Regulatory RNAs in Cardiovascular Development and Disease
A topical collection in Non-Coding RNA (ISSN 2311-553X). This collection belongs to the section "Evolution of Non-Coding RNA".
Viewed by 62958Editors
Interests: microRNAs; cardiovascular disease
Special Issues, Collections and Topics in MDPI journals
Interests: non-coding RNAs; cardiovascular diseases; infectious diseases; cell-to-cell communication; circulating RNAs; biomarkers
Special Issues, Collections and Topics in MDPI journals
Interests: microRNA; endothelial cells; angiogenesis; vascular biology; ischemic injury; extracellular vesicles
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
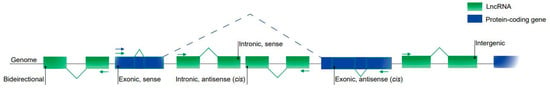
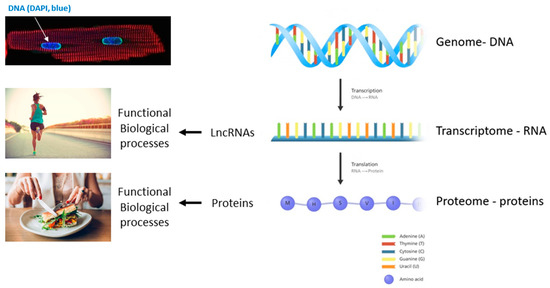
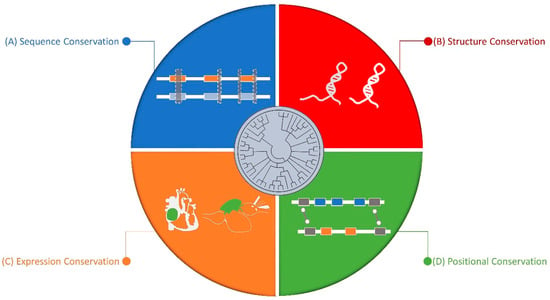
Regulatory RNAs, including microRNAs (miRNAs), long non-coding RNAs (lncRNAs) and circular RNAs (circRNAs), have attracted a great deal of attention from the cardiovascular community. They can be expressed in an organ-specific manner and participate in the regulation of important biological processes.
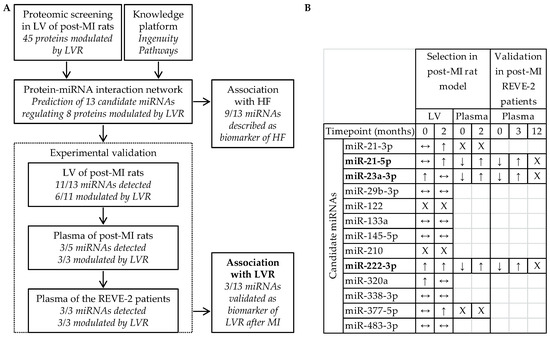
Several regulatory RNAs are dysregulated in many cardiovascular diseases, such as heart failure, hypertension, coronary artery disease, and myocardial infarction, suggesting that they might play an important role in regulating the expression of genes involved in such diseases.
In the last few years, different studies have demonstrated the importance of regulatory RNAs in cardiac development and cell differentiation.
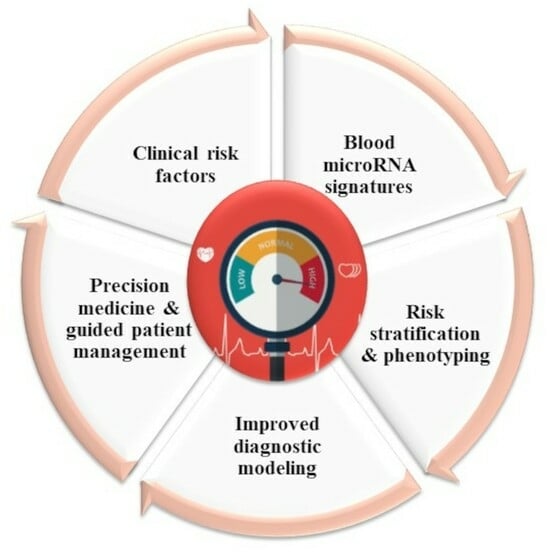
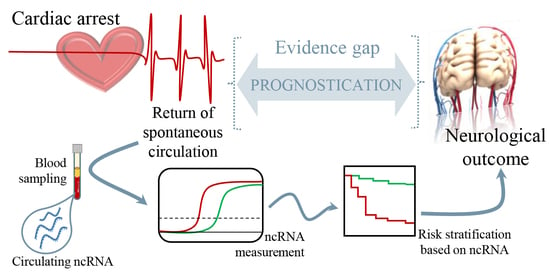
The observation that regulatory RNAs can be found circulating in the blood supports their potential as disease markers. Recent studies present encouraging results for disease prognosis and therapy.
This Topical Collection is focused on the understanding of the role of regulatory RNAs (miRNAs, lncRNAs, circRNAs) in cardiovascular development and disease and their potential as biomarkers and therapeutic targets. We will consider original manuscripts, as well as reviews. Topics of interest include:
- Regulatory RNAs in cardiovascular development
- Regulatory RNAs in cardiovascular disease
- Cardiovascular disease therapeutics involving regulatory RNAs
- Regulatory RNAs as cardiovascular disease biomarkers
Collection Editor
Prof. Dr. Francisco J. Enguita
Dr. Andrea Caporali
Co-Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Non-Coding RNA is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 1800 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- heart
- vascular system
- cardiovascular disease
- non-coding RNAs
- regulatory RNAs
- miRNAs
- lncRNAs
- circRNAs
- biomarkers
- functional genomics
- transcriptomics
- regulation of gene expression
- collaboration
- networking
- precision medicine