Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation?
Abstract
1. Introduction
2. Results
2.1. Differentially Expressed piRNAs
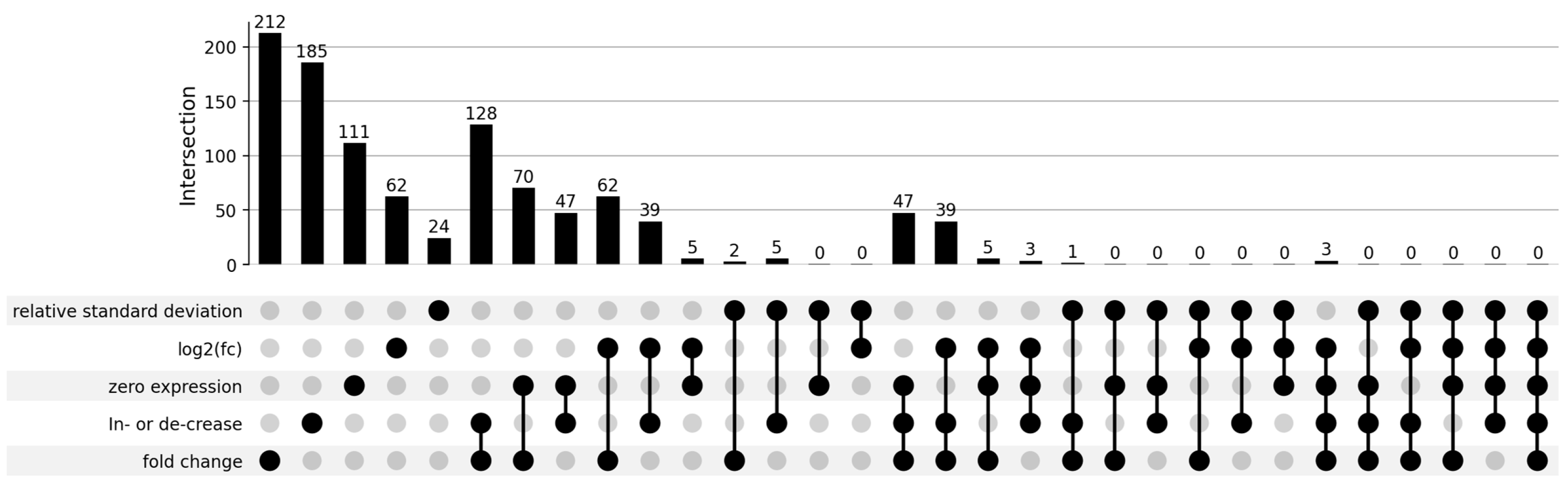
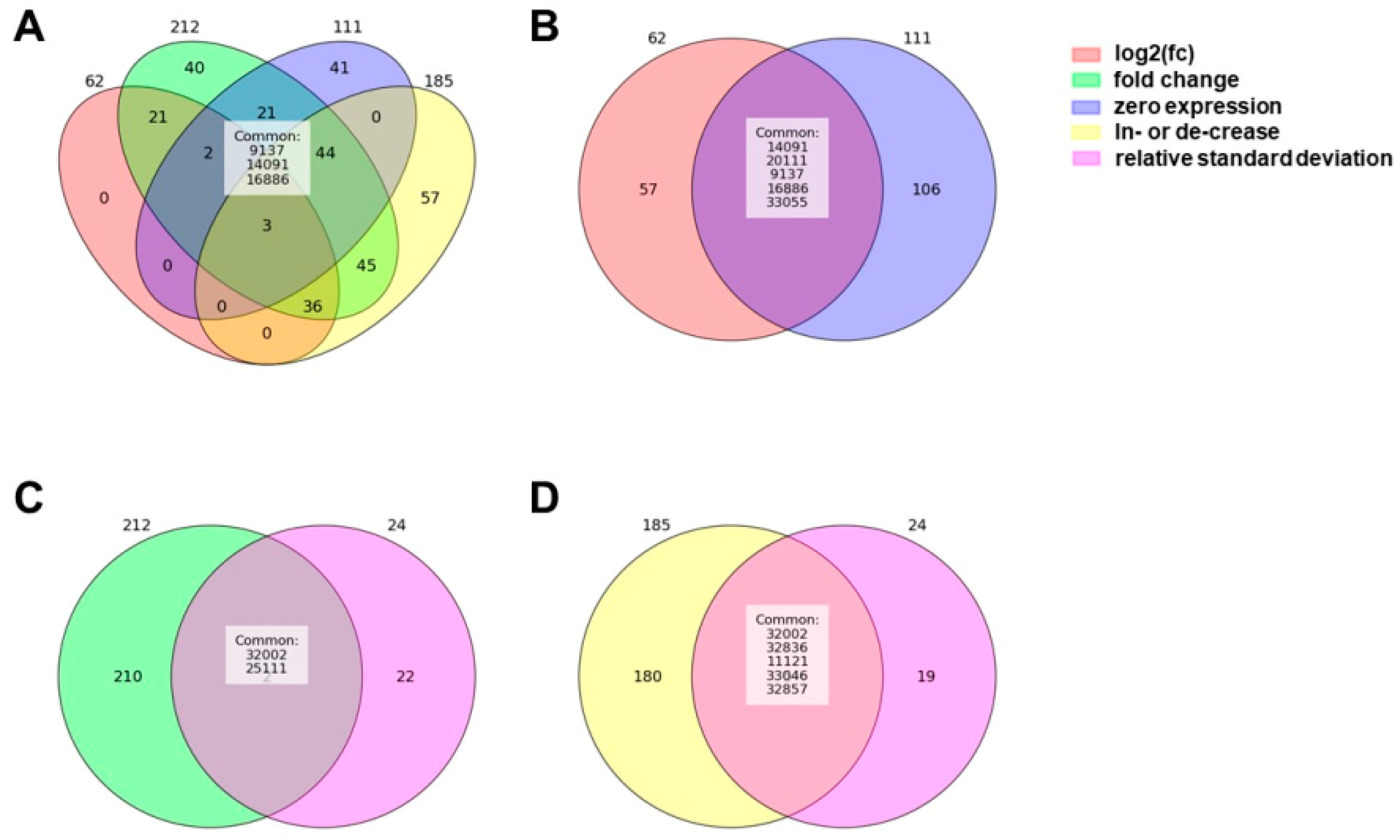
2.2. Plot-Based piRNA Selection
2.3. piRNA Characterization
2.4. Correlation with ΔV̇O2max
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Study Participants
4.2. Training Intervention Protocols
4.3. Diagnostics Blocks
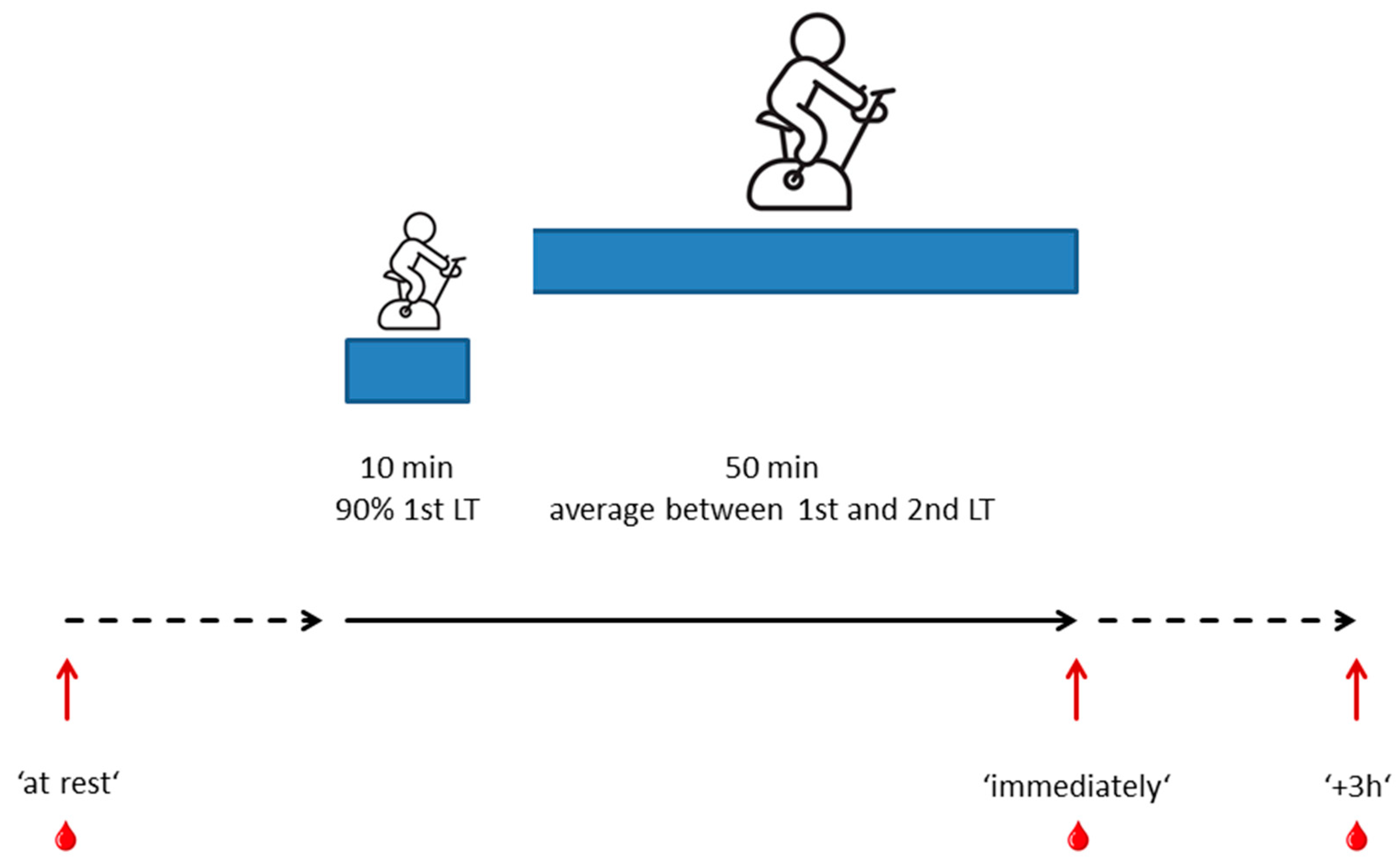
4.4. Standardized Reference Training Protocol and Collection of Blood Samples
4.5. Next-Generation Sequencing (NGS)
4.6. Criteria Selection for Evaluation of Differentially Expressed piRNAs
4.7. piRNA Selection
4.8. piRNA Characterization
4.9. Correlation Analysis
4.10. Data Visualization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PIWI | A family of proteins interacting with piRNAs |
| piRNA | PIWI-interacting RNA |
| piRNAdb | PIWI-interacting RNA Database |
| piRBase | A piRNA database with different naming conventions |
| HIIT | High-intensity interval training |
| MICT | Moderate-intensity continuous training |
| rRNA | Ribosomal RNA |
| sncRNA | Small non-coding RNA |
| miRNA | MicroRNA |
| lncRNA | Long non-coding RNA |
| tRNA | Transfer RNA |
| p-ERM | Protein-ERM (Ezrin, Radixin, Moesin) complex, related to cytoskeleton regulation |
| piRNA-Ls | piRNA-like small RNAs |
| hsa-piR-xxx | Human piRNA identification (e.g., hsa-piR-1677, hsa-piR-11119) |
| RER | Respiratory exchange ratio |
| NGS | Next-generation sequencing |
| HRmax | Maximum heart rate |
| V̇O2max | Maximal oxygen uptake |
| log2(fc) | Logarithmic base 2 fold change |
| qPCR | Quantitative polymerase chain reaction |
| NCBI | National Center for Biotechnology Information |
References
- Thiel, A.; Sudeck, G.; Gropper, H.; Maturana, F.M.; Schubert, T.; Srismith, D.; Widmann, M.; Behrens, S.; Martus, P.; Munz, B.; et al. The iReAct Study—A Biopsychosocial Analysis of the Individual Response to Physical Activity. Contemp. Clin. Trials Commun. 2020, 17, 100508. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Bredin, S.S.D. Reflections on Physical Activity and Health: What Should We Recommend? Can. J. Cardiol. 2016, 32, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Health Benefits of Physical Activity: The Evidence. Cmaj 2006, 174, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.E.R.; Nicol, C.W.; Bredin, S.S.D. Prescribing Exercise as Preventive Therapy. Cmaj 2006, 174, 961–974. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- WHO Guidelines on Physical Activity and Sedentary Behaviour. Available online: https://www.who.int/multi-media/details/who-guidelines-on-physical-activity-and-sedentary-behaviour (accessed on 17 December 2024).
- Dindorf, C.; Fröhlich, M. Zur Konnotation und Denotation des Trainingsbegriffs in Theorie und Praxis des Sports. Ger. J. Exerc. Sport Res. 2020, 50, 297–307. [Google Scholar] [CrossRef]
- Fröhlich, M. Überlegungen zum Trainingsbegriff und zur Theorie des Trainings aus Ökonomischer Perspektive; Lehmanns Media: Cologne, Germany, 2009. [Google Scholar]
- Buchheit, M.; Laursen, P.B. High-Intensity Interval Training, Solutions to the Programming Puzzle: Part I: Cardiopulmonary Emphasis. Sports Med. 2013, 43, 313–338. [Google Scholar] [CrossRef]
- Brauner, T.; Müller, J.; Beinert, K. Sportwissenschaft; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; ISBN 978-3-437-06007-6. [Google Scholar]
- Binder, R.K.; Wonisch, M.; Corra, U.; Cohen-Solal, A.; Vanhees, L.; Saner, H.; Schmid, J.-P. Methodological Approach to the First and Second Lactate Threshold in Incremental Cardiopulmonary Exercise Testing. Eur. J. Cardiovasc. Prev. Rehabil. 2008, 15, 726–734. [Google Scholar] [CrossRef]
- Caen, K.; Pogliaghi, S.; Lievens, M.; Vermeire, K.; Bourgois, J.G.; Boone, J. Ramp vs. Step Tests: Valid Alternatives to Determine the Maximal Lactate Steady-State Intensity? Eur. J. Appl. Physiol. 2021, 121, 1899–1907. [Google Scholar] [CrossRef]
- Mattioni Maturana, F.; Soares, R.N.; Murias, J.M.; Schellhorn, P.; Erz, G.; Burgstahler, C.; Widmann, M.; Munz, B.; Thiel, A.; Nieß, A.M. Responders and Non-Responders to Aerobic Exercise Training: Beyond the Evaluation of V̇O2max. Physiol. Rep. 2021, 9, e14951. [Google Scholar] [CrossRef]
- Widmann, M.; Mattioni Maturana, F.; Burgstahler, C.; Erz, G.; Schellhorn, P.; Fragasso, A.; Schmitt, A.; Nieß, A.M.; Munz, B. miRNAs as Markers for the Development of Individualized Training Regimens: A Pilot Study. Physiol. Rep. 2022, 10, e15217. [Google Scholar] [CrossRef] [PubMed]
- Wackerhage, H.; Woods, N.M. Exercise-Induced Signal Transduction and Gene Regulation in Skeletal Muscle. J. Sports Sci. Med. 2002, 1, 103–114. [Google Scholar]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in Human Disease and Prospects for Epigenetic Therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Castel, S.E.; Martienssen, R.A. RNA Interference in the Nucleus: Roles for Small RNAs in Transcription, Epigenetics and Beyond. Nat. Rev. Genet. 2013, 14, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sanjurjo, M.; de Gonzalo-Calvo, D.; Fernández-García, B.; Díez-Robles, S.; Martínez-Canal, Á.; Olmedillas, H.; Dávalos, A.; Iglesias-Gutiérrez, E. Circulating microRNA as Emerging Biomarkers of Exercise. Exerc. Sport Sci. Rev. 2018, 46, 160. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Senderowska, D.; Laguette, M.-J.N.; Jegier, A.; Cięszczyk, P.; September, A.V.; Brzeziańska-Lasota, E. MicroRNA Profile and Adaptive Response to Exercise Training: A Review. Int. J. Sports Med. 2019, 40, 227–235. [Google Scholar] [CrossRef]
- Sapp, R.M.; Shill, D.D.; Roth, S.M.; Hagberg, J.M. Circulating microRNAs in Acute and Chronic Exercise: More than Mere Biomarkers. J. Appl. Physiol. 2017, 122, 702–717. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Dávalos, A.; Montero, A.; García-González, Á.; Tyshkovska, I.; González-Medina, A.; Soares, S.M.A.; Martínez-Camblor, P.; Casas-Agustench, P.; Rabadán, M.; et al. Circulating Inflammatory miRNA Signature in Response to Different Doses of Aerobic Exercise. J. Appl. Physiol. 2015, 119, 124–134. [Google Scholar] [CrossRef]
- Brennecke, J.; Aravin, A.A.; Stark, A.; Dus, M.; Kellis, M.; Sachidanandam, R.; Hannon, G.J. Discrete Small RNA-Generating Loci as Master Regulators of Transposon Activity in Drosophila. Cell 2007, 128, 1089–1103. [Google Scholar] [CrossRef]
- Gainetdinov, I.; Colpan, C.; Arif, A.; Cecchini, K.; Zamore, P.D. A Single Mechanism of Biogenesis, Initiated and Directed by PIWI Proteins, Explains piRNA Production in Most Animals. Mol. Cell 2018, 71, 775–790.e5. [Google Scholar] [CrossRef]
- Hirakata, S.; Siomi, M.C. piRNA Biogenesis in the Germline: From Transcription of piRNA Genomic Sources to piRNA Maturation. Biochim. Biophys. Acta 2016, 1859, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S.; Siomi, M.C.; Siomi, H. piRNA Clusters and Open Chromatin Structure. Mob. DNA 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.Z.; Jiang, Y.; Kakumani, P.K. Somatic piRNA and PIWI-Mediated Post-Transcriptional Gene Regulation in Stem Cells and Disease. Front. Cell Dev. Biol. 2024, 12, 1495035. [Google Scholar] [CrossRef]
- Belicard, T.; Jareosettasin, P.; Sarkies, P. The piRNA Pathway Responds to Environmental Signals to Establish Intergenerational Adaptation to Stress. BMC Biol. 2018, 16, 103. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Porto, W.F.; Palu, C.C.; Pereira, L.M.; Petriz, B.; Almeida, J.A.; Viana, J.; Filho, N.N.A.; Franco, O.L.; Pereira, R.W. Effects of Acute Aerobic Exercise on Rats Serum Extracellular Vesicles Diameter, Concentration and Small RNAs Content. Front. Physiol. 2018, 9, 532. [Google Scholar] [CrossRef]
- Ingerslev, L.R.; Donkin, I.; Fabre, O.; Versteyhe, S.; Mechta, M.; Pattamaprapanont, P.; Mortensen, B.; Krarup, N.T.; Barrès, R. Endurance Training Remodels Sperm-Borne Small RNA Expression and Methylation at Neurological Gene Hotspots. Clin. Epigenetics 2018, 10, 12. [Google Scholar] [CrossRef]
- PIWI-Interacting RNA (piRNA) Database—piRNAdb. Available online: https://www.pirnadb.org/ (accessed on 27 January 2025).
- Mei, Y.; Wang, Y.; Kumari, P.; Shetty, A.C.; Clark, D.; Gable, T.; MacKerell, A.D.; Ma, M.Z.; Weber, D.J.; Yang, A.J.; et al. A piRNA-like Small RNA Interacts with and Modulates p-ERM Proteins in Human Somatic Cells. Nat. Commun. 2015, 6, 7316. [Google Scholar] [CrossRef]
- Girard, A.; Sachidanandam, R.; Hannon, G.J.; Carmell, M.A. A Germline-Specific Class of Small RNAs Binds Mammalian Piwi Proteins. Nature 2006, 442, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, F.; Rinaldi, A.; Marchese, G.; Coviello, E.; Sellitto, A.; Cordella, A.; Giurato, G.; Nassa, G.; Ravo, M.; Tarallo, R.; et al. Specific Patterns of PIWI-Interacting Small Noncoding RNA Expression in Dysplastic Liver Nodules and Hepatocellular Carcinoma. Oncotarget 2016, 7, 54650–54661. [Google Scholar] [CrossRef]
- Gainetdinov, I.V.; Skvortsova, Y.V.; Kondratieva, S.A.; Klimov, A.; Tryakin, A.A.; Azhikina, T.L. Assessment of piRNA Biogenesis and Function in Testicular Germ Cell Tumors and Their Precursor Germ Cell Neoplasia in Situ. BMC Cancer 2018, 18, 20. [Google Scholar] [CrossRef]
- Yin, J.; Qi, W.; Ji, C.-G.; Zhang, D.-X.; Xie, X.-L.; Ding, Q.; Jiang, X.-Y.; Han, J.; Jiang, H.-Q. Small RNA Sequencing Revealed Aberrant piRNA Expression Profiles in Colorectal Cancer. Oncol. Rep. 2019, 42, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Betts, S.D.; Basu, S.; Bolar, J.; Booth, R.; Chang, S.; Cigan, A.M.; Farrell, J.; Gao, H.; Harkins, K.; Kinney, A.; et al. Uniform Expression and Relatively Small Position Effects Characterize Sister Transformants in Maize and Soybean. Front. Plant Sci. 2019, 10, 1209. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, Y.; Lu, Q.; Wei, J.; Yang, H.; Gu, M. Detection of Stably Expressed piRNAs in Human Blood. Int. J. Clin. Exp. Med. 2015, 8, 13353–13358. [Google Scholar] [PubMed]
- Freedman, J.E.; Gerstein, M.; Mick, E.; Rozowsky, J.; Levy, D.; Kitchen, R.; Das, S.; Shah, R.; Danielson, K.; Beaulieu, L.; et al. Diverse Human Extracellular RNAs Are Widely Detected in Human Plasma. Nat. Commun. 2016, 7, 11106. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-Q.; Zhang, Y.-H.; Liu, F.; Ponnusamy, M.; Zhao, X.-M.; Zhou, L.-Y.; Zhai, M.; Liu, C.-Y.; Li, X.-M.; Wang, M.; et al. The piRNA CHAPIR Regulates Cardiac Hypertrophy by Controlling METTL3-Dependent N6-Methyladenosine Methylation of Parp10 mRNA. Nat. Cell Biol. 2020, 22, 1319–1331. [Google Scholar] [CrossRef] [PubMed]
- Martone, J.; Mariani, D.; Desideri, F.; Ballarino, M. Non-Coding RNAs Shaping Muscle. Front. Cell Dev. Biol. 2019, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Tosar, J.P.; Rovira, C.; Cayota, A. Non-Coding RNA Fragments Account for the Majority of Annotated piRNAs Expressed in Somatic Non-Gonadal Tissues. Commun. Biol. 2018, 1, 2. [Google Scholar] [CrossRef]
- Harriss, D.J.; MacSween, A.; Atkinson, G. Ethical Standards in Sport and Exercise Science Research: 2020 Update. Int. J. Sports Med. 2019, 40, 813–817. [Google Scholar] [CrossRef]
- Grieb, A.; Schmitt, A.; Fragasso, A.; Widmann, M.; Mattioni Maturana, F.; Burgstahler, C.; Erz, G.; Schellhorn, P.; Nieß, A.M.; Munz, B. Skeletal Muscle MicroRNA Patterns in Response to a Single Bout of Exercise in Females: Biomarkers for Subsequent Training Adaptation? Biomolecules 2023, 13, 884. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]


| ID | Group | Gender | Age | Baseline Weight [kg] | Baseline Height [cm] | Baseline BMI [kg/m2] | Baseline Absolute V̇O2max [L/min] | Baseline Relative V̇O2max [L/kgxmin] | FU1 Absolute V̇O2max [L/min] | FU1 Relative V̇O2max [L/kgxmin] |
|---|---|---|---|---|---|---|---|---|---|---|
| IR0005 | MICT-HIIT | Female | 22 | 61.2 | 167.5 | 21.80 | 2.03 | 33.2 | 2.39 | 38.9 |
| IR0010 | MICT-HIIT | Female | 21 | 66.5 | 163 | 25.03 | 2.04 | 30.65 | 2.23 | 34.02 |
| IR0030 | MICT-HIIT | Female | 29 | 60.9 | 159 | 24.09 | 2.04 | 33.55 | 2.19 | 36.24 |
| IR0046 | MICT-HIIT | Female | 26 | 66.2 | 174 | 21.8 | 2.03 | 30.71 | 2.28 | 34.13 |
| IR0008 | HIIT-MICT | Female | 20 | 59.3 | 164.5 | 21.9 | 1.95 | 32.82 | 2.28 | 37.62 |
| IR0012 | HIIT-MICT | Female | 21 | 68.9 | 173.5 | 22.8 | 2.01 | 29.15 | 2.25 | 33.16 |
| IR0015 | HIIT-MICT | Female | 28 | 61.7 | 174.2 | 20.3 | 1.97 | 31.93 | 2.30 | 36.62 |
| IR0042 | HIIT-MICT | Female | 27 | 57.6 | 160.4 | 22.3 | 1.77 | 30.8 | 1.94 | 34.97 |
| 1st |
| Reduce data set to piRNA data |
| 2nd |
| Calculate descriptive statistics |
| sum |
| mean |
| standard deviation |
| variance |
| fold change |
| log2(fc) |
| 3rd |
| Testing several selection criteria |
| relative standard deviation |
| log2(fc) |
| zero expression |
| increase/decrease |
| fold change |
| 4th |
| Comparing piRNA lists of different criteria |
| 5th |
| Deciding on criteria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedel, C.E.; Ibáñez, J.; Fragasso, A.; Schmitt, A.; Widmann, M.; Mattioni Maturana, F.; Niess, A.M.; Munz, B. Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation? Non-Coding RNA 2025, 11, 46. https://doi.org/10.3390/ncrna11030046
Riedel CE, Ibáñez J, Fragasso A, Schmitt A, Widmann M, Mattioni Maturana F, Niess AM, Munz B. Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation? Non-Coding RNA. 2025; 11(3):46. https://doi.org/10.3390/ncrna11030046
Chicago/Turabian StyleRiedel, Caroline Eva, Javier Ibáñez, Annunziata Fragasso, Angelika Schmitt, Manuel Widmann, Felipe Mattioni Maturana, Andreas M. Niess, and Barbara Munz. 2025. "Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation?" Non-Coding RNA 11, no. 3: 46. https://doi.org/10.3390/ncrna11030046
APA StyleRiedel, C. E., Ibáñez, J., Fragasso, A., Schmitt, A., Widmann, M., Mattioni Maturana, F., Niess, A. M., & Munz, B. (2025). Patterns of Circulating piRNAs in the Context of a Single Bout of Exercise: Potential Biomarkers of Exercise-Induced Adaptation? Non-Coding RNA, 11(3), 46. https://doi.org/10.3390/ncrna11030046






