Insights into miRNAs of the Stingless Bee Melipona quadrifasciata
Abstract
1. Introduction
2. Results
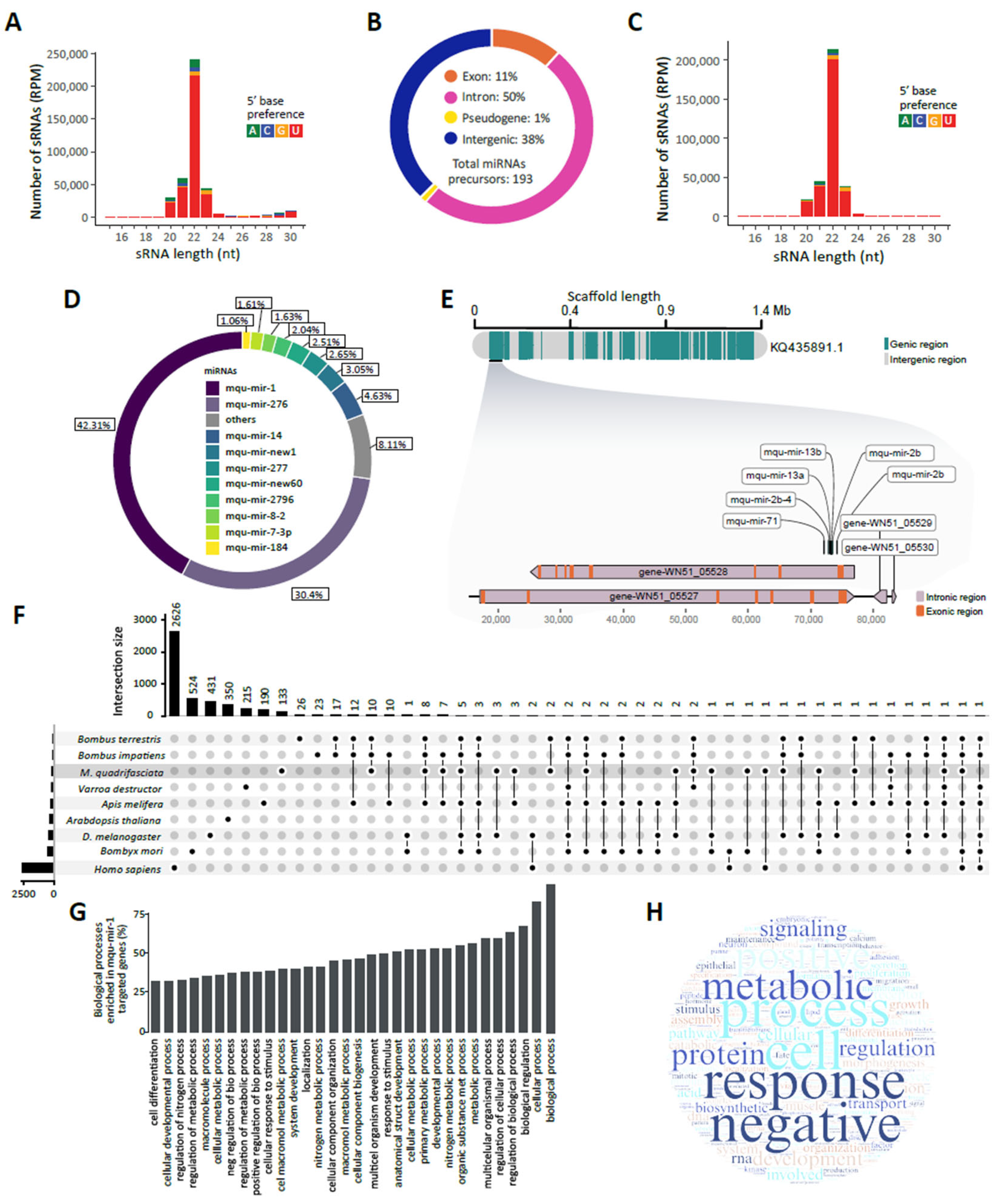
2.1. Overview of Melipona quadrifasciata Small RNAs
2.2. Small RNA-Based Identification and Characterization of miRNAs
2.3. Analysis of miRNA Conservation
2.4. miR-1 Target Prediction and Gene Ontology
3. Discussion
4. Materials and Methods
4.1. Sample, Library Preparation, RNA Deep Sequencing, and Pre-Processing
4.2. Characterization of miRNA Populations and Genomic Analysis
4.3. miRNA Conservation
4.4. Target and Functional Prediction of M. quadrifasciata miRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antonini, Y.; Martins, R.P. Urbanization Affects the Occurrence of a Large Stingless Bee Species in a Large City; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Fikadu, Z. The Contribution of Managed Honey Bees to Crop Pollination, Food Security, and Economic Stability: Case of Ethiopia. Open Agric. J. 2019, 13, 175–181. [Google Scholar] [CrossRef]
- Hristov, P.; Neov, B.; Shumkova, R.; Palova, N. Significance of Apoidea as Main Pollinators. Ecological and Economic Impact and Implications for Human Nutrition. Diversity 2020, 12, 280. [Google Scholar] [CrossRef]
- Aristizábal, N.; Mora-Mena, S.E.; Martínez-Salinas, A.; Chain-Guadarrama, A.; Castillo, D.; Murillo, J.B.; Porras, J.; Ricketts, T.H. Bee Pollination Affects Coffee Quality, Yield, and Trade-Offs within Them. Agric. Ecosyst. Environ. 2025, 377, 109258. [Google Scholar] [CrossRef]
- Thomas, G.; Rusman, Q.; Morrison, W.R.; Magalhães, D.M.; Dowell, J.A.; Ngumbi, E.; Osei-Owusu, J.; Kansman, J.; Gaffke, A.; Pagadala Damodaram, K.J.; et al. Deciphering Plant-Insect-Microorganism Signals for Sustainable Crop Production. Biomolecules 2023, 13, 997. [Google Scholar] [CrossRef]
- Ghosh, S.; Koley, B.; Das, R. Non-Apis Bee Pollinators: A Way out to the Future Pollinators’ Challenge. Int. J. Adv. Biochem. Res. 2024, 8, 116–124. [Google Scholar] [CrossRef]
- Kulhanek, K.; Steinhauer, N.; Rennich, K.; Caron, D.M.; Sagili, R.R.; Pettis, J.S.; Ellis, J.D.; Wilson, M.E.; Wilkes, J.T.; Tarpy, D.R.; et al. A National Survey of Managed Honey Bee 2015–2016 Annual Colony Losses in the USA. J. Apic. Res. 2017, 56, 328–340. [Google Scholar] [CrossRef]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of Managed Honey Bees and Beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Wagner, D.L. Insect Declines in the Anthropocene. Annu. Rev. Entomol. 2020, 65, 457–480. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide Occurrence Records Suggest a Global Decline in Bee Species Richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Bronkhorst, A.W.; Van Rij, R.P. The Long and Short of Antiviral Defense: Small RNA-Based Immunity in Insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and dsRNA-Triggered Transcriptional Responses Reveal Key Components of Honey Bee Antiviral Defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Huang, Q. Interactions Among Host–Parasite MicroRNAs During Nosema Ceranae Proliferation in Apis Mellifera. Front. Microbiol. 2018, 9, 698. [Google Scholar] [CrossRef]
- Narayanan, R.; Schratt, G. miRNA Regulation of Social and Anxiety-Related Behaviour. Cell. Mol. Life Sci. 2020, 77, 4347–4364. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; An, J.; Zheng, W.; He, S. Vibrio Cholerae Pathogen from the Freshwater-Cultured Whiteleg Shrimp Penaeus Vannamei and Control with Bdellovibrio Bacteriovorus. J. Invertebr. Pathol. 2015, 130, 13–20. [Google Scholar] [CrossRef]
- Sharma, R.; Upadhyay, S.; Bhattacharya, S.; Singh, A. Abiotic Stress-Responsive miRNA and Transcription Factor-Mediated Gene Regulatory Network in Oryza Sativa: Construction and Structural Measure Study. Front. Genet. 2021, 12, 618089. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, J.; Zhang, N.; Wu, J.; Si, H. Roles of microRNAs in Abiotic Stress Response and Characteristics Regulation of Plant. Front. Plant Sci. 2022, 13, 919243. [Google Scholar] [CrossRef] [PubMed]
- Lucas, K.J.; Zhao, B.; Liu, S.; Raikhel, A.S. Regulation of Physiological Processes by microRNAs in Insects. Curr. Opin. Insect Sci. 2015, 11, 1–7. [Google Scholar] [CrossRef]
- De Araujo, C.B.; Pereira, P.V.R.; Sousa, T.G.; Filho, A.C.F.; Silva, G.C.; Do Amaral, L.R.; Gandolfi, P.E.; Bonetti, A.M.; Ueira-Vieira, C.; Bertarini, P.L.L.; et al. Unveiling the World of Bee microRNAs: Computational Identification and Characterization of Pathway Genes, Conserved microRNAs, and Their Targets. Int. J. Trop Insect Sci. 2024, 44, 237–251. [Google Scholar] [CrossRef]
- Kapheim, K.M.; Pan, H.; Li, C.; Salzberg, S.L.; Puiu, D.; Magoc, T.; Robertson, H.M.; Hudson, M.E.; Venkat, A.; Fischman, B.J.; et al. Genomic Signatures of Evolutionary Transitions from Solitary to Group Living. Science 2015, 348, 1139–1143. [Google Scholar] [CrossRef]
- Díaz, S.; De Souza Urbano, S.; Caesar, L.; Blochtein, B.; Sattler, A.; Zuge, V.; Haag, K.L. Report on the Microbiota of Melipona Quadrifasciata Affected by a Recurrent Disease. J. Invertebr. Pathol. 2017, 143, 35–39. [Google Scholar] [CrossRef]
- Kulza, R.A.; Galhardo, D.; Moreira, D.R.; Gigliolli, A.A.S.; Aparecida Dos Santos, S.; de Toledo, V.D.A.A.; Ruvolo-Takasusuki, M.C.C. Analysis of the Population Structure of Tetragonisca (Hymenoptera, Meliponini) by Microsatellite Markers and Network Interactions. Res. Soc. Dev. 2022, 11, e4711424811. [Google Scholar] [CrossRef]
- Cunha, M.S.; Garcia, M.V.B.; Campos, L.A.O.; Lopes, D.M. Cytotaxonomy and Karyotype Evolution in Neotropical Meliponini (Hymenoptera: Apidae) Inferred by Chromosomal Mapping of 18S rDNA and Five Microsatellites. J. Apic. Res. 2024, 63, 208–218. [Google Scholar] [CrossRef]
- Farder-Gomes, C.F.; De Oliveira, M.A.; Malaspina, O.; Nocelli, R.F.C. Exposure of the Stingless Bee Melipona Scutellaris to Imidacloprid, Pyraclostrobin, and Glyphosate, Alone and in Combination, Impair Its Walking Activity and Fat Body Morphology and Physiology. Environ. Pollut. 2024, 348, 123783. [Google Scholar] [CrossRef]
- Roldão-Sbordoni, Y.S.; Hrncir, M.; Nascimento, F.S. Brood Thermogenesis Effects on the Thermal Dynamics in Stingless Bee Nests (Melipona Scutellaris). Insect. Soc. 2024, 71, 185–195. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, C.; Zhang, J.; Lu, S.; Zhao, H.; Jiang, Y.; Ma, W. Regulatory Roles of Long Non-Coding RNAs in Short-Term Heat Stress in Adult Worker Bees. BMC Genom. 2024, 25, 506. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, S.; Wang, J.; Hu, W. microRNA Profiles and Functions in Mosquitoes. PLoS Negl. Trop Dis. 2018, 12, e0006463. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Li, W.; Li, Z.; Pan, J.; Yan, L.; Zhang, S.; Huang, Z.Y.; Su, S. Differences in microRNAs and Their Expressions between Foraging and Dancing Honey Bees, Apis Mellifera L. J. Insect Physiol. 2012, 58, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Ylla, G.; Fromm, B.; Piulachs, M.-D.; Belles, X. The microRNA Toolkit of Insects. Sci. Rep. 2016, 6, 37736. [Google Scholar] [CrossRef]
- Saini, H.K.; Griffiths-Jones, S.; Enright, A.J. Genomic Analysis of Human microRNA Transcripts. Proc. Natl. Acad. Sci. USA 2007, 104, 17719–17724. [Google Scholar] [CrossRef]
- Vilimova, M.; Pfeffer, S. Post-transcriptional Regulation of Polycistronic microRNAs. WIREs RNA 2023, 14, e1749. [Google Scholar] [CrossRef]
- Zondag, L.; Dearden, P.K.; Wilson, M.J. Deep Sequencing and Expression of microRNAs from Early Honeybee (Apis Mellifera) Embryos Reveals a Role in Regulating Early Embryonic Patterning. BMC Evol. Biol. 2012, 12, 211. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [PubMed]
- Bushati, N.; Stark, A.; Brennecke, J.; Cohen, S.M. Temporal Reciprocity of miRNAs and Their Targets during the Maternal-to-Zygotic Transition in Drosophila. Curr. Biol. 2008, 18, 501–506. [Google Scholar] [CrossRef]
- Lee, C.-T.; Risom, T.; Strauss, W.M. Evolutionary Conservation of MicroRNA Regulatory Circuits: An Examination of MicroRNA Gene Complexity and Conserved MicroRNA-Target Interactions through Metazoan Phylogeny. DNA Cell Biol. 2007, 26, 209–218. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, Y.; Jagadeeswaran, G.; Ren, R.; Sunkar, R.; Jiang, H. Identification of Conserved and Novel microRNAs in Manduca Sexta and Their Possible Roles in the Expression Regulation of Immunity-Related Genes. Insect Biochem. Mol. Biol. 2014, 47, 12–22. [Google Scholar] [CrossRef]
- Liu, S.; Li, D.; Li, Q.; Zhao, P.; Xiang, Z.; Xia, Q. MicroRNAs of Bombyx Mori Identified by Solexa Sequencing. BMC Genom. 2010, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Patalano, S.; Vlasova, A.; Wyatt, C.; Ewels, P.; Camara, F.; Ferreira, P.G.; Asher, C.L.; Jurkowski, T.P.; Segonds-Pichon, A.; Bachman, M.; et al. Molecular Signatures of Plastic Phenotypes in Two Eusocial Insect Species with Simple Societies. Proc. Natl. Acad. Sci. USA 2015, 112, 13970–13975. [Google Scholar] [CrossRef]
- Macedo, L.M.F.; Nunes, F.M.F.; Freitas, F.C.P.; Pires, C.V.; Tanaka, E.D.; Martins, J.R.; Piulachs, M.-D.; Cristino, A.S.; Pinheiro, D.G.; Simões, Z.L.P. MicroRNA Signatures Characterizing Caste-independent Ovarian Activity in Queen and Worker Honeybees (A Pis Mellifera L.). Insect Mol. Biol. 2016, 25, 216–226. [Google Scholar] [CrossRef]
- Song, J.; Li, W.; Gao, L.; Yan, Q.; Zhang, X.; Liu, M.; Zhou, S. miR-276 and miR-182013-5p Modulate Insect Metamorphosis and Reproduction via Dually Regulating Juvenile Hormone Acid Methyltransferase. Commun Biol. 2024, 7, 1604. [Google Scholar] [CrossRef]
- Chen, X.; Rosbash, M. Mir-276a Strengthens Drosophila Circadian Rhythms by Regulating Timeless Expression. Proc. Natl. Acad. Sci. USA 2016, 113, E2965–E2972. [Google Scholar] [CrossRef]
- Lampe, L.; Jentzsch, M.; Kierszniowska, S.; Levashina, E.A. Metabolic Balancing by miR-276 Shapes the Mosquito Reproductive Cycle and Plasmodium Falciparum Development. Nat. Commun. 2019, 10, 5634. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Ge, X.; Li, Z.; Zeng, B.; Xu, J.; Chen, X.; Shang, P.; James, A.A.; Huang, Y.; Tan, A. MiR-2 Family Targets Awd and Fng to Regulate Wing Morphogenesis in Bombyx Mori. RNA Biol. 2015, 12, 742–748. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Y.; Dong, W. MicroRNA miR-927 Targets the Juvenile Hormone Primary Response Gene Krüppel Homolog1 to Control Drosophila Developmental Growth. Insect Mol. Biol. 2020, 29, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Saedi, H.; Waro, G.; Giacchetta, L.; Tsunoda, S. miR-137 Regulates PTP61F, Affecting Insulin Signaling, Metabolic Homeostasis, and Starvation Resistance in Drosophila. Proc. Natl. Acad. Sci. USA 2024, 121, e2319475121. [Google Scholar] [CrossRef]
- Abbas, M.N.; Kausar, S.; Asma, B.; Ran, W.; Li, J.; Lin, Z.; Li, T.; Cui, H. MicroRNAs Reshape the Immunity of Insects in Response to Bacterial Infection. Front. Immunol. 2023, 14, 1176966. [Google Scholar] [CrossRef]
- Bortolomeazzi, M.; Gaffo, E.; Bortoluzzi, S. A Survey of Software Tools for microRNA Discovery and Characterization Using RNA-Seq. Brief. Bioinform. 2019, 20, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Du, Y.; Chen, H.; Fan, Y.; Fan, X.; Zhu, Z.; Wang, J.; Xiong, C.; Zheng, Y.; Hou, C.; et al. Comparative Identification of MicroRNAs in Apis Cerana Cerana Workers’ Midguts in Response to Nosema Ceranae Invasion. Insects 2019, 10, 258. [Google Scholar] [CrossRef]
- Ferreira, L.Y.M.; Santos, J.P.N.; Souza, D.G.D.N.; Orellana, L.C.B.; De Santana, S.F.; Sousa, A.G.; Fonseca, P.L.C.; Silva, A.G.S.; Santos, V.C.; De Faria, I.J.D.S.; et al. Potential Effect of Wolbachia on Virus Restriction in the Spider Mite T. Truncatus. Front. Microbiol. 2025, 16, 1570606. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10. [Google Scholar] [CrossRef]
- Langmead, B. Aligning Short Sequencing Reads with Bowtie. Curr. Protoc. Bioinform. 2010, 32, 11–17. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 Accurately Identifies Known and Hundreds of Novel microRNA Genes in Seven Animal Clades. Nucleic Acids Research 2012, 40, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, P.L.C.; Mucherino, M.J.J.; Porto, J.A.M.; Armache, J.N.; De Almeida, J.P.P.; Da Silva, F.F.; Olmo, R.P.; Faria, I.J.D.S.; De Carvalho, D.S.; Góes-Neto, A.; et al. Genome-Wide Identification of miRNAs and Target Regulatory Network in the Invasive Ectoparasitic Mite Varroa Destructor. Genomics 2021, 113, 2290–2303. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, A.R.; Hall, I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.; Hackenberg, M.; Langenberger, D.; Frishman, D. TargetSpy: A Supervised Machine Learning Approach for microRNA Target Prediction. BMC Bioinform. 2010, 11, 292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, D.O.; Ferreira, L.Y.M.; Rodrigues, G.V.P.; Santos, J.P.N.; Lopes, Í.S.; Amorim Conceição, L.B.d.; Chagas Moura, T.; de Faria, I.J.d.S.; Olmo, R.P.; Santana, W.C.; et al. Insights into miRNAs of the Stingless Bee Melipona quadrifasciata. Non-Coding RNA 2025, 11, 48. https://doi.org/10.3390/ncrna11030048
Soares DO, Ferreira LYM, Rodrigues GVP, Santos JPN, Lopes ÍS, Amorim Conceição LBd, Chagas Moura T, de Faria IJdS, Olmo RP, Santana WC, et al. Insights into miRNAs of the Stingless Bee Melipona quadrifasciata. Non-Coding RNA. 2025; 11(3):48. https://doi.org/10.3390/ncrna11030048
Chicago/Turabian StyleSoares, Dalliane Oliveira, Lucas Yago Melo Ferreira, Gabriel Victor Pina Rodrigues, João Pedro Nunes Santos, Ícaro Santos Lopes, Lucas Barbosa de Amorim Conceição, Tatyana Chagas Moura, Isaque João da Silva de Faria, Roenick Proveti Olmo, Weyder Cristiano Santana, and et al. 2025. "Insights into miRNAs of the Stingless Bee Melipona quadrifasciata" Non-Coding RNA 11, no. 3: 48. https://doi.org/10.3390/ncrna11030048
APA StyleSoares, D. O., Ferreira, L. Y. M., Rodrigues, G. V. P., Santos, J. P. N., Lopes, Í. S., Amorim Conceição, L. B. d., Chagas Moura, T., de Faria, I. J. d. S., Olmo, R. P., Santana, W. C., Costa, M. A., & Rocha Aguiar, E. R. G. (2025). Insights into miRNAs of the Stingless Bee Melipona quadrifasciata. Non-Coding RNA, 11(3), 48. https://doi.org/10.3390/ncrna11030048







