Role of ncRNAs in the Development of Chronic Pain
Abstract
1. Introduction
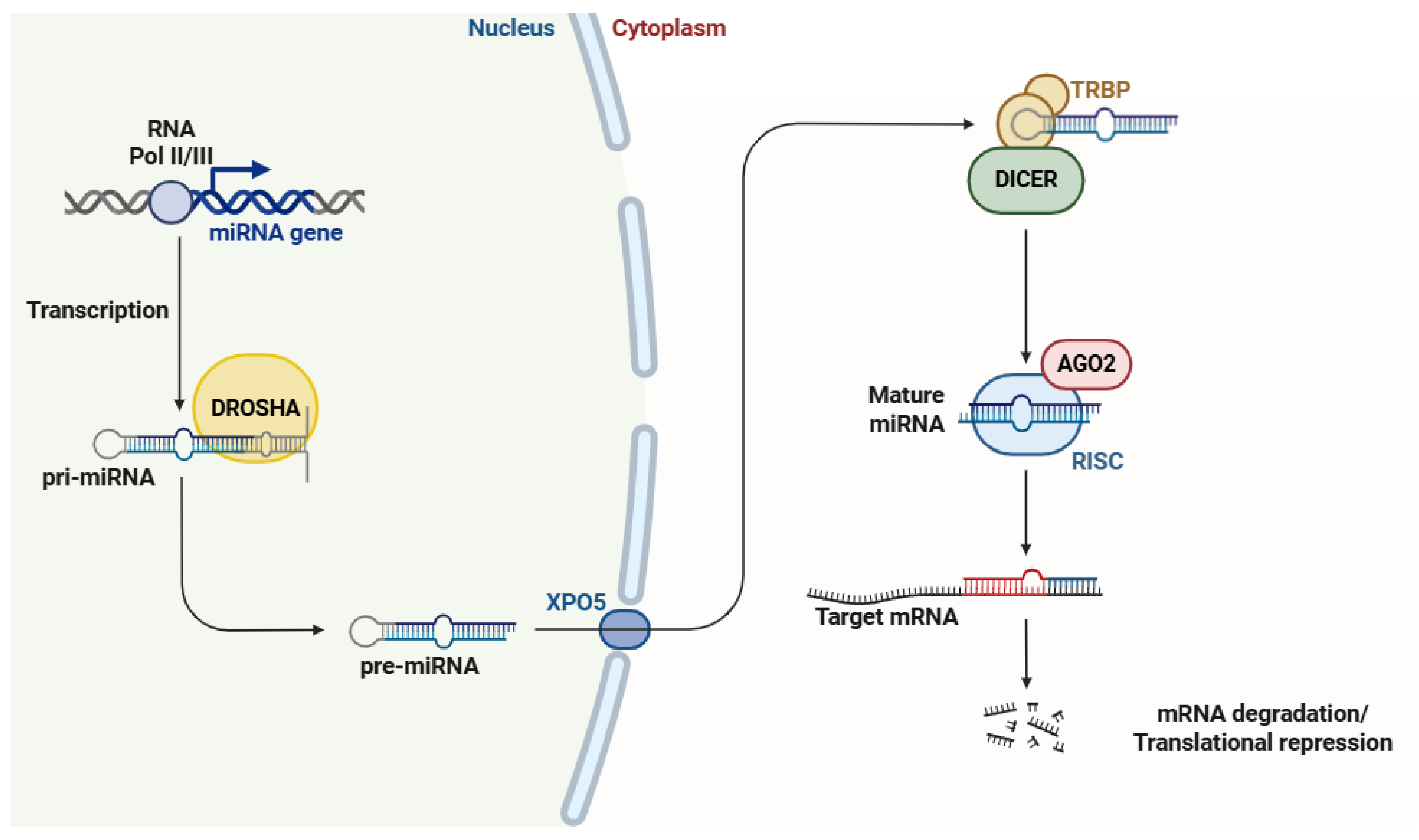
2. MicroRNAs (miRNAs)
3. Small Interfering RNAs (siRNAs)
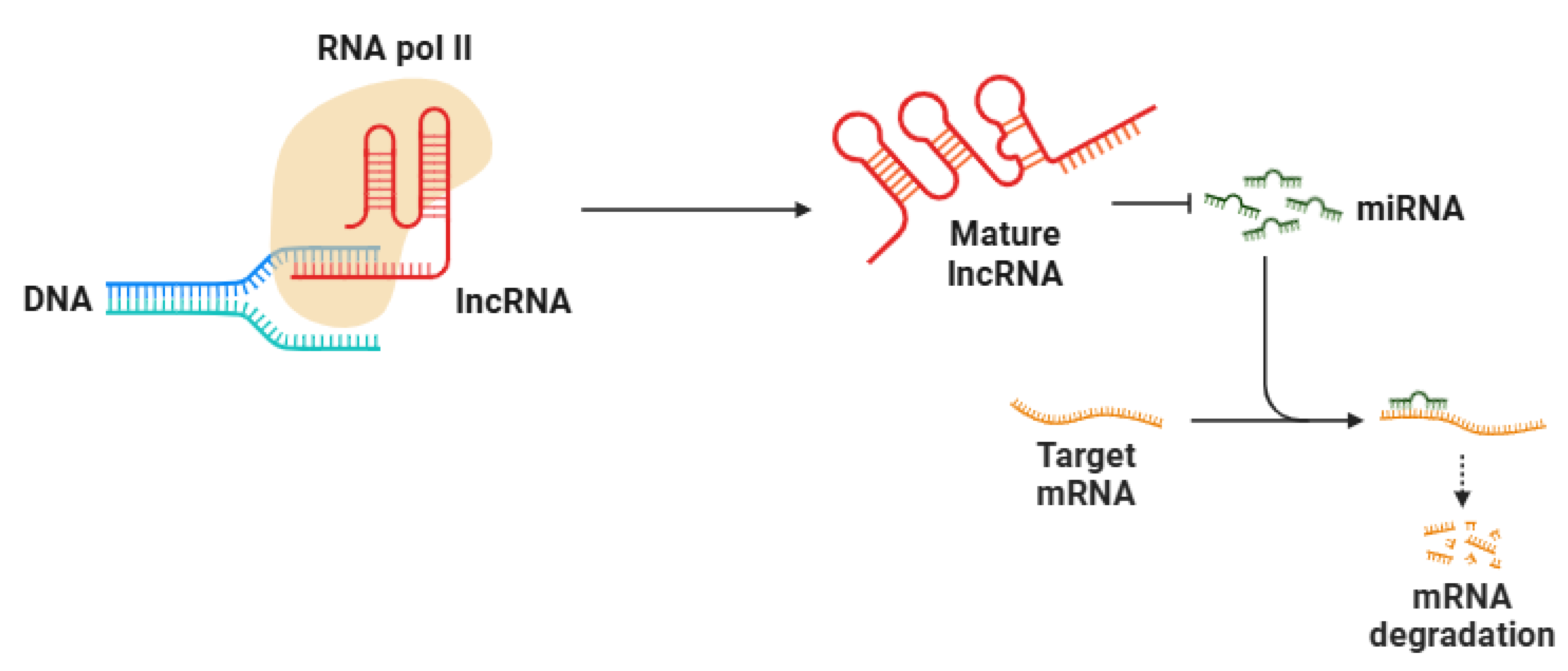
4. Long Non-Coding RNAs (lncRNAs)
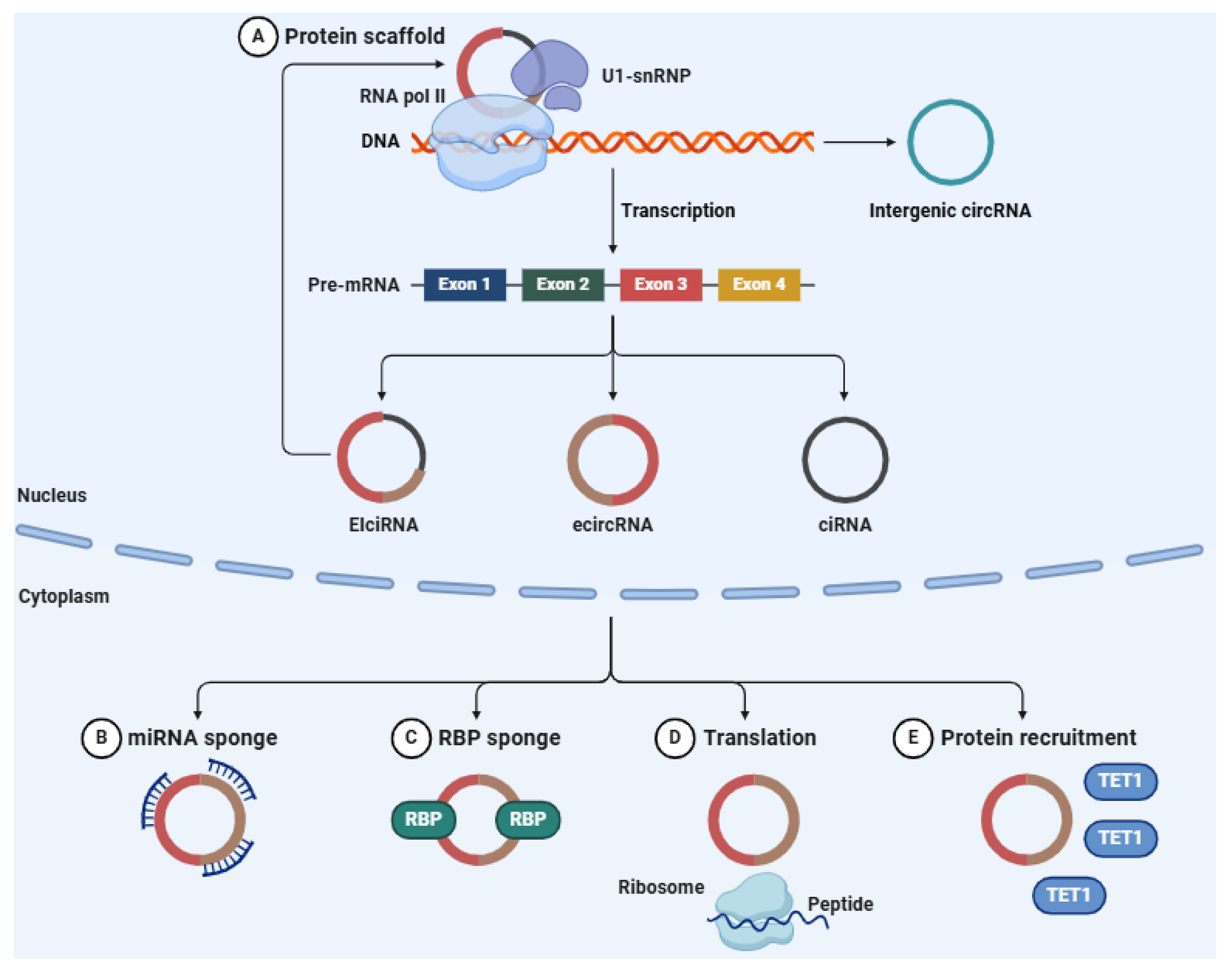
5. Circular RNAs (circRNAs)
6. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3′-UTR | 3′-untranslated region |
| AAV | Adeno-associated virus |
| AGO | Argonaute |
| AGO2 | Argonaute 2 |
| AIA | Adjuvant-induced arthritis |
| AKT | Protein kinase B |
| ALB | Albumin |
| AP-1 | Activator protein 1 |
| ASIC | Acid-sensing ion channel |
| BAI1 | Brain-specific angiogenesis inhibitor 1 |
| bCCI | Bilateral chronic constriction injury |
| BCP | Bone cancer pain |
| BTZ | Bortezomib |
| CACNA1H | Calcium voltage-gated channel subunit alpha1 H |
| CCD | Chronic compression of the DRG |
| CCI | Chronic constriction injury |
| CCI-IoN | Chronic constriction injury of the infraorbital nerve |
| CCL2 | C-C motif chemokine ligand 2 |
| ceRNA | Competing endogenous RNA |
| CFA | Complete Freund’s adjuvant |
| c-Fos | FBJ murine osteosarcoma viral oncogene homolog |
| ChIRP | Chromatin isolation by RNA purification |
| ciRNA | Circular intronic RNA |
| circRNA | Circular RNA |
| CIPN | Chemotherapy-induced neuropathic pain |
| CIVP | Chronic inflammatory visceral pain |
| CLIP | Class II-associated invariant chain peptide |
| CNS | Central nervous system |
| COX-2 | Cyclooxygenase 2 |
| CRC | Colorectal cancer |
| CREB | cAMP response element binding protein |
| CXCL13 | C-X-C motif chemokine ligand 13 |
| CXCL9 | C-X-C motif chemokine ligand 9 |
| CXCR5 | C-X-C chemokine receptor type 5 |
| DGCR8 | DiGeorge Syndrome critical region 8 |
| DNA | Deoxyribonucleic acid |
| DPN | Diabetic peripheral neuropathy |
| DRG | Dorsal root ganglion |
| DHX9 | DEAH-box helicase 9 |
| dsRNA | Double-strand RNA |
| ecircRNA | Exonic circRNA |
| EIciRNA | Exon-intron circRNA |
| ENO1 | Enolase 1 |
| ERK | Extracellular signal-regulated kinase |
| EZH2 | Enhancer of zeste homolog 2 |
| FUS | Fused in sarcoma |
| GAD65 | Glutamate decarboxylase 65 |
| GFAP | Glial fibrillary acidic protein |
| GTP | Guanosine triphosphate |
| IASP | International Association for the Study of Pain |
| IBA-1 | Ionized calcium binding adapter molecule 1 |
| IFT52 | Intraflagellar transport 52 |
| IFT88 | Intraflagellar transport 88 |
| IKBKB | Inhibitor of NF-κB |
| IL-1β | Interleukin 1 beta |
| IL-10 | Interleukin 10 |
| IL-6 | Interleukin 6 |
| IRES | Internal ribosome entry site |
| IST1 | Increased sodium tolerance 1 |
| JAK2 | Janus kinase 2 |
| JMJD1A | Jumonji domain containing 1A |
| KCC2 | Potassium chloride cotransporter 2 |
| KCNK1 | Potassium channel, two-pore domain subfamily K, member 1 |
| L5-VRT | L5 ventral root transection |
| lncRNA | Long non-coding RNA |
| LC3-II | Microtubule-associated protein 1 light chain 3-II |
| LPAR3 | Lysophosphatidic acid receptor 3 |
| LPS | Lipopolysaccharide |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| m6A | N6-methyladenosine |
| MBL | Mannose-binding lectin |
| MEG3 | Maternally expressed gene 3 |
| miRNA | MicroRNA |
| mRNA | Messenger RNA |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| ncRNA | Non-coding RNA |
| NEAT1 | Nuclear enriched abundant transcript 1 |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NK1R | Neurokinin 1 receptor |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NR2B | N-methyl-D-aspartate receptor subunit 2B |
| ORF | Open reading frame |
| OX42 | OX42 antigen/CD11b |
| p62 | Sequestosome 1 (SQSTM1) |
| PANX1 | Pannexin 1 |
| PARP1 | Poly(ADP-ribose) polymerase 1 |
| p-ERK | Phosphorylated extracellular signal-regulated kinase |
| PDN | Painful diabetic neuropathy |
| PI3K | Phosphoinositide 3-kinase |
| PI3KCB | Phosphoinositide 3-kinase catalytic subunit beta |
| PKB | Protein kinase B |
| PKC | Protein kinase C |
| PKM2 | Pyruvate kinase M2 |
| PNS | Peripheral nervous system |
| PRC2 | Polycomb repressive complex 2 |
| pre-mRNA | Precursor messenger RNA |
| pre-miRNA | Precursor microRNA |
| pri-miRNA | Primary microRNA |
| pSNL | Partial spinal nerve ligation |
| PVT1 | Plasmacytoma variant translocation 1 |
| Ran | Ras-related nuclear protein |
| RBP | RNA-binding protein |
| RdRPs | RNA-dependent RNA polymerase |
| RISC | RNA-induced silencing complex |
| RNA | Ribonucleic acid |
| RNA Pol II | RNA polymerase II |
| RNA Pol III | RNA polymerase III |
| RNA-seq | RNA sequencing |
| SCI | Spinal cord injury |
| SDH | Spinal dorsal horn |
| SGK3 | Serum/glucocorticoid regulated kinase family member 3 |
| siRNA | Small interfering RNA |
| SLICK | Sequence like an intermediate conductance K channel |
| SNHG1 | Small nucleolar RNA host gene 1 |
| SNI | Spared nerve injury |
| SNL | Spinal nerve ligation |
| SOCS3 | Suppressor of cytokine signaling 3 |
| STAT3 | Signal transducer and activator of transcription 3 |
| STZ | Streptozotocin |
| TET1 | Ten-eleven translocation methylcytosine dioxygenase 1 |
| TLR4 | Toll-like receptor 4 |
| TNFAIP1 | Tumor necrosis factor alpha induced protein 1 |
| TNF-α | Tumor necrosis factor alpha |
| TRBP | Transactivation response element RNA-binding protein |
| TRPV1 | Transient receptor potential vanilloid 1 |
| U1-snNRP | U1 small nuclear ribonucleoprotein |
| UBR5 | Ubiquitin protein ligase E3 component N-recognin 5 |
| VEGFB | Vascular endothelial growth factor B |
| WNT5A | Wnt family member 5A |
| Ybx1 | Y-Box binding protein 1 |
| XPO5 | Exportin 5 |
References
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Vader, K.; Bostick, G.P.; Carlesso, L.C.; Hunter, J.; Mesaroli, G.; Perreault, K.; Tousignant-Laflamme, Y.; Tupper, S.; Walton, D.M.; Wideman, T.H.; et al. The Revised IASP Definition of Pain and Accompanying Notes: Considerations for the Physiotherapy Profession. Physiother. Can. 2021, 73, 103–106. [Google Scholar] [CrossRef]
- Malfliet, A.; Coppieters, I.; Van Wilgen, P.; Kregel, J.; De Pauw, R.; Dolphens, M.; Ickmans, K. Brain changes associated with cognitive and emotional factors in chronic pain: A systematic review. Eur. J. Pain 2017, 21, 769–786. [Google Scholar] [CrossRef]
- Lee, G.I.; Neumeister, M.W. Pain: Pathways and physiology. Clin. Plast. Surg. 2020, 47, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, P.; Hu, Y.; Meng, J. Pain Modulates Responses to Emotional Stimuli. Front. Psychol. 2020, 11, 595987. [Google Scholar] [CrossRef]
- Nikolenko, V.N.; Shelomentseva, E.M.; Tsvetkova, M.M.; Abdeeva, E.I.; Giller, D.B.; Babayeva, J.V.; Achkasov, E.E.; Gavryushova, L.V.; Sinelnikov, M.Y. Nociceptors: Their Role in Body’s Defenses, Tissue Specific Variations and Anatomical Update. J. Pain Res. 2022, 15, 867–877. [Google Scholar] [CrossRef]
- Middleton, S.J.; Barry, A.M.; Comini, M.; Li, Y.; Ray, P.R.; Shiers, S.; Themistocleous, A.C.; Uhelski, M.L.; Yang, X.; Dougherty, P.M.; et al. Studying human nociceptors: From fundamentals to clinic. Brain 2021, 144, 1312–1335. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef]
- De Ridder, D.; Adhia, D.; Vanneste, S. The anatomy of pain and suffering in the brain and its clinical implications. Neurosci. Biobehav. Rev. 2021, 130, 125–146. [Google Scholar] [CrossRef]
- Orr, P.M.; Shank, B.C.; Black, A.C. The Role of Pain Classification Systems in Pain Management. Crit. Care Nurs. Clin. N. Am. 2017, 29, 407–418. [Google Scholar] [CrossRef]
- Mears, L.; Mears, J. The pathophysiology, assessment, and management of acute pain. Br. J. Nurs. 2023, 32, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.E.E.; Nicolson, K.P.; Smith, B.H. Chronic pain: A review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019, 123, e273–e283. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Robbins, S.R.; McDougall, J.J. Osteoarthritis: The genesis of pain. Rheumatology 2018, 57, iv43–iv50. [Google Scholar] [CrossRef]
- Nicol, V.; Verdaguer, C.; Daste, C.; Bisseriex, H.; Lapeyre, É.; Lefèvre-Colau, M.M.; Rannou, F.; Rören, A.; Facione, J.; Nguyen, C. Chronic Low Back Pain: A Narrative Review of Recent International Guidelines for Diagnosis and Conservative Treatment. J. Clin. Med. 2023, 12, 1685. [Google Scholar] [CrossRef]
- García-Domínguez, M. A Comprehensive Analysis of Fibromyalgia and the Role of the Endogenous Opioid System. Biomedicines 2025, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Mestdagh, F.; Steyaert, A.; Lavand’homme, P. Cancer Pain Management: A Narrative Review of Current Concepts, Strategies, and Techniques. Curr. Oncol. 2023, 30, 6838–6858. [Google Scholar] [CrossRef]
- Crofford, L.J. Chronic Pain: Where the Body Meets the Brain. Trans. Am. Clin. Climatol. Assoc. 2015, 126, 167–183. [Google Scholar]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef]
- Larsson, C.; Hansson, E.E.; Sundquist, K.; Jakobsson, U. Chronic pain in older adults: Prevalence, incidence, and risk factors. Scand. J. Rheumatol. 2017, 46, 317–325. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Tinnirello, A.; Mazzoleni, S.; Santi, C. Chronic Pain in the Elderly: Mechanisms and Distinctive Features. Biomolecules 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Chronic pain in the elderly: Exploring cellular and molecular mechanisms and therapeutic perspectives. Front. Aging 2024, 5, 1477017. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.R.; Davis, K.D. Sex and gender differences in pain. Int. Rev. Neurobiol. 2022, 164, 277–307. [Google Scholar]
- Khan, J.S.; Hah, J.M.; Mackey, S.C. Effects of smoking on patients with chronic pain: A propensity-weighted analysis on the Collaborative Health Outcomes Information Registry. Pain 2019, 160, 2374–2379. [Google Scholar] [CrossRef]
- Karimi, R.; Mallah, N.; Nedjat, S.; Beasley, M.J.; Takkouche, B. Association between alcohol consumption and chronic pain: A systematic review and meta-analysis. Br. J. Anaesth. 2022, 129, 355–365. [Google Scholar] [CrossRef]
- Okifuji, A.; Hare, B.D. The association between chronic pain and obesity. J. Pain Res. 2015, 8, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm. 2023, 4, e292. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-coding RNAs in disease: From mechanisms to therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Nirvanie-Persaud, L.; Millis, R.M. Epigenetics and Pain: New Insights to an Old Problem. Cureus 2022, 14, e29353. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Mostafavi, E.; Aref, A.R.; Sethi, G.; Wang, L.; Tergaonkar, V. Non-coding RNA-based regulation of inflammation. Semin. Immunol. 2022, 59, 101606. [Google Scholar] [CrossRef]
- Nayak, M.; Das, D.; Pradhan, J.; Ahmed, R.G.; Laureano-Melo, R.; Dandapat, J. Epigenetic signature in neural plasticity: The journey so far and journey ahead. Heliyon 2022, 8, e12292. [Google Scholar] [CrossRef]
- Giordano, R.; Kjær-Staal Petersen, K.; Arendt-Nielsen, L. The link between epigenetics, pain sensitivity and chronic pain. Scand. J. Pain 2022, 22, 664–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, R.; Zhou, R.; Chen, H.; Liu, C.; Zhu, T.; Chen, C. The emerging power and promise of non-coding RNAs in chronic pain. Front. Mol. Neurosci. 2022, 15, 1037929. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sahoo, S.S.; Chauss, D.; Kazemian, M.; Afzali, B. Non-coding RNAs in immunoregulation and autoimmunity: Technological advances and critical limitations. J. Autoimmun. 2023, 134, 102982. [Google Scholar] [CrossRef] [PubMed]
- Musgrove, M.R.B.; Mikhaylova, M.; Bredy, T.W. Fundamental Neurochemistry Review: At the intersection between the brain and the immune system: Non-coding RNAs spanning learning, memory and adaptive immunity. J. Neurochem. 2024, J168, 961–976. [Google Scholar] [CrossRef]
- Li, X.; Jin, D.S.; Eadara, S.; Caterina, M.J.; Meffert, M.K. Regulation by noncoding RNAs of local translation, injury responses, and pain in the peripheral nervous system. Neurobiol. Pain 2023, 13, 100119. [Google Scholar] [CrossRef]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, Homeostasis, and Degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef]
- Mishra, R.; Kumar, A.; Ingle, H.; Kumar, H. The Interplay Between Viral-Derived miRNAs and Host Immunity During Infection. Front. Immunol. 2020, 10, 3079. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat. Rev. Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004, 23, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Huang, C.; Xia, X.G. A tightly regulated Pol III promoter for synthesis of miRNA genes in tandem. Biochim. Biophys. Acta 2008, 1779, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.; Qin, Y.; Macara, I.G.; Cullen, B.R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003, 17, 3011–3016. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Liu, C.P.; Wang, H.W.; Xu, R.M. Structural Basis for pri-miRNA Recognition by Drosha. Mol. Cell 2020, 78, 423–433.e5. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, H.; Kim, H.; Kim, V.N.; Roh, S.H. Structure of the human DICER-pre-miRNA complex in a dicing state. Nature 2023, 615, 331–338. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Gu, S.; Jin, L.; Zhang, F.; Sarnow, P.; Kay, M.A. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nat. Struct. Mol. Biol. 2009, 16, 144–150. [Google Scholar] [CrossRef]
- Buhagiar, A.F.; Kleaveland, B. To kill a microRNA: Emerging concepts in target-directed microRNA degradation. Nucleic Acids Res. 2024, 52, 1558–1574. [Google Scholar] [CrossRef]
- Naeli, P.; Winter, T.; Hackett, A.P.; Alboushi, L.; Jafarnejad, S.M. The intricate balance between microRNA-induced mRNA decay and translational repression. FEBS J. 2023, 290, 2508–2524. [Google Scholar] [CrossRef]
- Tuli, H.S.; Garg, V.K.; Bhushan, S.; Uttam, V.; Sharma, U.; Jain, A.; Sak, K.; Yadav, V.; Lorenzo, J.M.; Dhama, K.; et al. Natural flavonoids exhibit potent anticancer activity by targeting microRNAs in cancer: A signature step hinting towards clinical perfection. Transl. Oncol. 2023, 27, 101596. [Google Scholar] [CrossRef]
- Galagali, H.; Kim, J.K. The multifaceted roles of microRNAs in differentiation. Curr. Opin. Cell Biol. 2020, 67, 118–140. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.; Hajizadeh, F.; Ghaffarei, S.; Amin Doustvandi, M.; Hajizadeh, K.; Yaghoubi, S.M.; Mohammadnejad, F.; Khiabani, N.A.; Mousavi, P.; Baradaran, B. MicroRNAs and their vital role in apoptosis in hepatocellular carcinoma: miRNA-based diagnostic and treatment methods. Gene 2023, 888, 147803. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Sharp, P.A. MicroRNA functions in stress responses. Mol. Cell 2010, 40, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gaál, Z. Role of microRNAs in Immune Regulation with Translational and Clinical Applications. Int. J. Mol. Sci. 2024, 25, 1942. [Google Scholar] [CrossRef]
- Rahimian, N.; Nahand, J.S.; Hamblin, M.R.; Mirzaei, H. Exosomal MicroRNA Profiling. Methods Mol. Biol. 2023, 2595, 13–47. [Google Scholar]
- Shu, Z.; Tan, J.; Miao, Y.; Zhang, Q. The role of microvesicles containing microRNAs in vascular endothelial dysfunction. J. Cell. Mol. Med. 2019, 23, 7933–7945. [Google Scholar] [CrossRef]
- Layne, T.R.; Green, R.A.; Lewis, C.A.; Nogales, F.; Dawson Cruz, T.C.; Zehner, Z.E.; Seashols-Williams, S.J. microRNA Detection in Blood, Urine, Semen, and Saliva Stains After Compromising Treatments. J. Forensic Sci. 2019, 64, 1831–1837. [Google Scholar] [CrossRef]
- Klein, M.; Chandradoss, S.D.; Depken, M.; Joo, C. Why Argonaute is needed to make microRNA target search fast and reliable. Semin. Cell. Dev. Biol. 2017, 65, 20–28. [Google Scholar] [CrossRef]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef]
- Li, S.; Lei, Z.; Sun, T. The role of microRNAs in neurodegenerative diseases: A review. Cell. Biol. Toxicol. 2023, 39, 53–83. [Google Scholar] [CrossRef] [PubMed]
- Searles, C.D. MicroRNAs and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2024, 26, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, H.; Zhao, M.; Chang, C.; Lu, Q. Clinical significance of miRNAs in autoimmunity. J. Autoimmun. 2020, 109, 102438. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Zhang, R.; Dai, X.; Chen, W.; Xing, D. Circulating microRNAs: Biomarkers of disease. Clin. Chim. Acta 2021, 516, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ekiz Kanik, F.; Celebi, I.; Sevenler, D.; Tanriverdi, K.; Lortlar Ünlü, N.; Freedman, J.E.; Ünlü, M.S. Attomolar sensitivity microRNA detection using real-time digital microarrays. Sci. Rep. 2022, 12, 16220. [Google Scholar] [CrossRef]
- Androvic, P.; Benesova, S.; Rohlova, E.; Kubista, M.; Valihrach, L. Small RNA-Sequencing for Analysis of Circulating miRNAs: Benchmark Study. J. Mol. Diagn. 2022, 24, 386–394. [Google Scholar] [CrossRef]
- Stebel, S.; Breuer, J.; Rossbach, O. Studying miRNA-mRNA Interactions: An Optimized CLIP-Protocol for Endogenous Ago2-Protein. Methods Protoc. 2022, 5, 96. [Google Scholar] [CrossRef]
- Riolo, G.; Cantara, S.; Marzocchi, C.; Ricci, C. miRNA Targets: From Prediction Tools to Experimental Validation. Methods Protoc. 2020, 4, 1. [Google Scholar] [CrossRef]
- Martino, E.; D’Onofrio, N.; Anastasio, C.; Abate, M.; Zappavigna, S.; Caraglia, M.; Balestrieri, M.L. MicroRNA-nanoparticles against cancer: Opportunities and challenges for personalized medicine. Mol. Ther. Nucleic Acids 2023, 32, 371–384. [Google Scholar] [CrossRef]
- Sabina, S.; Panico, A.; Mincarone, P.; Leo, C.G.; Garbarino, S.; Grassi, T.; Bagordo, F.; De Donno, A.; Scoditti, E.; Tumolo, M.R. Expression and Biological Functions of miRNAs in Chronic Pain: A Review on Human Studies. Int. J. Mol. Sci. 2022, 23, 6016. [Google Scholar] [CrossRef]
- López-González, M.J.; Landry, M.; Favereaux, A. MicroRNA and chronic pain: From mechanisms to therapeutic potential. Pharmacol. Ther. 2017, 180, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kiyosawa, N.; Watanabe, K.; Toyama, K.; Ishizuka, H. Circulating miRNA Signature as a Potential Biomarker for the Prediction of Analgesic Efficacy of Hydromorphone. Int. J. Mol. Sci. 2019, 20, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Shenoda, B.B.; Ajit, S.K. Overview of microRNA Modulation in Analgesic Research. Curr. Protoc. Pharmacol. 2017, 79, 9.25.1–9.25.10. [Google Scholar] [CrossRef]
- Luo, M.; Li, L.; Ding, M.; Niu, Y.; Xu, X.; Shi, X.; Shan, N.; Qiu, Z.; Piao, F.; Zhang, C. Long-term potentiation and depression regulatory microRNAs were highlighted in Bisphenol A induced learning and memory impairment by microRNA sequencing and bioinformatics analysis. PLoS ONE 2023, 18, e0279029. [Google Scholar] [CrossRef]
- Ramanathan, S.; Ajit, S.K. MicroRNA-Based Biomarkers in Pain. Adv. Pharmacol. 2016, 75, 5–62. [Google Scholar]
- Sakai, A.; Saitow, F.; Miyake, N.; Miyake, K.; Shimada, T.; Suzuki, H. miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain 2013, 136, 2738–2750. [Google Scholar] [CrossRef]
- Cai, L.; Liu, X.; Guo, Q.; Huang, Q.; Zhang, Q.; Cao, Z. MiR-15a attenuates peripheral nerve injury-induced neuropathic pain by targeting AKT3 to regulate autophagy. Genes Genom. 2020, 42, 77–85. [Google Scholar] [CrossRef]
- Ito, N.; Sakai, A.; Miyake, N.; Maruyama, M.; Iwasaki, H.; Miyake, K.; Okada, T.; Sakamoto, A.; Suzuki, H. miR-15b mediates oxaliplatin-induced chronic neuropathic pain through BACE1 down-regulation. Br. J. Pharmacol. 2017, 174, 386–395. [Google Scholar] [CrossRef]
- Chen, W.; Guo, S.; Wang, S. MicroRNA-16 Alleviates Inflammatory Pain by Targeting Ras-Related Protein 23 (RAB23) and Inhibiting p38 MAPK Activation. Med. Sci. Monit. 2016, 22, 3894–3901. [Google Scholar] [CrossRef]
- Li, T.; Wan, Y.; Sun, L.; Tao, S.; Chen, P.; Liu, C.; Wang, K.; Zhou, C.; Zhao, G. Inhibition of MicroRNA-15a/16 Expression Alleviates Neuropathic Pain Development through Upregulation of G Protein-Coupled Receptor Kinase 2. Biomol. Ther. 2019, 27, 414–422. [Google Scholar] [CrossRef]
- You, H.; Zhang, L.; Chen, Z.; Liu, W.; Wang, H.; He, H. MiR-20b-5p relieves neuropathic pain by targeting Akt3 in a chronic constriction injury rat model. Synapse 2019, 73, e22125. [Google Scholar] [CrossRef] [PubMed]
- Zeboudj, L.; Sideris-Lampretsas, G.; Silva, R.; Al-Mudaris, S.; Picco, F.; Fox, S.; Chambers, D.; Malcangio, M. Silencing miR-21-5p in sensory neurons reverses neuropathic allodynia via activation of TGF-β-related pathway in macrophages. J. Clin. Investig. 2023, 133, e164472. [Google Scholar] [CrossRef]
- Reinhold, A.K.; Krug, S.M.; Salvador, E.; Sauer, R.S.; Karl-Schöller, F.; Malcangio, M.; Sommer, C.; Rittner, H.L. MicroRNA-21-5p functions via RECK/MMP9 as a proalgesic regulator of the blood nerve barrier in nerve injury. Ann. N. Y. Acad. Sci. 2022, 1515, 184–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Liu, H.L.; Li, L.; Wei, M.; Ge, D.J.; Zhang, Z.J. Effects of miR-26a-5p on neuropathic pain development by targeting MAPK6 in in CCI rat models. Biomed. Pharmacother. 2018, 107, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, M.; Guo, X.; Wang, P.; Zeng, F.; Wang, H.; Tang, J.; Qin, Z.; Tao, T. miR-26a-5p alleviates CFA-induced chronic inflammatory hyperalgesia through Wnt5a/CaMKII/NFAT signaling in mice. CNS Neurosci. Ther. 2023, 29, 1254–1271. [Google Scholar] [CrossRef]
- Liu, C.C.; Hung, K.C.; Li, Y.Y.; Yi-Kung Huang, E.; Chu, C.C.; Chow, L.H.; Tan, P.H. The concerted actions of microRNA-29a and interferon-β modulate complete Freund’s adjuvant-induced inflammatory pain by regulating the expression of type 1 interferon receptor, interferon-stimulated gene 15, and p-extracellular signal-regulated kinase. BJA Open 2025, 13, 100376. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Zhang, Y.; Xia, F.; Xu, S.; Shen, X. Downregulation of microRNA-29c reduces pain after child delivery by activating the oxytocin-GABA pathway. Mol. Med. Rep. 2020, 22, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yao, Y.; Ma, Y.; Chen, Y. MiR-30a-5p ameliorates LPS-induced inflammatory injury in human A549 cells and mice via targeting RUNX2. Innate Immun. 2021, 27, 41–49. [Google Scholar] [CrossRef]
- Liao, J.; Liu, J.; Long, G.; Lv, X. MiR-30b-5p attenuates neuropathic pain by the CYP24A1-Wnt/β-catenin signaling in CCI rats. Exp. Brain Res. 2022, 240, 263–277. [Google Scholar] [CrossRef]
- Tramullas, M.; Francés, R.; de la Fuente, R.; Velategui, S.; Carcelén, M.; García, R.; Llorca, J.; Hurlé, M.A. MicroRNA-30c-5p modulates neuropathic pain in rodents. Sci. Transl. Med. 2018, 10, eaao6299. [Google Scholar] [CrossRef]
- Brandenburger, T.; Johannsen, L.; Prassek, V.; Kuebart, A.; Raile, J.; Wohlfromm, S.; Köhrer, K.; Huhn, R.; Hollmann, M.W.; Hermanns, H. MiR-34a is differentially expressed in dorsal root ganglia in a rat model of chronic neuropathic pain. Neurosci. Lett. 2019, 708, 134365. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Q.; Jiang, W.; Yu, S.; Zhang, S. MiR-34c Ameliorates Neuropathic Pain by Targeting NLRP3 in a Mouse Model of Chronic Constriction Injury. Neuroscience 2019, 399, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Gu, Y.; Dai, Q.; He, Y.; Wang, J. Spinal miR-34a regulates inflammatory pain by targeting SIRT1 in complete Freund’s adjuvant mice. Biochem. Biophys. Res. Commun. 2019, 516, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Shao, A.; Tang, X.; Feng, M.; Wang, J.; Qiu, Y. MiR-34a affects dexmedetomidine-inhibited chronic inflammatory visceral pain by targeting to HDAC2. BMC Anesthesiol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, B.; Mo, Y.; Wang, X.; Zhong, L.; Han, X.; Mi, F. MiR-101 promotes pain hypersensitivity in rats with chronic constriction injury via the MKP-1 mediated MAPK pathway. J. Cell. Mol. Med. 2020, 24, 8986–8997. [Google Scholar] [CrossRef]
- Xie, T.; Zhang, J.; Kang, Z.; Liu, F.; Lin, Z. miR-101 down-regulates mTOR expression and attenuates neuropathic pain in chronic constriction injury rat models. Neurosci. Res. 2020, 158, 30–36. [Google Scholar] [CrossRef]
- Favereaux, A.; Thoumine, O.; Bouali-Benazzouz, R.; Roques, V.; Papon, M.A.; Salam, S.A.; Drutel, G.; Léger, C.; Calas, A.; Nagy, F.; et al. Bidirectional integrative regulation of Cav1.2 calcium channel by microRNA miR-103: Role in pain. EMBO J. 2011, 30, 3830–3841. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, R.; Xu, M.J.; Sha, J.; Xu, G.Y.; Wu, J.; Zhang, P.A. MiRNA-107 contributes to inflammatory pain by down-regulating GLT-1 expression in rat spinal dorsal horn. Eur. J. Pain 2021, 25, 1254–1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.L.; An, L.J.; Li, L.; Wei, M.; Ge, D.J.; Su, Z. miR-124-3p attenuates neuropathic pain induced by chronic sciatic nerve injury in rats via targeting EZH2. J. Cell. Biochem. 2019, 120, 5747–5755. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X.; Wang, X.; Xu, F.; Zhang, J.; Li, L.; Xie, X.; Wang, L.; Yang, Y.; Xu, J.T. MicroRNA-124-3p attenuates the development of nerve injury-induced neuropathic pain by targeting early growth response 1 in the dorsal root ganglia and spinal dorsal horn. J. Neurochem. 2021, 158, 928–942. [Google Scholar] [CrossRef]
- Liu, C.C.; Cheng, J.T.; Li, T.Y.; Tan, P.H. Integrated analysis of microRNA and mRNA expression profiles in the rat spinal cord under inflammatory pain conditions. Eur. J. Neurosci. 2017, 46, 2713–2728. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, P.; Ni, Y.; Zhao, J.; Liu, Z. Decreased microRNA-125a-3p contributes to upregulation of p38 MAPK in rat trigeminal ganglions with orofacial inflammatory pain. PLoS ONE 2014, 9, e111594. [Google Scholar] [CrossRef] [PubMed]
- Kasimu, A.; Apizi, X.; Talifujiang, D.; Ma, X.; Fang, L.; Zhou, X. miR-125a-5p in astrocytes attenuates peripheral neuropathy in type 2 diabetic mice through targeting TRAF6. Endocrinol. Diabetes Nutr. 2022, 69, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Geng, X.; Guo, X.; Wang, T.; Xu, J.; Jiang, L.; Zhen, H. microRNA-125b-5p alleviated CCI-induced neuropathic pain and modulated neuroinflammation via targeting SOX11. Synapse 2024, 78, e22306. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Cai, W.; Liu, Y.; Liu, H.; Zhang, Z.; Su, Z. MicroRNA-128-3p Alleviates Neuropathic Pain Through Targeting ZEB1. Neurosci. Lett. 2020, 729, 134946. [Google Scholar] [CrossRef]
- Xian, S.; Ding, R.; Li, M.; Chen, F. LncRNA NEAT1/miR-128-3p/AQP4 axis regulating spinal cord injury-induced neuropathic pain progression. J. Neuroimmunol. 2021, 351, 577457. [Google Scholar] [CrossRef]
- Yao, L.; Guo, Y.; Wang, L.; Li, G.; Qian, X.; Zhang, J.; Liu, H.; Liu, G. Knockdown of miR-130a-3p alleviates spinal cord injury induced neuropathic pain by activating IGF-1/IGF-1R pathway. J. Neuroimmunol. 2021, 351, 577458. [Google Scholar] [CrossRef]
- Dong, J.; Xu, C.; Xia, R.; Zhang, Z. Upregulating miR-130a-5p relieves astrocyte over activation-induced neuropathic pain through targeting C-X-C motif chemokine receptor 12/C-X-C motif chemokine receptor 4 axis. NeuroReport 2021, 32, 135–143. [Google Scholar] [CrossRef]
- Leinders, M.; Üçeyler, N.; Pritchard, R.A.; Sommer, C.; Sorkin, L.S. Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp. Neurol. 2016, 283, 276–286. [Google Scholar] [CrossRef]
- Ji, L.J.; Su, J.; Xu, A.L.; Pang, B.; Huang, Q.M. MiR-134-5p attenuates neuropathic pain progression through targeting Twist1. J. Cell Biochem. 2019, 120, 1694–1701. [Google Scholar] [CrossRef]
- Ni, J.; Gao, Y.; Gong, S.; Guo, S.; Hisamitsu, T.; Jiang, X. Regulation of μ-opioid type 1 receptors by microRNA134 in dorsal root ganglion neurons following peripheral inflammation. Eur. J. Pain 2013, 17, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; He, H.; Wang, P.J. A critical role for miR-135a-5p-mediated regulation of SLC24A2 in neuropathic pain. Mol. Med. Rep. 2020, 22, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, Y.; Su, Z. ciRS-7 targeting miR-135a-5p promotes neuropathic pain in CCI rats via inflammation and autophagy. Gene 2020, 736, 144386. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Yan, H.; Chen, J.; Liu, C.; Chen, Z. MiR-135-5p Alleviates Bone Cancer Pain by Regulating Astrocyte-Mediated Neuroinflammation in Spinal Cord through JAK2/STAT3 Signaling Pathway. Mol. Neurobiol. 2021, 58, 4802–4815. [Google Scholar] [CrossRef]
- Zhang, Y.; Mou, J.; Cao, L.; Zhen, S.; Huang, H.; Bao, H. MicroRNA-142-3p relieves neuropathic pain by targeting high mobility group box 1. Int. J. Mol. Med. 2018, 41, 501–510. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Zhu, C.; Wang, D.; Li, Y.; Hao, X.; Cao, L.; Fan, Y.; Fang, B. Biosynthesis inhibition of miR-142-5p in a N6-methyladenosine-dependent manner induces neuropathic pain through CDK5/TRPV1 signaling. Cell. Mol. Biol. Lett. 2025, 30, 16. [Google Scholar] [CrossRef]
- Lu, Y.; Cao, D.L.; Jiang, B.C.; Yang, T.; Gao, Y.J. MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav. Immun. 2015, 49, 119–129. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, F.; Wei, M.; Qiu, Y.; Ma, C.; Shen, L.; Huang, Y. Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J. Neuroinflamm. 2018, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Garo, L.P.; Ajay, A.K.; Fujiwara, M.; Gabriely, G.; Raheja, R.; Kuhn, C.; Kenyon, B.; Skillin, N.; Kadowaki-Saga, R.; Saxena, S.; et al. MicroRNA-146a limits tumorigenic inflammation in colorectal cancer. Nat. Commun. 2021, 12, 2419. [Google Scholar] [CrossRef]
- Dou, L.; Lin, H.; Wang, K.; Zhu, G.; Zou, X.; Chang, E.; Zhu, Y. Long non-coding RNA CCAT1 modulates neuropathic pain progression through sponging miR-155. Oncotarget 2017, 8, 89949–89957. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Liu, W.; Yan, B.; Hu, X.; Yang, F. miRNA-155 silencing reduces sciatic nerve injury in diabetic peripheral neuropathy. J. Mol. Endocrinol. 2019, 63, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.; Sun, Y. LncRNA p21, downregulating miR-181b, aggravates neuropathic pain by upregulating Tnfaip1 and inhibit the AKT/CREB axis. Brain Res. Bull. 2021, 171, 150–161. [Google Scholar] [CrossRef]
- Zhang, Y.U.; Ye, G.; Zhao, J.; Chen, Y.; Kong, L.; Sheng, C.; Yuan, L. Exosomes carried miR-181c-5p alleviates neuropathic pain in CCI rat models. An. Acad. Bras. Cienc. 2022, 94, e20210564. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Ma, L.; Xi, K.; Zhang, W.; Fan, D. MicroRNA-183 Suppresses Neuropathic Pain and Expression of AMPA Receptors by Targeting mTOR/VEGF Signaling Pathway. Cell Physiol. Biochem. 2017, 41, 181–192. [Google Scholar] [CrossRef]
- Huang, L.; Wang, L. Upregulation of miR-183 represses neuropathic pain through inhibiton of MAP3K4 in CCI rat models. J. Cell Physiol. 2020, 235, 3815–3822. [Google Scholar] [CrossRef]
- Yang, D.; Yang, Q.; Wei, X.; Liu, Y.; Ma, D.; Li, J.; Wan, Y.; Luo, Y. The role of miR-190a-5p contributes to diabetic neuropathic pain via targeting SLC17A6. J. Pain Res. 2017, 10, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Shi, J.; Liu, K.; Liu, N.; Wang, Y.; Fu, Z.; Ding, J.; Jia, L.; Yuan, W. Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia 2013, 61, 504–512. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, T.; He, M.; Liang, H.; Wang, H.; Xu, L.; Chen, S.; Xu, M. Inhibition of MicroRNA-195 Alleviates Neuropathic Pain by Targeting Patched1 and Inhibiting SHH Signaling Pathway Activation. Neurochem. Res. 2019, 44, 1690–1702. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Li, Z. Low-Level miR-199 Contribute to Neuropathic Low Back Pain via TRPV1 by Regulating the Production of Pro-Inflammatory Cytokines on Macrophage. Turk. Neurosurg. 2024, 34, 299–307. [Google Scholar]
- Saadh, M.J.; Rashed, A.B.; Jamal, A.; Castillo-Acobo, R.Y.; Kamal, M.A.; Cotrina-Aliaga, J.C.; Gonzáles, J.L.A.; Alothaim, A.S.; Alhoqail, W.A.; Ahmad, F.; et al. miR-199a-3p suppresses neuroinflammation by directly targeting MyD88 in a mouse model of bone cancer pain. Life Sci. 2023, 333, 122139. [Google Scholar] [CrossRef]
- Li, H.; Huang, Y.; Ma, C.; Yu, X.; Zhang, Z.; Shen, L. MiR-203 involves in neuropathic pain development and represses Rap1a expression in nerve growth factor differentiated neuronal PC12 cells. Clin. J. Pain 2015, 31, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Fu, Z.; Zhou, Q. MicroRNA-212-3p Attenuates Neuropathic Pain via Targeting Sodium Voltage-gated Channel Alpha Subunit 3 (NaV 1.3). Curr. Neurovasc. Res. 2019, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Luan, L.; Li, J.; Yang, L. MiR-212-3p improves rat functional recovery and inhibits neurocyte apoptosis in spinal cord injury models via PTEN downregulation-mediated activation of AKT/mTOR pathway. Brain Res. 2021, 1768, 147576. [Google Scholar] [CrossRef]
- Pan, Z.; Zhu, L.J.; Li, Y.Q.; Hao, L.Y.; Yin, C.; Yang, J.X.; Guo, Y.; Zhang, S.; Hua, L.; Xue, Z.Y.; et al. Epigenetic modification of spinal miR-219 expression regulates chronic inflammation pain by targeting CaMKIIγ. J. Neurosci. 2014, 34, 9476–9483. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, J.; Xu, J. miR-223 Inhibits the Polarization and Recruitment of Macrophages via NLRP3/IL-1β Pathway to Meliorate Neuropathic pain. Pain Res. Manag. 2021, 2021, 6674028. [Google Scholar] [CrossRef]
- Huang, B.; Guo, S.; Zhang, Y.; Lin, P.; Lin, C.; Chen, M.; Zhu, S.; Huang, L.; He, J.; Zhang, L.; et al. MiR-223-3p alleviates trigeminal neuropathic pain in the male mouse by targeting MKNK2 and MAPK/ERK signaling. Brain Behav. 2022, 12, e2634. [Google Scholar] [CrossRef] [PubMed]
- Manners, M.T.; Tian, Y.; Zhou, Z.; Ajit, S.K. MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio 2015, 5, 733–740. [Google Scholar] [CrossRef]
- Lu, Z.; Zhang, Y.; Li, Y. LncRNA FTX ameliorates neuropathic pain by targeting miR-320a in a rat model of chronic constriction injury. Folia Neuropathol. 2023, 61, 291–300. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, C.; Su, Z.; Lin, D. DGCR5 attenuates neuropathic pain through sponging miR-330-3p and regulating PDCD4 in CCI rat models. J. Cell. Physiol. 2019, 234, 7292–7300. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Peng, Y.; Xu, H.; Zhou, X. METTL3 regulates inflammatory pain by modulating m6A-dependent pri-miR-365-3p processing. FASEB J. 2020, 34, 122–132. [Google Scholar] [CrossRef]
- Xiang, W.; Jiang, L.; Zhou, Y.; Li, Z.; Zhao, Q.; Wu, T.; Cao, Y.; Zhou, J. The lncRNA Ftx/miR-382-5p/Nrg1 axis improves the inflammation response of microglia and spinal cord injury repair. Neurochem. Int. 2021, 143, 104929. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Shen, H.; Mei, S.; Wang, Z.; Xie, Q.; Cui, H.; Chu, Y.; Feng, B. Down-regulation of microRNA-382-5p reduces neuropathic pain by targeting regulation of dual specificity phosphatase-1. Korean J. Pain 2024, 37, 320–331. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Wei, J.Y.; Ou-Yang, H.D.; Li, D.; Xu, T.; Wu, S.L.; Zhang, X.L.; Liu, C.C.; Ma, C.; Xin, W.J. mir-500-Mediated GAD67 Downregulation Contributes to Neuropathic Pain. J. Neurosci. 2016, 36, 6321–6331. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Shen, W.; Hu, Y. The Role of miR-539 in the Anterior Cingulate Cortex in Chronic Neuropathic pain. Pain Med. 2017, 18, 2433–2442. [Google Scholar] [CrossRef]
- Li, H.; Shen, L.; Ma, C.; Huang, Y. Differential expression of miRNAs in the nervous system of a rat model of bilateral sciatic nerve chronic constriction injury. Int. J. Mol. Med. 2013, 32, 219–226. [Google Scholar] [CrossRef]
- Liu, Y.; Jeon, S.M.; Caterina, M.J.; Qu, L. miR-544-3p mediates arthritis pain through regulation of FcγRI. Pain 2022, 163, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Du, X.J.; Zhao, Y.; Xia, D.L. XIST/miR-544 axis induces neuropathic pain by activating STAT3 in a rat model. J. Cell Physiol. 2018, 233, 5847–5855. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, L.; Xu, T.; Gou, B.; Mai, J.W.; Luo, D.X.; Xin, W.J.; Wu, J.Y. MiR-672-5p-Mediated Upregulation of REEP6 in Spinal Dorsal Horn Participates in Bortezomib-Induced Neuropathic Pain in Rats. Neurochem. Res. 2023, 48, 229–237. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, B.; Gan, C.; Sun, H.; Zhang, J.; Feng, L. A Comprehensive Review of Small Interfering RNAs (siRNAs): Mechanism, Therapeutic Targets, and Delivery Strategies for Cancer Therapy. Int. J. Nanomed. 2023, 18, 7605–7635. [Google Scholar] [CrossRef]
- Wang, Q.; Xue, Y.; Zhang, L.; Zhong, Z.; Feng, S.; Wang, C.; Xiao, L.; Yang, Z.; Harris, C.J.; Wu, Z.; et al. Mechanism of siRNA production by a plant Dicer-RNA complex in dicing-competent conformation. Science 2021, 374, 1152–1157. [Google Scholar] [CrossRef]
- Lee, H.Y.; Zhou, K.; Smith, A.M.; Noland, C.L.; Doudna, J.A. Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 2013, 41, 6568–6576. [Google Scholar] [CrossRef]
- Xu, R.; Njumbe Ediage, E.; Verhaeghe, T.; Snoeys, J.; Dillen, L. Therapeutic siRNA Loaded to RISC as Single and Double Strands Requires an Appropriate Quantitative Assay for RISC PK Assessment. Nucleic Acid. Ther. 2024, 34, 199–210. [Google Scholar] [CrossRef]
- Saddique, M.N.; Qadri, M.; Ain, N.U.; Farhan, E.; Shahid, F.; Benyamin, J.; Bashir, M.A.; Jain, H.; Iqbal, J. Safety and effectiveness of interference RNA (RNAi) based therapeutics in cardiac failure: A systematic review. Heart Lung 2024, 68, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, P.J.; Ameres, S.L.; Kueng, S.; Martinez, J. Cleavage of the siRNA passenger strand during RISC assembly in human cells. EMBO Rep. 2006, 7, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, R.A.; Onizuka, K.; Ball-Jones, A.A.; Hu, T.; Suter, S.R.; Beal, P.A. Guide Strand 3′-End Modifications Regulate siRNA Specificity. Chembiochem. 2016, 17, 2340–2345. [Google Scholar] [CrossRef] [PubMed]
- Meister, G.; Landthaler, M.; Patkaniowska, A.; Dorsett, Y.; Teng, G.; Tuschl, T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell 2004, 15, 185–197. [Google Scholar] [CrossRef]
- Qi, X.; Bao, F.S.; Xie, Z. Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 2009, 4, e4971. [Google Scholar] [CrossRef]
- Marker, S.; Le Mouël, A.; Meyer, E.; Simon, M. Distinct RNA-dependent RNA polymerases are required for RNAi triggered by double-stranded RNA versus truncated transgenes in Paramecium tetraurelia. Nucleic Acids Res. 2010, 38, 4092–4107. [Google Scholar] [CrossRef]
- Qureshi, A.; Tantray, V.G.; Kirmani, A.R.; Ahangar, A.G. A review on current status of antiviral siRNA. Rev. Med. Virol. 2018, 28, e1976. [Google Scholar] [CrossRef]
- Wallen, M.; Aqil, F.; Kandimalla, R.; Jeyabalan, J.; Auwardt, S.; Tyagi, N.; Schultz, D.J.; Spencer, W.; Gupta, R.C. A model system for antiviral siRNA therapeutics using exosome-based delivery. Mol. Ther. Nucleic Acids 2022, 29, 691–704. [Google Scholar] [CrossRef]
- Yang, Q.; Ye, Q.A.; Liu, Y. Mechanism of siRNA production from repetitive DNA. Genes Dev. 2015, 29, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Barreto, G.; Sathyapalan, T.; Sahebkar, A. siRNA Therapeutics: Future Promise for Neurodegenerative Diseases. Curr. Neuropharmacol. 2021, 19, 1896–1911. [Google Scholar] [CrossRef] [PubMed]
- Wittrup, A.; Lieberman, J. Knocking down disease: A progress report on siRNA therapeutics. Nat. Rev. Genet. 2015, 16, 543–552. [Google Scholar] [CrossRef]
- Dalmay, T. Short RNAs in environmental adaptation. Proc. Biol. Sci. 2006, 273, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Takada, T.; Nemoto, K.; Yamashita, A.; Kato, M.; Kondo, Y.; Torii, R. Efficient gene silencing and cell differentiation using siRNA in mouse and monkey ES cells. Biochem. Biophys. Res. Commun. 2005, 331, 1039–1044. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, J.; Zhu, G.; Huang, Y.; Jin, L. SiRNA directed against NF-κB inhibits mononuclear macrophage cells releasing proinflammatory cytokines in vitro. Mol. Med. Rep. 2017, 16, 9060–9066. [Google Scholar] [CrossRef]
- Dong, X.W.; Goregoaker, S.; Engler, H.; Zhou, X.; Mark, L.; Crona, J.; Terry, R.; Hunter, J.; Priestley, T. Small interfering RNA-mediated selective knockdown of Na(V)1.8 tetrodotoxin-resistant sodium channel reverses mechanical allodynia in neuropathic rats. Neuroscience 2007, 146, 812–821. [Google Scholar] [CrossRef]
- Ganju, P.; Hall, J. Potential applications of siRNA for pain therapy. Expert Opin. Biol. Ther. 2004, 4, 531–542. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.; Fan, B.; Xu, X.; Chen, Y.; Lu, R.; Xu, Z.; Liu, X. PKM2 is involved in neuropathic pain by regulating ERK and STAT3 activation in rat spinal cord. J. Headache Pain 2018, 19, 7. [Google Scholar] [CrossRef]
- Peng, J.; Ma, J.; Yang, X.; He, H.; Wu, H.; Ma, T.; Lu, J. Water-Soluble Polymer Assists N-Methyl-D-Aspartic Acid Receptor 2B siRNA Delivery to Relieve Chronic Inflammatory Pain In Vitro and In Vivo. Pain Res. Manag. 2018, 2018, 7436060. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, J.; Chen, X.; Ren, J.; Wu, C.; Shen, H.; Li, X.; Yu, J.; Jiang, B.; Yu, L. NAMPT/NAD+/PARP1 Pathway Regulates CFA-Induced Inflammatory Pain via NF-κB Signaling in Rodents. Adv. Biol. 2024, 8, e2400028. [Google Scholar] [CrossRef] [PubMed]
- Moqbel Redhwan, M.A.; Hariprasad, M.G.; Samaddar, S.; Bafail, D.; Hard, S.A.A.A.; Guha, S.; Dhavale, A. siRNA targeting PARP-1 alleviates diabetic peripheral neuropathy in a streptozotocin-induced rat model. J. Drug Target. 2025, 33, 424–435. [Google Scholar] [CrossRef]
- Fitzsimons, L.A.; Staurengo-Ferrari, L.; Khomula, E.V.; Bogen, O.; Araldi, D.; Bonet, I.J.M.; Green, P.G.; Jordan, E.E.; Sclafani, F.; Nowak, C.E.; et al. The Nociceptor Primary Cilium Contributes to Mechanical Nociceptive Threshold and Inflammatory and Neuropathic Pain. J. Neurosci. 2024, 44, e1265242024. [Google Scholar] [CrossRef]
- Lee, S.; Shin, H.J.; Noh, C.; Kim, S.I.; Ko, Y.K.; Lee, S.Y.; Lim, C.; Hong, B.; Yang, S.Y.; Kim, D.W.; et al. IKBKB siRNA-Encapsulated Poly (Lactic-co-Glycolic Acid) Nanoparticles Diminish Neuropathic Pain by Inhibiting Microglial Activation. Int. J. Mol. Sci. 2021, 22, 5657. [Google Scholar] [CrossRef]
- Pan, R.; Di, H.; Zhang, J.; Huang, Z.; Sun, Y.; Yu, W.; Wu, F. Inducible Lentivirus-Mediated siRNA against TLR4 Reduces Nociception in a Rat Model of Bone Cancer Pain. Mediat. Inflamm. 2015, 2015, 523896. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.Y.; Ling, Z.M.; Chen, G.; Wei, Z.Y. Inhibition of Schwann cell pannexin 1 attenuates neuropathic pain through the suppression of inflammatory responses. J. Neuroinflamm. 2022, 19, 244. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Yang, Y.; Li, Y.; Zhang, J.; Fan, K.; Guo, Y.; Chen, J.; Chen, Y.; Zhu, P.; Huang, L.; et al. Targeting TANK-binding kinase 1 attenuates painful diabetic neuropathy via inhibiting microglia pyroptosis. Cell. Commun. Signal. 2024, 22, 368. [Google Scholar] [CrossRef]
- Xu, L.; Feng, Q.; Deng, H.; Zhang, X.; Ni, H.; Yao, M. Neurexin-2 is a potential regulator of inflammatory pain in the spinal dorsal horn of rats. J. Cell. Mol. Med. 2020, 24, 13623–13633. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Cao, D.L.; Ma, L.J.; Gao, Y.J. TRAF6 Contributes to CFA-Induced Spinal Microglial Activation and Chronic Inflammatory Pain in Mice. Cell. Mol. Neurobiol. 2022, 42, 1543–1555. [Google Scholar] [CrossRef]
- Peng, S.; Lu, Y.; Li, P.; Liu, P.; Shi, X.; Liu, C.; Zhang, Y.; Liu, S.; Wang, J. The short interference RNA (siRNA) targeting NMUR2 relieves nociception in a bone cancer pain model of rat through PKC-ERK and PI3K-AKT pathways. Biochem. Biophys. Res. Commun. 2019, 512, 616–622. [Google Scholar] [CrossRef]
- Kamata, Y.; Kambe, T.; Chiba, T.; Yamamoto, K.; Kawakami, K.; Abe, K.; Taguchi, K. Paclitaxel Induces Upregulation of Transient Receptor Potential Vanilloid 1 Expression in the Rat Spinal Cord. Int. J. Mol. Sci. 2020, 21, 4341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, J.; Meng, Q. AAV-mediated siRNA against TRPV1 reduces nociception in a rat model of bone cancer pain. Neurol. Res. 2019, 41, 972–979. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y. Suppressing BRD4 exhibits protective effects against vincristine-induced peripheral neuropathy by alleviating inflammation and oxidative stress. Biochem. Biophys. Res. Commun. 2020, 532, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Chia, Y.Y.; Chow, L.H.; Chen, J.J.; Yang, L.C.; Hung, K.C.; Chen, H.S.; Kuo, C.H. Gene knockdown of the N-methyl-D-aspartate receptor NR1 subunit with subcutaneous small interfering RNA reduces inflammation-induced nociception in rats. Anesthesiology 2010, 112, 1482–1493. [Google Scholar] [CrossRef]
- Liu, X.; Tian, Y.; Lu, N.; Gin, T.; Cheng, C.H.; Chan, M.T. Stat3 inhibition attenuates mechanical allodynia through transcriptional regulation of chemokine expression in spinal astrocytes. PLoS ONE 2013, 8, e75804. [Google Scholar] [CrossRef]
- Hang, L.H.; Yang, J.P.; Yin, W.; Wang, L.N.; Guo, F.; Ji, F.H.; Shao, D.H.; Xu, Q.N.; Wang, X.Y.; Zuo, J.L. Activation of spinal TDAG8 and its downstream PKA signaling pathway contribute to bone cancer pain in rats. Eur. J. Neurosci. 2012, 36, 2107–2117. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.X.; Wang, J.; Ma, F.J.; Liu, M.M.; Chen, S.H.; Wei, Y.; Xiao, Y.F.; Lv, P.Y.; Liu, X.; Qu, J.Q.; et al. Rab11a in the spinal cord: An essential contributor to complete Freund’s adjuvant-induced inflammatory pain in mice. Mol. Brain 2023, 16, 70. [Google Scholar] [CrossRef]
- Yang, L.; Bai, H.H.; Zhang, Z.Y.; Liu, J.P.; Suo, Z.W.; Yang, X.; Hu, X.D. Disruption of SHP1/NMDA receptor signaling in spinal cord dorsal horn alleviated inflammatory pain. Neuropharmacology 2018, 137, 104–113. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S.; Li, L.; Ji, X.; Wang, M.; Zhou, J. Spinal IL-36γ/IL-36R participates in the maintenance of chronic inflammatory pain through astroglial JNK pathway. Glia 2019, 67, 438–451. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Z.; Wang, K.; Chen, Z.; Shen, H. Suppression of microglial Ccl2 reduces neuropathic pain associated with chronic spinal compression. Front. Immunol. 2023, 14, 1191188. [Google Scholar] [CrossRef]
- Lin, C.R.; Cheng, J.K.; Wu, C.H.; Chen, K.H.; Liu, C.K. Epigenetic suppression of potassium-chloride co-transporter 2 expression in inflammatory pain induced by complete Freund’s adjuvant (CFA). Eur. J. Pain 2017, 21, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.L.; Qian, B.; Zhang, Z.J.; Gao, Y.J.; Wu, X.B. Chemokine receptor CXCR2 in dorsal root ganglion contributes to the maintenance of inflammatory pain. Brain Res. Bull. 2016, 127, 219–225. [Google Scholar] [CrossRef]
- Kwon, A.; Jeon, S.M.; Hwang, S.H.; Kim, J.H.; Cho, H.J. Expression and functional role of metallothioneins I and II in the spinal cord in inflammatory and neuropathic pain models. Brain Res. 2013, 1523, 37–48. [Google Scholar] [CrossRef]
- Wang, H.; Song, T.; Wang, W.; Zhang, Z. TRPM2 participates the transformation of acute pain to chronic pain during injury-induced neuropathic pain. Synapse 2019, 73, e22117. [Google Scholar] [CrossRef]
- Deval, E.; Noël, J.; Lay, N.; Alloui, A.; Diochot, S.; Friend, V.; Jodar, M.; Lazdunski, M.; Lingueglia, E. ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 2008, 27, 3047–3055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qian, Z.; Jiang, D.; Sun, Y.; Gao, S.; Jiang, X.; Wang, H.; Tao, J. Neuromedin B receptor stimulation of Cav3.2 T-type Ca2+ channels in primary sensory neurons mediates peripheral pain hypersensitivity. Theranostics 2021, 11, 9342–9357. [Google Scholar] [CrossRef]
- Noh, C.; Shin, H.J.; Lee, S.; Kim, S.I.; Kim, Y.H.; Lee, W.H.; Kim, D.W.; Lee, S.Y.; Ko, Y.K. CX3CR1-Targeted PLGA Nanoparticles Reduce Microglia Activation and Pain Behavior in Rats with Spinal Nerve Ligation. Int. J. Mol. Sci. 2020, 21, 3469. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Chen, L.; Lu, X.; Zhang, J.; Bao, G.; Xu, G.; Sun, Y.; Guo, X.; Jiang, J.; Gu, H.; et al. Vimentin Promotes Astrocyte Activation After Chronic Constriction Injury. J. Mol. Neurosci. 2017, 63, 91–99. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, J.; He, M.; Liu, R.; Belegu, V.; Dai, P.; Liu, W.; Wang, W.; Xia, Q.J.; Shang, F.F.; et al. Mechanisms of PDGF siRNA-mediated inhibition of bone cancer pain in the spinal cord. Sci. Rep. 2016, 6, 27512. [Google Scholar] [CrossRef]
- Huang, H.J.; Zhang, M. Downregulation of PI3Kcb utilizing adenovirus-mediated transfer of siRNA attenuates bone cancer pain. Int. J. Clin. Exp. Pathol. 2014, 7, 8127–8135. [Google Scholar]
- Gao, N.; Li, Y.; Li, J.; Gao, Z.; Yang, Z.; Li, Y.; Liu, H.; Fan, T. Long Non-Coding RNAs: The Regulatory Mechanisms, Research Strategies, and Future Directions in Cancers. Front. Oncol. 2020, 10, 598817. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Chang, C.P. Long non-coding RNA and chromatin remodeling. RNA Biol. 2015, 12, 1094–1098. [Google Scholar] [CrossRef]
- Vance, K.W.; Ponting, C.P. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends. Genet. 2014, 30, 348–355. [Google Scholar] [CrossRef]
- He, R.Z.; Luo, D.X.; Mo, Y.Y. Emerging roles of lncRNAs in the post-transcriptional regulation in cancer. Genes Dis. 2019, 6, 6–15. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ren, Y.; Hu, W.; Paliouras, A.R.; Zhang, W.; Zhong, L.; Yang, K.; Su, L.; Wang, P.; Li, Y.; et al. Long non-coding RNA-encoded micropeptides: Functions, mechanisms and implications. Cell Death Discov. 2024, 10, 450. [Google Scholar] [CrossRef]
- Nojima, T.; Proudfoot, N.J. Mechanisms of lncRNA biogenesis as revealed by nascent transcriptomics. Nat. Rev. Mol. Cell Biol. 2022, 23, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Chen, L.L. Life without A tail: New formats of long noncoding RNAs. Int. J. Biochem. Cell. Biol. 2014, 54, 338–349. [Google Scholar] [CrossRef]
- Xu, S.M.; Curry-Hyde, A.; Sytnyk, V.; Janitz, M. RNA polyadenylation patterns in the human transcriptome. Gene 2022, 816, 146133. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Zhao, Z.; Lu, J.; Chen, H.; Ding, N.; Wang, G.; Xu, J.; Li, X. Identifying and functionally characterizing tissue-specific and ubiquitously expressed human lncRNAs. Oncotarget. 2016, 7, 7120–7133. [Google Scholar] [CrossRef] [PubMed]
- Davidovich, C.; Cech, T.R. The recruitment of chromatin modifiers by long noncoding RNAs: Lessons from PRC2. RNA 2015, 21, 2007–2022. [Google Scholar] [CrossRef]
- Teo, W.W.; Cao, X.; Wu, C.S.; Tan, H.K.; Zhou, Q.; Gao, C.; Vanuytsel, K.; Kumar, S.S.; Murphy, G.J.; Yang, H.; et al. Non-coding RNA LEVER sequestration of PRC2 can mediate long range gene regulation. Commun. Biol. 2022, 5, 343. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, J.; Dimitrova, N. Transcription regulation by long non-coding RNAs: Mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 2024, 25, 396–415. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Kim, K.M.; McClusky, W.G.; Abdelmohsen, K.; Gorospe, M. Cytoplasmic functions of long noncoding RNAs. Wiley Interdiscip. Rev. RNA 2018, 9, e1471. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, H.; Li, L.; Gao, Y.; Yu, B.; Ma, G.; Jin, X.; Sun, Y. New mechanism of LncRNA: In addition to act as a ceRNA. Noncoding RNA Res. 2024, 9, 1050–1060. [Google Scholar] [CrossRef]
- Peng, Y.; Long, X.D. The role of the ceRNA network mediated by lncRNA SNHG3 in the progression of cancer. Discov. Oncol. 2024, 15, 514. [Google Scholar] [CrossRef]
- Mirzadeh Azad, F.; Polignano, I.L.; Proserpio, V.; Oliviero, S. Long Noncoding RNAs in Human Stemness and Differentiation. Trends Cell. Biol. 2021, 31, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhang, X.; Gu, X.; Li, X.; Shang, L. Progress in understanding the role of lncRNA in programmed cell death. Cell Death Discov. 2021, 7, 30. [Google Scholar] [CrossRef]
- Tüncel, Ö.; Kara, M.; Yaylak, B.; Erdoğan, İ.; Akgül, B. Noncoding RNAs in apoptosis: Identification and function. Turk. J. Biol. 2021, 46, 1–40. [Google Scholar]
- Bocchetti, M.; Scrima, M.; Melisi, F.; Luce, A.; Sperlongano, R.; Caraglia, M.; Zappavigna, S.; Cossu, A.M. LncRNAs and Immunity: Coding the Immune System with Noncoding Oligonucleotides. Int. J. Mol. Sci. 2021, 22, 1741. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, J.; Yang, L.; Wu, M.; Ma, Q. The Role of Long Non-coding RNAs in Human Imprinting Disorders: Prospective Therapeutic Targets. Front. Cell. Dev. Biol. 2021, 9, 730014. [Google Scholar] [CrossRef]
- Dragomir, M.; Chen, B.; Calin, G.A. Exosomal lncRNAs as new players in cell-to-cell communication. Transl. Cancer Res. 2018, 7, S243–S252. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Weiswald, L.B.; Poulain, L.; Denoyelle, C.; Meryet-Figuiere, M. Involvement of lncRNAs in cancer cells migration, invasion and metastasis: Cytoskeleton and ECM crosstalk. J. Exp. Clin. Cancer Res. 2023, 42, 173. [Google Scholar] [CrossRef]
- Anilkumar, A.K.; Vij, P.; Lopez, S.; Leslie, S.M.; Doxtater, K.; Khan, M.M.; Yallapu, M.M.; Chauhan, S.C.; Maestre, G.E.; Tripathi, M.K. Long Non-Coding RNAs: New Insights in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2268. [Google Scholar] [CrossRef] [PubMed]
- Kohlmaier, A.; Holdt, L.M.; Teupser, D. Long noncoding RNAs in cardiovascular disease. Curr. Opin. Cardiol. 2023, 38, 179–192. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Lin, J.D. Long Noncoding RNAs: A New Regulatory Code in Metabolic Control. Trends Biochem. Sci. 2015, 40, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Nadhan, R.; Isidoro, C.; Song, Y.S.; Dhanasekaran, D.N. LncRNAs and the cancer epigenome: Mechanisms and therapeutic potential. Cancer Lett. 2024, 605, 217297. [Google Scholar] [CrossRef]
- Arrigoni, A.; Ranzani, V.; Rossetti, G.; Panzeri, I.; Abrignani, S.; Bonnal, R.J.; Pagani, M. Analysis RNA-seq and Noncoding RNA. Methods Mol. Biol. 2016, 1480, 125–135. [Google Scholar]
- Chu, C.; Qu, K.; Zhong, F.L.; Artandi, S.E.; Chang, H.Y. Genomic maps of long noncoding RNA occupancy reveal principles of RNA-chromatin interactions. Mol. Cell 2011, 44, 667–678. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, X.; Hu, Y. Identification of lncRNA-Protein Interactions by CLIP and RNA Pull-Down Assays. Methods Mol. Biol. 2021, 2348, 231–242. [Google Scholar] [PubMed]
- Habib, A.M.; Cox, J.J.; Okorokov, A.L. Out of the dark: The emerging roles of lncRNAs in pain. Trends Genet. 2024, 40, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Jian, W.; Xue, Q.; Liu, Z. Roles of Long Non-coding RNAs in the Development of Chronic pain. Front. Mol. Neurosci. 2021, 14, 760964. [Google Scholar] [CrossRef]
- Videira, R.F.; da Costa Martins, P.A.; Falcão-Pires, I. Non-Coding RNAs as Blood-Based Biomarkers in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 9285. [Google Scholar] [CrossRef]
- Garofalo, M.; Pandini, C.; Sproviero, D.; Pansarasa, O.; Cereda, C.; Gagliardi, S. Advances with Long Non-Coding RNAs in Alzheimer’s Disease as Peripheral Biomarker. Genes 2021, 12, 1124. [Google Scholar] [CrossRef]
- Wong, D.T. Salivary extracellular noncoding RNA: Emerging biomarkers for molecular diagnostics. Clin. Ther. 2015, 37, 540–551. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, X.; Han, X.; Pandey, V.; Lobie, P.E.; Zhu, T. The potential of long noncoding RNAs for precision medicine in human cancer. Cancer Lett. 2021, 501, 12–19. [Google Scholar] [CrossRef]
- Peng, J.W.; Gu, Y.Y.; Wei, J.; Sun, Y.; Zhu, C.L.; Zhang, L.; Song, Y.; Chen, L.; Chen, X.; Wang, Q.; et al. LncRNA MEG3-TRPV1 signaling regulates chronic inflammatory pain in rats. Mol. Pain 2022, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Sakai, A.; Fukunaga, T.; Miyagawa, Y.; Okada, T.; Hamada, M.; Suzuki, H. Neat1 lncRNA organizes the inflammatory gene expressions in the dorsal root ganglion in neuropathic pain caused by nerve injury. Front. Immunol. 2023, 14, 1185322. [Google Scholar] [CrossRef]
- Sun, W.; Ma, M.; Yu, H.; Yu, H. Inhibition of lncRNA X inactivate-specific transcript ameliorates inflammatory pain by suppressing satellite glial cell activation and inflammation by acting as a sponge of miR-146a to inhibit Nav 1.7. J. Cell. Biochem. 2018, 119, 9888–9898. [Google Scholar] [CrossRef]
- Dou, Q.; Ba, F.; Hu, S.; Xu, G.Y.; Wei, J.; Jiang, G.Q. LncRNA NONRATT014888.2 contributes to cancer-induced bone pain through downregulation of natriuretic peptide receptor 3 in rats. Biochem. Biophys. Res. Commun. 2023, 683, 149114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, C.; Wang, X.; Wang, L.; Chen, J.; Ji, F. LncRNA NONRATT009773.2 promotes bone cancer pain progression through the miR-708-5p/CXCL13 axis. Eur. J. Neurosci. 2022, 55, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, H.; Ji, Z. Downregulating lncRNA PVT1 Relieves Astrocyte Overactivation Induced Neuropathic Pain Through Targeting miR-186-5p/CXCL13/CXCR5 Axis. Neurochem. Res. 2021, 46, 1457–1469. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, D.; Li, Q. LncRNA CRNDE exacerbates neuropathic pain in chronic constriction injury-induced(CCI) rats through regulating miR-146a-5p/WNT5A pathway. Bioengineered 2021, 12, 7348–7359. [Google Scholar] [CrossRef]
- Huo, M.; Zheng, X.; Bai, N.; Xu, R.; Yang, G.; Zhao, Z. LncRNA PCAT19 Regulates Neuropathic Pain via Regulation of miR-182-5p/JMJD1A in a Rat Model of Chronic Constriction Injury. Neuroimmunomodulation 2022, 29, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, L.; Ren, S.; Zhang, Y.; Xue, J. Inhibition of lncRNA DILC attenuates neuropathic pain via the SOCS3/JAK2/STAT3 pathway. Biosci. Rep. 2020, 40, BSR20194486. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Lv, D.B.; Su, Y.N.; Wang, X.L.; Sheng, W.C.; Yang, G.; Li, L.X.; Gao, X.; Gao, Y.Z.; Li, J.T. LncRNA SNHG1 attenuates neuropathic pain following spinal cord injury by regulating CDK4 level. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12034–12040. [Google Scholar]
- Zhang, Z.; Sun, X.; Zhao, G.; Ma, Y.; Zeng, G. LncRNA embryonic stem cells expressed 1 (Lncenc1) is identified as a novel regulator in neuropathic pain by interacting with EZH2 and downregulating the expression of Bai1 in mouse microglia. Exp. Cell Res. 2021, 399, 112435. [Google Scholar] [CrossRef]
- Sun, R.M.; Wei, J.; Wang, S.S.; Xu, G.Y.; Jiang, G.Q. Upregulation of lncRNA-NONRATT021203.2 in the dorsal root ganglion contributes to cancer-induced pain via CXCL9 in rats. Biochem. Biophys. Res. Commun. 2020, 524, 983–989. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Liu, P.; He, Q.; Zhang, S.; Liu, Z.; Ni, C.; Chen, L.; Zhi, T.; Xu, L.; et al. LncRNA 51325 Alleviates Bone Cancer Induced Hyperalgesia Through Inhibition of Pum2. J. Pain Res. 2024, 17, 265–284. [Google Scholar] [CrossRef]
- Ni, H.; Xu, M.; Kuang, J.; Xu, C.; He, Q.; Luo, G.; Fu, J.; Zhu, J.; Ni, C.; Zhao, B.; et al. Upregulation of LncRNA71132 in the spinal cord regulates hypersensitivity in a rat model of bone cancer pain. Pain 2023, 164, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Li, J.; Zhang, K.Q. Structure, Regulation, and Function of Linear and Circular Long Non-Coding RNAs. Front. Genet. 2020, 11, 150. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Khanabdali, R.; Kalionis, B.; Tai, X.; Xia, S. Circular RNAs: Isolation, characterization and their potential role in diseases. RNA Biol. 2017, 14, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Tan, S.; Xin, J.; Yuan, Q.; Xu, H.; Xu, X.; Liang, Q.; Christiani, D.C.; Wang, M.; et al. Circular RNAs in body fluids as cancer biomarkers: The new frontier of liquid biopsies. Mol. Cancer 2021, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wilusz, J.E.; Chen, L.L. Biogenesis and Regulatory Roles of Circular RNAs. Annu. Rev. Cell. Dev. Biol. 2022, 38, 263–289. [Google Scholar] [CrossRef]
- Rogalska, M.E.; Vivori, C.; Valcárcel, J. Regulation of pre-mRNA splicing: Roles in physiology and disease, and therapeutic prospects. Nat. Rev. Genet. 2023, 24, 251–269. [Google Scholar] [CrossRef]
- Chen, I.; Chen, C.Y.; Chuang, T.J. Biogenesis, identification, and function of exonic circular RNAs. Wiley Interdiscip. Rev. RNA 2015, 6, 563–579. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.O.; Chen, T.; Xiang, J.F.; Yin, Q.F.; Xing, Y.H.; Zhu, S.; Yang, L.; Chen, L.L. Circular intronic long noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Y.; Chen, L. EIciRNAs in focus: Current understanding and future perspectives. RNA Biol. 2025, 22, 1–12. [Google Scholar] [CrossRef]
- Das, A.; Sinha, T.; Shyamal, S.; Panda, A.C. Emerging Role of Circular RNA-Protein Interactions. Noncoding RNA 2021, 7, 48. [Google Scholar] [CrossRef]
- Eger, N.; Schoppe, L.; Schuster, S.; Laufs, U.; Boeckel, J.N. Circular RNA Splicing. Adv. Exp. Med. Biol. 2018, 1087, 41–52. [Google Scholar] [PubMed]
- Pamudurti, N.R.; Patop, I.L.; Krishnamoorthy, A.; Bartok, O.; Maya, R.; Lerner, N.; Ashwall-Fluss, R.; Konakondla, J.V.V.; Beatus, T.; Kadener, S. circMbl functions in cis and in trans to regulate gene expression and physiology in a tissue-specific fashion. Cell Rep. 2022, 39, 110740. [Google Scholar] [CrossRef] [PubMed]
- Colantoni, A.; Capauto, D.; Alfano, V.; D’Ambra, E.; D’Uva, S.; Tartaglia, G.G.; Morlando, M. FUS Alters circRNA Metabolism in Human Motor Neurons Carrying the ALS-Linked P525L Mutation. Int. J. Mol. Sci. 2023, 24, 3181. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 39, 81–491. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.C. Circular RNAs Act as miRNA Sponges. Adv. Exp. Med. Biol. 2018, 1087, 67–79. [Google Scholar]
- Xiao, J.; Joseph, S.; Xia, M.; Teng, F.; Chen, X.; Huang, R.; Zhai, L.; Deng, W. Circular RNAs Acting as miRNAs’ Sponges and Their Roles in Stem Cells. J. Clin. Med. 2022, 11, 2909. [Google Scholar] [CrossRef]
- Shao, T.; Pan, Y.H.; Xiong, X.D. Circular RNA: An important player with multiple facets to regulate its parental gene expression. Mol. Ther. Nucleic Acids 2020, 23, 369–376. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Li, F.; Yu, K.; Bai, Y. The mechanism and detection of alternative splicing events in circular RNAs. PeerJ. 2020, 8, e10032. [Google Scholar] [CrossRef]
- Sinha, T.; Panigrahi, C.; Das, D.; Chandra Panda, A. Circular RNA translation, a path to hidden proteome. Wiley Interdiscip. Rev. RNA 2022, 13, e1685. [Google Scholar] [CrossRef]
- Chen, C.K.; Cheng, R.; Demeter, J.; Chen, J.; Weingarten-Gabbay, S.; Jiang, L.; Snyder, M.P.; Weissman, J.S.; Segal, E.; Jackson, P.K.; et al. Structured elements drive extensive circular RNA translation. Mol. Cell 2021, 81, 4300–4318.e13. [Google Scholar] [CrossRef]
- Di Timoteo, G.; Dattilo, D.; Centrón-Broco, A.; Colantoni, A.; Guarnacci, M.; Rossi, F.; Incarnato, D.; Oliviero, S.; Fatica, A.; Morlando, M.; et al. Modulation of circRNA Metabolism by m6A Modification. Cell Rep. 2020, 31, 107641. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.X.; Chen, L.L. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022, 185, 2016–2034. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.M.; Chinnaiyan, A.M.; Conn, S.J. Circular RNA in cancer. Nat. Rev. Cancer 2024, 24, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, P.; Qi, S.; Zhou, J.; Amalraj, J.; Wang, J.; Ding, Z. The clinical perspective of circular RNAs in neurodegenerative diseases: Potential diagnostic tools and therapeutic targets. Front. Cell. Neurosci. 2024, 18, 1470641. [Google Scholar] [CrossRef]
- Long, Q.; Lv, B.; Jiang, S.; Lin, J. The Landscape of Circular RNAs in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 4571. [Google Scholar] [CrossRef]
- Kurtović, Z.; Sandor, K.; Ter Heegde, F.; Rudjito, R.; Svensson, C.I.; Palada, V. circRNA landscape in dorsal root ganglia from mice with collagen antibody-induced arthritis. Neurobiol. Pain 2023, 14, 100142. [Google Scholar] [CrossRef]
- Siddiq, M.M.; Toro, C.A.; Johnson, N.P.; Hansen, J.; Xiong, Y.; Mellado, W.; Tolentino, R.E.; Johnson, K.; Jayaraman, G.; Suhail, Z.; et al. Spinal cord injury regulates circular RNA expression in axons. Front. Mol. Neurosci. 2023, 16, 1183315. [Google Scholar] [CrossRef]
- Chen, X.; Song, Y.; Chen, G.; Zhang, B.; Bai, Y.; Sun, C.; Fan, D.; Chen, Z. Circular RNA CircFOXO3 Functions as a Competitive Endogenous RNA for Acid-Sensing Ion Channel Subunit 1 Mediating Oxeiptosis in Nucleus Pulposus. Biomedicines 2024, 12, 678. [Google Scholar] [CrossRef]
- Benitez, M.B.M.; Navarro, Y.P.; Azuara-Liceaga, E.; Cruz, A.T.; Flores, J.V.; Lopez-Canovas, L. Circular RNAs and the regulation of gene expression in diabetic nephropathy (Review). Int. J. Mol. Med. 2024, 53, 44. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, S.; Liao, X.; Li, M.; Chen, S.; Li, X.; Wu, X.; Yang, M.; Tang, M.; Hu, Y.; et al. Circular RNA circIKBKB promotes breast cancer bone metastasis through sustaining NF-κB/bone remodeling factors signaling. Mol. Cancer 2021, 20, 98. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, C.; Zheng, Y. Involvement of circRNAs in Proinflammatory Cytokines-Mediated β-Cell Dysfunction. Mediat. Inflamm. 2021, 2021, 5566453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Xiao, D.; Wu, C.; Yang, J.; Peng, X.; Chen, L.; Zhang, J.; Zha, G.; Li, W.; Ju, R.; et al. Circular RNA-based therapy provides sustained and robust neuroprotection for retinal ganglion cells. Mol. Ther. Nucleic Acids 2024, 35, 102258. [Google Scholar] [CrossRef]
- Qadir, J.; Wen, S.Y.; Yuan, H.; Yang, B.B. CircRNAs regulate the crosstalk between inflammation and tumorigenesis: The bilateral association and molecular mechanisms. Mol. Ther. 2023, 31, 1514–1532. [Google Scholar] [CrossRef] [PubMed]
- Giusti, S.A.; Pino, N.S.; Pannunzio, C.; Ogando, M.B.; Armando, N.G.; Garrett, L.; Zimprich, A.; Becker, L.; Gimeno, M.L.; Lukin, J.; et al. A brain-enriched circular RNA controls excitatory neurotransmission and restricts sensitivity to aversive stimuli. Sci. Adv. 2024, 10, eadj8769. [Google Scholar] [CrossRef]
- You, X.; Vlatkovic, I.; Babic, A.; Will, T.; Epstein, I.; Tushev, G.; Akbalik, G.; Wang, M.; Glock, C.; Quedenau, C.; et al. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat. Neurosci. 2015, 18, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Dong, X.; Li, X.; Yang, Y.; Li, H.; Hong, Y.; Yang, G.; Kong, X.; Wang, X.; Ma, X. Moxibustion ameliorates chronic inflammatory visceral pain via spinal circRNA-miRNA-mRNA networks: A central mechanism study. Mol. Brain 2024, 17, 23. [Google Scholar] [CrossRef]
- Yang, S.Q.; Peng, L.; Lin, L.D.; Chen, Y.Z.; Liu, M.Z.; Zhang, C.; Chen, J.W.; Luo, D.Y. Identification of circRNA-miRNA-mRNA network as biomarkers for interstitial cystitis/bladder pain syndrome. Aging 2023, 15, 12155–12170. [Google Scholar] [CrossRef]
- He, A.T.; Liu, J.; Li, F.; Yang, B.B. Targeting circular RNAs as a therapeutic approach: Current strategies and challenges. Signal Transduct. Target. Ther. 2021, 6, 185. [Google Scholar] [CrossRef]
- Qi, D.; Ke, R.; Huang, J.H.; Wu, E. Forging the future of circRNA therapeutics: Unleashing synthetic potential and conquering challenges. Mol. Ther. Nucleic Acids 2023, 33, 42–43. [Google Scholar] [CrossRef]
- Pan, Z.; Li, G.F.; Sun, M.L.; Xie, L.; Liu, D.; Zhang, Q.; Yang, X.X.; Xia, S.; Liu, X.; Zhou, H.; et al. MicroRNA-1224 Splicing CircularRNA-Filip1l in an Ago2-Dependent Manner Regulates Chronic Inflammatory Pain via Targeting Ubr5. J. Neurosci. 2019, 39, 2125–2143. [Google Scholar] [CrossRef]
- Wang, L.; Luo, T.; Bao, Z.; Li, Y.; Bu, W. Intrathecal circHIPK3 shRNA alleviates neuropathic pain in diabetic rats. Biochem. Biophys. Res. Commun. 2018, 505, 644–650. [Google Scholar] [CrossRef]
- Wang, K.; Shen, Z.; Peng, X.; Wu, X.; Mao, L. Circular RNA-GRIN2B Suppresses Neuropathic Pain by Targeting the NF-κB/SLICK Pathway. Neuromol. Med. 2024, 26, 12. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Song, X.; Ge, Q. Circular RNA SMEK1 promotes neuropathic pain in rats through targeting microRNA-216a-5p to mediate Thioredoxin Interacting Protein (TXNIP) expression. Bioengineered 2021, 12, 5540–5551. [Google Scholar] [CrossRef]
- Li, L.; Luo, Y.; Zhang, Y.; Wei, M.; Zhang, M.; Liu, H.; Su, Z. CircZNF609 aggravates neuropathic pain via miR-22-3p/ENO1 axis in CCI rat models. Gene 2020, 763, 145069. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Jin, T.; Tao, Y.; Zhang, M.; Zheng, H.L.; Liu, Q.Q.; Yang, K.H.; Wei, R.N.; Li, S.Y.; Huang, Y.; et al. DHX9/DNA-tandem repeat-dependent downregulation of ciRNA-Fmn1 in the dorsal horn is required for neuropathic pain. Acta Pharmacol. Sin. 2023, 44, 1748–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, T.; Li, X.; Wen, C.C.; Yan, X.T.; Peng, C.; Xiao, Y. Circ_0005075 targeting miR-151a-3p promotes neuropathic pain in CCI rats via inducing NOTCH2 expression. Gene 2021, 767, 145079. [Google Scholar] [CrossRef]
- Tang, Q.; Fang, Z.; Liao, H.; Zhang, Y.; Li, C.; Zhou, C.; Liu, F.; Shen, J. Reduced circ_lrrc49 in trigeminal ganglion contributes to neuropathic pain in mice by downregulating Ist1 and impairing autophagy. J. Neurochem. 2024, 168, 1265–1280. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, M.; Liu, Q.; Wei, R.; Sun, M.; Zhang, Q.; Hao, L.; Xue, Z.; Wang, Q.; Yang, L.; et al. Downregulation of ciRNA-Kat6b in dorsal spinal horn is required for neuropathic pain by regulating Kcnk1 in miRNA-26a-dependent manner. CNS Neurosci. Ther. 2023, 29, 2955–2971. [Google Scholar] [CrossRef]
- Xu, T.; Li, Z.Y.; Liu, M.; Zhang, S.B.; Ding, H.H.; Wu, J.Y.; Lin, S.Y.; Liu, J.; Wei, J.Y.; Zhang, X.Q.; et al. CircFhit Modulates GABAergic Synaptic Transmission via Regulating the Parental Gene Fhit Expression in the Spinal Dorsal Horn in a Rat Model of Neuropathic pain. Neurosci. Bull. 2023, 39, 947–961. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lin, S.Y.; Liu, M.; Liu, C.C.; Ding, H.H.; Sun, Y.; Ma, C.; Guo, R.X.; Lv, Y.Y.; Wu, S.L.; et al. CircAnks1a in the spinal cord regulates hypersensitivity in a rodent model of neuropathic pain. Nat. Commun. 2019, 10, 4119. [Google Scholar] [CrossRef]
- Wei, M.; Li, L.; Zhang, Y.; Zhang, M.; Su, Z. Downregulated circular RNA zRANB1 mediates Wnt5a/β-Catenin signaling to promote neuropathic pain via miR-24-3p/LPAR3 axis in CCI rat models. Gene 2020, 761, 145038. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.W.; Zhang, X.X.; Peng, Z.D.; Xing, Z.M.; Zhang, Y.W.; Li, Y.L. The circular RNA circSlc7a11 promotes bone cancer pain pathogenesis in rats by modulating LLC-WRC 256 cell proliferation and apoptosis. Mol. Cell Biochem. 2021, 476, 1751–1763. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, L.; Liang, Y.; Lei, H.; Qin, M.; Li, X.; Ren, Y. MicroRNA therapeutic delivery strategies: A review. J. Drug Deliv. Sci. Technol. 2024, 93, 105430. [Google Scholar] [CrossRef]
- Ali Zaidi, S.S.; Fatima, F.; Ali Zaidi, S.A.; Zhou, D.; Deng, W.; Liu, S. Engineering siRNA therapeutics: Challenges and strategies. J. Nanobiotechnology 2023, 21, 381. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Zhu, S.X.; Pu, K.J.; Huang, H.J.; Chen, Y.Q.; Wang, W.T. New insight into circRNAs: Characterization, strategies, and biomedical applications. Exp. Hematol. Oncol. 2023, 12, 91. [Google Scholar] [CrossRef]
- Han, S.; Chen, X.; Huang, L. The tumor therapeutic potential of long non-coding RNA delivery and targeting. Acta Pharm. Sin. B 2023, 13, 1371–1382. [Google Scholar] [CrossRef]
- Hossam Abdelmonem, B.; Kamal, L.T.; Wardy, L.W.; Ragheb, M.; Hanna, M.M.; Elsharkawy, M.; Abdelnaser, A. Non-coding RNAs: Emerging biomarkers and therapeutic targets in cancer and inflammatory diseases. Front. Oncol. 2025, 15, 1534862. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Chu, H.; Ma, J.; Yan, Y.; Zhang, X.; Liang, Y. The Regulatory Mechanisms and Therapeutic Potential of MicroRNAs: From Chronic Pain to Morphine Tolerance. Front. Mol. Neurosci. 2018, 11, 80. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.; Jin, X.; Hua, N.; Liu, H.; Qi, R.; Huang, Z.; Sun, Y.; Jiang, D.; Snutch, T.P.; et al. Epigenetic regulation of beta-endorphin synthesis in hypothalamic arcuate nucleus neurons modulates neuropathic pain in a rodent pain model. Nat. Commun. 2023, 14, 7234. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long non-coding RNAs: From disease code to drug role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef]
- Li, Y.Z.; Ji, R.R. Gene therapy for chronic pain management. Cell Rep. Med. 2024, 5, 101756. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA therapeutics—Challenges and potential solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Galuppini, F.; Corbo, V.; Fassan, M. Current role of non-coding RNAs in the clinical setting. Noncoding RNA Res. 2019, 4, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Karthik, S.; Mohan, S.; Magesh, I.; Bharathy, A.; Kolipaka, R.; Ganesamoorthi, S.; Sathiya, K.; Shanmugavadivu, A.; Gurunathan, R.; Selvamurugan, N. Chitosan nanocarriers for non-coding RNA therapeutics: A review. Int. J. Biol. Macromol. 2024, 263, 130361. [Google Scholar] [CrossRef]
- Rincón-Riveros, A.; Morales, D.; Rodríguez, J.A.; Villegas, V.E.; López-Kleine, L. Bioinformatic Tools for the Analysis and Prediction of ncRNA Interactions. Int. J. Mol. Sci. 2021, 222, 11397. [Google Scholar] [CrossRef]
- Ito, M.; Miyata, Y.; Okada, M. Current clinical trials with non-coding RNA-based therapeutics in malignant diseases: A systematic review. Transl. Oncol. 2023, 31, 101634. [Google Scholar] [CrossRef]

| miRNA | Pain Condition | Pain Model | Effects on Pain | References |
|---|---|---|---|---|
| miR-7 | Neuropathic pain | SNL | Anti-hyperalgesic (miR-7a) | [76] |
| miR-15 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-15a) | [77] |
| CIPN (Oxaliplatin) | Hyperalgesic (miR-15b) | [78] | ||
| miR-16 | Inflammatory pain | CFA | Anti-hyperalgesic | [79] |
| Neuropathic pain | CCI | [80] | ||
| miR-20 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-20b-5p) | [81] |
| miR-21 | Neuropathic pain | SNI | Hyperalgesic (miR-21-5p) | [82,83] |
| miR-26 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-26a-5p) | [84] |
| Inflammatory pain | CFA | [85] | ||
| miR-29 | Inflammatory pain | CFA | Hyperalgesic (miR-29a) | [86] |
| Neuropathic pain | SNI | Hyperalgesic (miR-29c) | [87] | |
| Inflammatory pain | LPS | Anti-hyperalgesic (miR-30a-5p) | [88] | |
| miR-30 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-30b-5p) | [89] |
| SNI | Anti-hyperalgesic (miR-30c-5p) | [90] | ||
| miR-34 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-34a) | [91] |
| Anti-hyperalgesic (miR-34c) | [92] | |||
| Inflammatory pain | CFA | Anti-hyperalgesic (miR-34a) | [93] | |
| CIVP | [94] | |||
| miR-101 | Neuropathic pain | CCI | Hyperalgesic | [95] |
| Anti-hyperalgesic | [96] | |||
| miR-103 | Neuropathic pain | SNL | Anti-hyperalgesic | [97] |
| miR-107 | Inflammatory pain | CFA | Hyperalgesic | [98] |
| miR-124 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-124-3p) | [99] |
| SNL | [100] | |||
| Inflammatory pain | CFA | [101] | ||
| miR-125 | Inflammatory pain | CFA | Anti-hyperalgesic (miR-125a-3p) | [102] |
| Neuropathic pain | DPN | Anti-hyperalgesic (miR-125a-5p) | [103] | |
| CCI | Anti-hyperalgesic (miR-125b-5p) | [104] | ||
| miR-128 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-128-3p) | [105] |
| SCI | [106] | |||
| miR-130 | Neuropathic pain | SCI | Hyperalgesic (miR-130a-3p) | [107] |
| Hyperalgesic (miR-130a-5p) | [108] | |||
| miR-132 | Neuropathic pain | SNI | Hyperalgesic (miR-132-3p) | [109] |
| miR-134 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-134-5p) | [110] |
| Inflammatory pain | CFA | Hyperalgesic | [111] | |
| miR-135 | Neuropathic pain | CCI | Hyperalgesic (miR-135a-5p) | [112,113] |
| Cancer pain | BCP | Anti-hyperalgesic (miR-135-5p) | [114] | |
| miR-142 | Neuropathic pain | SNL | Anti-hyperalgesic (miR-142-3p) | [115] |
| CCI | Anti-hyperalgesic (miR-142-5p) | [116] | ||
| miR-146 | Neuropathic pain | SNL | Anti-hyperalgesic (miR-146-3p) | [117] |
| CCI | [118] | |||
| Cancer pain | CRC | Anti-hyperalgesic (miR-146a) | [119] | |
| miR-155 | Neuropathic pain | bCCI | Hyperalgesic | [120] |
| DPN | [121] | |||
| miR-181 | Neuropathic pain | SNL | Anti-hyperalgesic (miR-181b) | [122] |
| CCI | Anti-hyperalgesic (miR-181c-5p) | [123] | ||
| miR-183 | Neuropathic pain | CCI | Anti-hyperalgesic | [124,125] |
| miR-190 | Neuropathic pain | DPN | Anti-hyperalgesic (miR-190a-5p) | [126] |
| miR-195 | Neuropathic pain | SNL | Hyperalgesic | [127] |
| CCI-IoN | [128] | |||
| miR-199 | Neuropathic pain | CCD | Anti-hyperalgesic | [129] |
| Cancer pain | BCP | [130] | ||
| miR-203 | Neuropathic pain | bCCI | Hyperalgesic | [131] |
| miR-212 | Neuropathic pain | CCI | Anti-hyperalgesic (miR-212-3p) | [132] |
| SCI | [133] | |||
| miR-219 | Inflammatory pain | CFA | Anti-hyperalgesic | [134] |
| miR-223 | Neuropathic pain | CCI | Anti-hyperalgesic | [135] |
| CCI-IoN | Anti-hyperalgesic (miR-223-3p) | [136] | ||
| miR-301 | Neuropathic pain | SNI | Hyperalgesic | [137] |
| miR-320 | Neuropathic pain | CCI | Hyperalgesic (miR-320a) | [138] |
| miR-330 | Neuropathic pain | CCI | Hyperalgesic (miR-330-3p) | [139] |
| miR-365 | Inflammatory pain | CFA | Hyperalgesic (miR-365-3p) | [140] |
| miR-382 | Neuropathic pain | SCI | Hyperalgesic (miR-382-5p) | [141] |
| CCI | [142] | |||
| miR-500 | Neuropathic pain | CIPN (Paclitaxel) | Hyperalgesic | [143] |
| L5-VRT | ||||
| miR-539 | Neuropathic pain | CCI | Anti-hyperalgesic | [144] |
| miR-541 | Neuropathic pain | bCCI | Hyperalgesic | [145] |
| miR-544 | Inflammatory pain | AIA-CFA | Anti-hyperalgesic (miR-544-3p) | [146] |
| Neuropathic pain | CCI | Hyperalgesic | [147] | |
| miR-672 | Neuropathic pain | BTZ | Hyperalgesic (miR-672-5p) | [148] |
| siRNA | Pain Condition | Pain Model | Effects on Pain | References |
|---|---|---|---|---|
| PKM2-siRNA | Neuropathic pain | CCI | Reduced mechanical sensitivity and thermal pain response associated with decreased ERK and STAT3 activation. | [169] |
| NR2B-siRNA | Inflammatory pain | CFA | Attenuating nociceptive responses. | [170] |
| NAMPT-siRNA | Inflammatory pain | CFA | Pain relief is achieved through the inhibition of the NF-κB/IL-1β inflammatory pathway. | [171] |
| PARP1-siRNA | Neuropathic pain | DPN | PARP1 silencing reduces neuropathic symptoms. | [172] |
| IFT52-siRNA | Neuropathic pain | CIPN (Paclitaxel) | A decrease in primary cilia was correlated with an elevated mechanical nociceptive threshold. | [173] |
| IFT88-siRNA | ||||
| IKBKB-siRNA | Neuropathic pain | SNL | Mechanical allodynia was blocked, and the release of pro-inflammatory mediators driven by NF-κB was reduced. | [174] |
| TLR4-siRNA | Cancer pain | BCP | Intrathecal injection of TLR4-siRNA diminished nociception induced by Walker 256 cells. | [175] |
| PANX1-siRNA | Neuropathic pain | CCI | Knockdown of PANX1 in Schwann cells alleviated neuropathic pain. | [176] |
| TBK1-siRNA | Neuropathic pain | PDN | TBK1 activates the NF-κB pathway, triggers NLRP3 activation, causes microglia pyroptosis, all of which can be reversed by TBK1-siRNA injection. | [177] |
| Neurexin 2-siRNA | Inflammatory pain | CFA | Intrathecal neurexin 2-siRNA reduced CFA-induced mechanical and thermal hyperalgesia and decreased the expression of glutamate receptors in the SDH. | [178] |
| TRAF6-siRNA | Inflammatory pain | CFA | TRAF6-siRNA reduced CFA-induced allodynia and reversed the increase in IBA-1 expression. | [179] |
| NMUR2-siRNA | Cancer pain | BCP | NMUR2-siRNA alleviates BCP through the inactivation of the PKC/ERK and PI3K/PKB signaling pathways. | [180] |
| TRPV1-siRNA | Neuropathic pain | CIPN (Paclitaxel) | Intrathecal TRPV1-siRNA administration reduced paclitaxel-induced mechanical allodynia/hyperalgesia and thermal hyperalgesia. | [181] |
| Cancer pain | BCP | Intrathecal administration of AAV-mediated TRPV1-siRNA enhanced both mechanical and thermal thresholds. | [182] | |
| BRD4-siRNA | Neuropathic pain | CIPN (Vincristine) | The transfection of BRD-4-siRNA alleviated neuropathic pain caused by vincristine. | [183] |
| NR1-siRNA | Inflammatory pain | CFA | NR1-siRNA effectively reduced the nociceptive response induced by CFA stimulation. | [184] |
| STAT3-siRNA | Inflammatory pain | LPS | Blocking STAT3 activity reduced mechanical allodynia and was associated with fewer reactive astrocytes in the SDH. | [185] |
| TDAG8-siRNA | Cancer pain | BCP | Intrathecal siRNA-TDAG8 reduced BCP behaviors during both the onset and maintenance phases. | [186] |
| RAB11A-siRNA | Inflammatory pain | CFA | The injection of RAB11A-siRNA into the SDH led to a significant analgesic effect following CFA injection. | [187] |
| SHP1-siRNA | Inflammatory pain | CFA | SHP1-siRNA alleviated CFA-induced pain. | [188] |
| IL-36γ siRNA | Inflammatory pain | CFA | IL-36γ-siRNA significantly reduced chronic inflammatory pain behaviors induced by CFA. | [189] |
| CCL2-siRNA | Neuropathic pain | SCI | In vivo depletion of CCL2 reduced the intensity of chronic spinal compression and its associated pain. | [190] |
| KCC2-siRNA | Inflammatory pain | CFA | Intrathecal administration of KCC2-siRNA in naïve rats decreased KCC2 expression in the spinal cord, resulting in heightened pain behaviors and disrupted inhibitory synaptic transmission. | [191] |
| CXCR2-siRNA | Inflammatory pain | CFA | Perisciatic nerve injection of CXCR2-siRNA reduced CFA-induced mechanical allodynia and thermal hyperalgesia. | [192] |
| MT-I-siRNA | Inflammatory pain | CFA | Treatment with MT-I-siRNA prior to CFA injection or shortly after CCI significantly reduced mechanical allodynia and thermal hyperalgesia. | [193] |
| TRPM2-siRNA | Neuropathic pain | CCI | Treatment with TRPM2-siRNA during the early phase after CCI reduced injury-induced neuropathic pain. | [194] |
| ASIC3-siRNA | Inflammatory pain | CFA | ASIC3-siRNA exerts strong analgesic effects against primary inflammation-induced hyperalgesia. | [195] |
| CACNA1H-siRNA | Inflammatory pain | CFA | CACNA1H knockdown alleviated inflammatory pain. | [196] |
| CX3CR1-siRNA | Neuropathic pain | SNL | CX3CR1-siRNA treatment reduced microglial activation in the SDH, lowered pro-inflammatory mediators, and significantly decreased mechanical allodynia. | [197] |
| Vimentin-siRNA | Neuropathic pain | CCI | Vimentin knockdown reduced p-ERK upregulation, decreased vimentin expression, and lowered the release of pro-inflammatory cytokines. | [198] |
| PDGF-siRNA | Cancer pain | BCP | Intrathecal injection of PDGF-siRNA alleviated thermal and mechanical hyperalgesia in BCP rats. | [199] |
| PI3KCB-siRNA | Cancer pain | BCP | Silencing of PI3KCB using siRNA led to a reduction in the expression of GFAP and OX42. | [200] |
| lncRNA | Pain Condition | Pain Model | Effects on Pain | References |
|---|---|---|---|---|
| lncRNA MEG3 | Inflammatory pain | CFA | lncRNA MEG3 is negatively correlated with TRPV1 mRNA in the DRG and SDH of CFA-induced rats. Therefore, the intrathecal delivery of a lentivirus overexpressing MEG3 significantly suppressed TRPV1 expression and relieved chronic inflammatory pain. | [238] |
| lncRNA NEAT1 | Neuropathic pain | SNL | NEAT1 lncRNA regulated the expression of pro-inflammatory genes in the DRG of rats with neuropathic pain. NEAT1 increased the expression of pro-inflammatory genes by stabilizing its associated mRNAs in neuropathic pain. | [239] |
| lncRNA XIST | Inflammatory pain | CFA | The inhibition of the lncRNA XIST alleviated inflammatory pain by inhibiting satellite glial cell activation and inflammation. | [240] |
| lncRNA p21 | Neuropathic pain | SNL | LncRNA p21 aggravated neuropathic pain by increasing TNFAIP1 expression and suppressing the AKT/CREB pathway. | [122] |
| lncRNA NONRATT014888.2 | Cancer pain | BCP | lncRNA NONRATT014888.2 is highly expressed in tibia-related DRGs of BCP rats. Its downregulation by siRNA in BCP rats significantly reduced hind-paw mechanical pain hypersensitivity. | [241] |
| lncRNA NONRATT009773.2 | Cancer pain | BCP | lncRNA NONRATT009773.2 was significantly up-regulated in BCP model. Depletion of lncRNA NONRATT009773.2 reduced BCP, while its overexpression triggered pain-like symptoms in naïve rats. Additionally, lncRNA NONRATT009773.2 acted as a miRNA sponge to absorb miR-708-5p and up-regulated the downstream target CXCL13, which plays a crucial role in hyperalgesia. | [242] |
| lncRNA PVT | Neuropathic pain | SCI | In the SCI model, PVT1 depletion significantly alleviated neuropathic pain, astrocytic activation, and reduced the expression of CXCL13/CXCR5. | [243] |
| lncRNA CRNDE | Neuropathic pain | CCI | lncRNA CRNDE intensified neuropathic pain in CCI rats by regulating the miR-146a-5p/WNT5A signaling pathway. | [244] |
| lncRNA FTX | Neuropathic pain | CCI | lncRNA FTX alleviated neuropathic pain by targeting miR-320a. | [138] |
| lncRNA PCAT19 | Neuropathic pain | CCI | lncRNA PCAT19 influenced neuropathic pain by modulating the miR-182-5p/JMJD1A pathway. | [245] |
| lncRNA DILC | Neuropathic pain | bCCI | Suppression of lncRNA DILC alleviated neuropathic pain through the regulation of the SOCS3/JAK2/STAT3 pathway. | [246] |
| lncRNA SNHG1 | Neuropathic pain | SNL | The inhibition of SNHG1 reduced the progression of neuropathic pain, while its overexpression was sufficient to trigger neuropathic pain symptoms in naïve rats. | [247] |
| Lncenc1 | Neuropathic pain | pSNL | Knockdown of Lncenc1 reduced the development and maintenance of mechanical and thermal hyperalgesia in pSNL mice, accompanied by increased BAI1 expression and decreased EZH2 expression in microglia. | [248] |
| lncRNA NONRATT021203.2 | Cancer pain | BCP | lncRNA NONRATT021203.2 was increased in BCP rats and silencing it with siRNA reduced significantly hyperalgesia. lncRNA NONRATT021203.2 targeted CXCL9, which was also increased in BCP rats. | [249] |
| lncRNA 51325 | Cancer pain | BCP | The overexpression of lncRNA 51325 significantly alleviated mechanical allodynia in BCP rats, while its knockdown induced pain behaviors and anxiety-like responses in naïve rats. | [250] |
| lncRNA 71132 | Cancer pain | BCP | Spinal lncRNA 71132 was significantly upregulated in BCP. Its knockdown reversed BCP behaviors and reduced spinal c-Fos neuronal sensitization, while overexpression in naïve rats induced pain behaviors and heightened c-Fos sensitization. Additionally, lncRNA 71132 modulated BCP by inversely regulating miR-143-5p processing, with increased lncRNA 71132 expression leading to decreased miR-143 levels under BCP conditions. | [251] |
| circRNA | Pain Condition | Pain Model | Effects on Pain | References |
|---|---|---|---|---|
| circRNA_02767 | Inflammatory pain | CIVP | Moxibustion alleviated visceral pain in CIVP rats by modulating the circRNA_02767/rno-miR-483-3p/GFAP network in the spinal cord, thereby reducing central sensitization. | [286] |
| circRNA-Filip1l | Inflammatory pain | CFA | This study revealed that chronic inflammatory pain induced by CFA significantly upregulated circRNA-Filip1l expression in spinal neurons. Inhibiting this increase alleviated nociceptive behaviors, while its overexpression in naïve mice replicated pain responses, lowering thermal and mechanical nociceptive thresholds. | [290] |
| circHIPK3 | Neuropathic pain | DPN | The research found that circHIPK3 is highly abundant in the serum of diabetes patients with neuropathic pain and in the DRG of STZ-induced diabetic rats. Silencing circHIPK3 alleviated neuropathic pain in diabetic rats by modulating neuroinflammation. circHIPK3 negatively regulated miR-124. Notably, inhibiting miR-124 reversed the pain relief and neuroinflammation reduction caused by circHIPK3 knockdown in diabetic rats. | [291] |
| circGRIN2B | Neuropathic pain | CCI | Overexpression of circGRIN2B has been shown to alleviate neuropathic pain by reducing mechanical and thermal hyperalgesia. This upregulation also significantly decreases pro-inflammatory cytokine levels (IL-1β, IL-6, and TNF-α) in the DRG. These findings suggest that circGRIN2B may mitigate neuropathic pain by modulating the NF-κB/SLICK pathway. | [292] |
| circSMEK1 | Neuropathic pain | CCI | The results demonstrated that circSMEK1 and TXNIP were upregulated in neuropathic pain. Knockdown of circSMEK1 increased the claw retraction threshold and decreased claw retraction latency in rats. Additionally, circSMEK1 knockout reduced pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6) in the spinal cord, suppressed microglial activation, and promoted microglial polarization toward the anti-inflammatory phenotype. Conversely, circSMEK1 upregulation had the opposite effects. | [293] |
| circZNF609 | Neuropathic pain | CCI | This study demonstrated that circZNF609 exacerbates neuropathic pain progression by promoting the expression of pro-inflammatory factors through the miR-22-3p/ENO1 axis. | [294] |
| ciRNA-Fmn1 | Neuropathic pain | CCI | The downregulation of ciRNA-Fmn1, resulting from altered DHX9 binding to DNA tandem repeats, contributed to the development of neuropathic pain by negatively regulating UBR5-mediated ALB expression in the spinal dorsal horn. | [295] |
| circ_0005075 | Neuropathic pain | CCI | circ_0005075 was upregulated in CCI rat models. Knockdown of circ_0005075 attenuated neuropathic pain behaviors, including mechanical and thermal hyperalgesia. Furthermore, loss of circ_0005075 reduced neuroinflammation by targeting COX-2, IL-6, and TNF-α, while promoting the expression of IL-10. | [296] |
| circ_lrrc49 | Neuropathic pain | CCI-IoN | Knockdown of circ_lrrc49 using siRNA decreased IST1 expression, elevated LC3-II and p62 levels, and increased the number of autophagosomes. This also induced orofacial mechanical hypersensitivity, an effect that could be reversed by IST1 overexpression. | [297] |
| ciRS-7 | Neuropathic pain | CCI | ciRS-7 is associated with the progression of neuropathic pain, partly by upregulating autophagy and inflammation in CCI rats. Furthermore, ciRS-7 regulated neuropathic pain progression by sponging miR-135a-5p. In CCI rats, inhibition of miR-135a-5p reduced autophagy and pro-inflammatory cytokines, thereby alleviating neuropathic pain. | [113] |
| ciRNA-Kat6b | Neuropathic pain | CCI | Peripheral nerve injury downregulated ciRNA-Kat6b in the spinal horn of male mice. Restoring ciRNA-Kat6b expression alleviated CCI-induced pain hypersensitivities. The downregulation of ciRNA-Kat6b decreased the binding of miRNA-26a to ciRNA-Kat6b, while increasing its binding to the 3′-UTR of KCNK1 mRNA, leading to the degradation of KCNK1 mRNA and a reduction in Kcnk1 protein levels in the dorsal horn of neuropathic pain mice. | [298] |
| circFhit | Neuropathic pain | SNI | circFhit, an exon-intron circRNA in GABAergic neurons, reduced inhibitory transmission in the spinal dorsal horn, contributing to neuropathic pain after SNI. CircFhit downregulated GAD65 expression and induced hyperexcitation in NK1R+ neurons. | [299] |
| circAnks1a | Neuropathic pain | SNL | SNL upregulated circAnks1a in dorsal horn neurons, enhancing VEGFB expression through dual mechanisms. In the nucleus, circAnks1a binds the VEGFB promoter, promoting Ybx1 recruitment and transcription. In the cytoplasm, it acts as a miR-324-3p sponge, preventing VEGFB downregulation. Elevated VEGFB protein enhances neuronal excitability, contributing to nerve injury-induced pain. | [300] |
| circRNA cZRANB1 | Neuropathic pain | CCI | cZRANB1 promoted neuropathic pain in CCI rat models by modulating Wnt5a/β-Catenin signaling via the miR-24-3p/LPAR3 axis. | [301] |
| circSlc7a11 | Cancer pain | BCP | The circRNA circSlc7a11 regulated BCP progression in rats by modulating Walker-256 cell proliferation and apoptosis through multiple signaling pathways. | [302] |
| ncRNA Class | Stability | Specificity | Delivery Challenges | Clinical Translation Strength | Limitations | References |
|---|---|---|---|---|---|---|
| miRNA | Moderate | High | Moderate (requires carriers) | Abundant data in pain models Good biomarker potential | Off-target effects (redundancy in miRNA-mRNA interactions) | [303] |
| siRNA | High | Very high | Moderate to high (delivery to neurons remains challenging) | Potent gene silencing | Immune activation Transient effects | [304] |
| circRNA | High | Moderate | Moderate (emerging delivery methods) | Excellent stability Novel biomarker potential | Function not understood Complex to manipulate | [305] |
| lncRNA | Low | High | High (large size and poor uptake limit therapeutic delivery) | Highly specific functions in neuronal and immune pathways | Limited in vivo data Complex structure- function relationships | [306] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. Role of ncRNAs in the Development of Chronic Pain. Non-Coding RNA 2025, 11, 51. https://doi.org/10.3390/ncrna11040051
García-Domínguez M. Role of ncRNAs in the Development of Chronic Pain. Non-Coding RNA. 2025; 11(4):51. https://doi.org/10.3390/ncrna11040051
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "Role of ncRNAs in the Development of Chronic Pain" Non-Coding RNA 11, no. 4: 51. https://doi.org/10.3390/ncrna11040051
APA StyleGarcía-Domínguez, M. (2025). Role of ncRNAs in the Development of Chronic Pain. Non-Coding RNA, 11(4), 51. https://doi.org/10.3390/ncrna11040051





