025
Special Issue in
ncRNA
Non-coding RNA as Biomarker in Cancer
Guest Editors: Luca Falzone, Daniela Calina, Giuseppe GattusoDeadline: 30 November 2025
Special Issue in
ncRNA
Non-Coding RNA: 10th Anniversary
Guest Editor: George A. CalinDeadline: 31 March 2026
Topical Collections
Topical Collection in
ncRNA
Regulatory RNAs in Cardiovascular Development and Disease
Collection Editors: Yvan Devaux, Francisco J. Enguita, Andrea Caporali
Topical Collection in
ncRNA
Non-Coding RNAs, COVID-19, and Long-COVID
Collection Editors: Gaetano Santulli, Jessica Gambardella
Topical Collection in
ncRNA
Role of microRNA in Neuroendocrine Neoplasms
Collection Editor: Neil Renwick








