Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice
Abstract
1. Introduction
2. Results
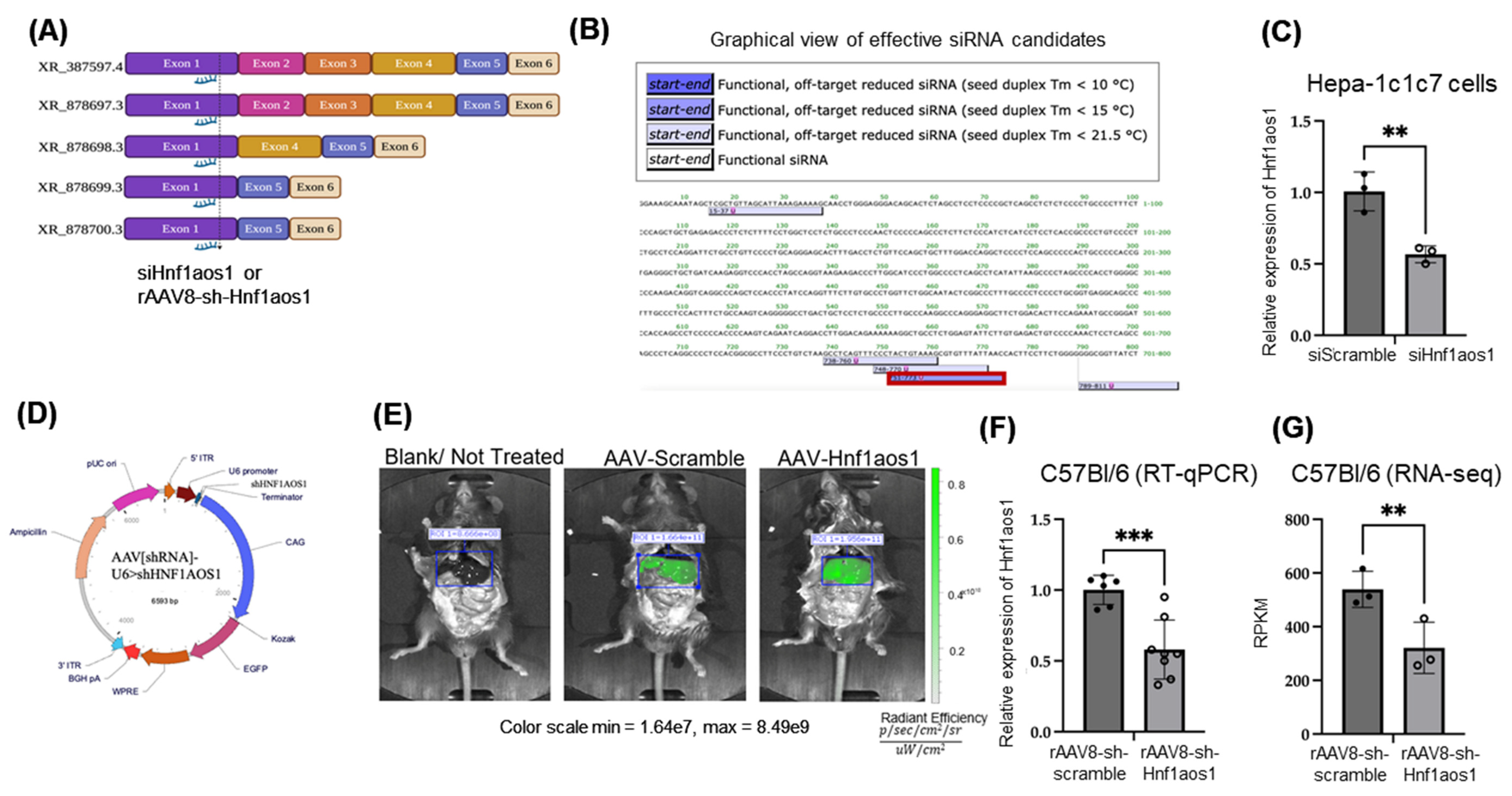
2.1. Effective Knockdown of lncRNA Hnf1aos1 in In Vitro Hepa1C1c7 Cells via siRNA and In Vivo Mouse Liver via rAAV8-sh-Hnf1aos1 Approaches
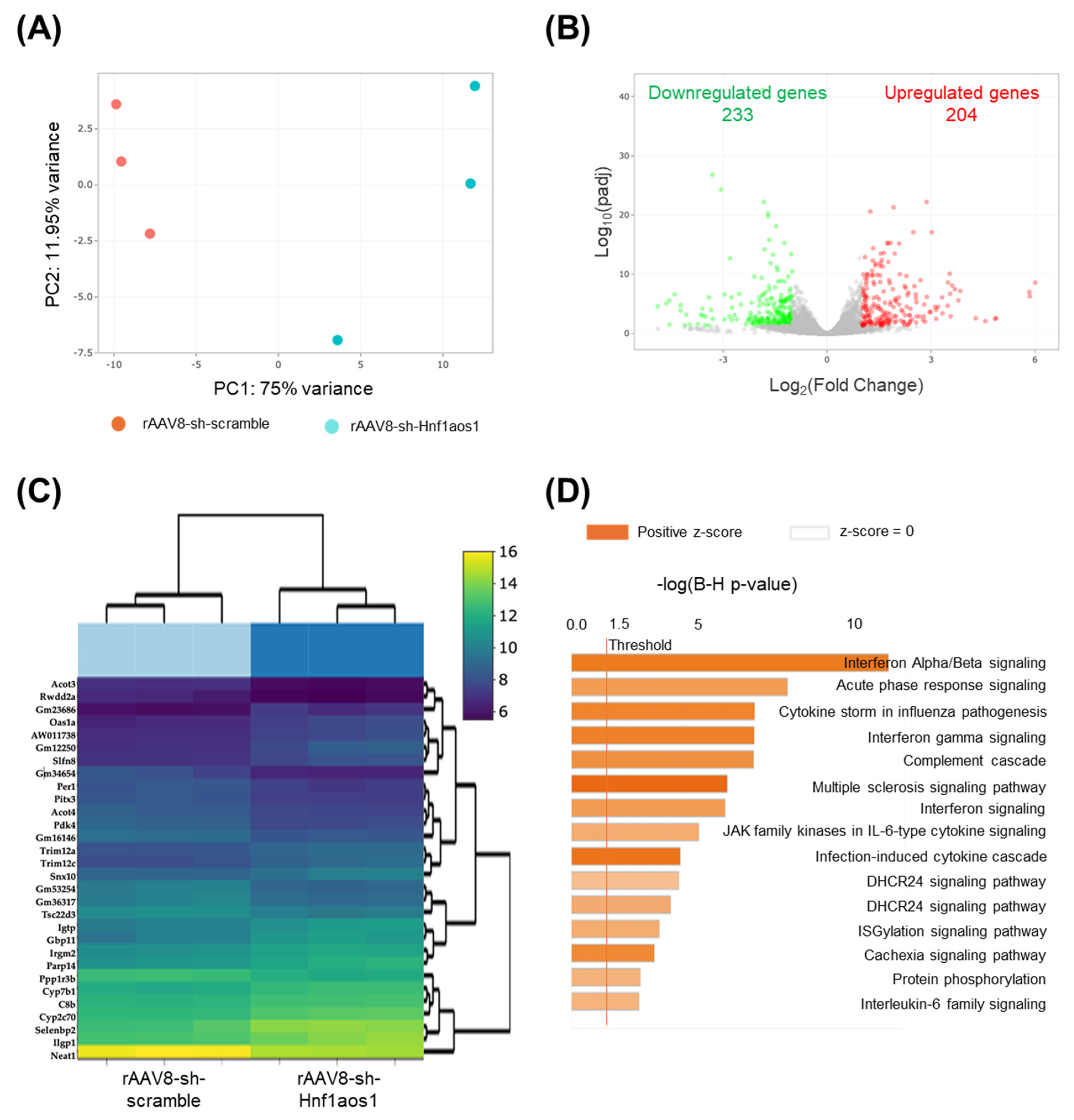
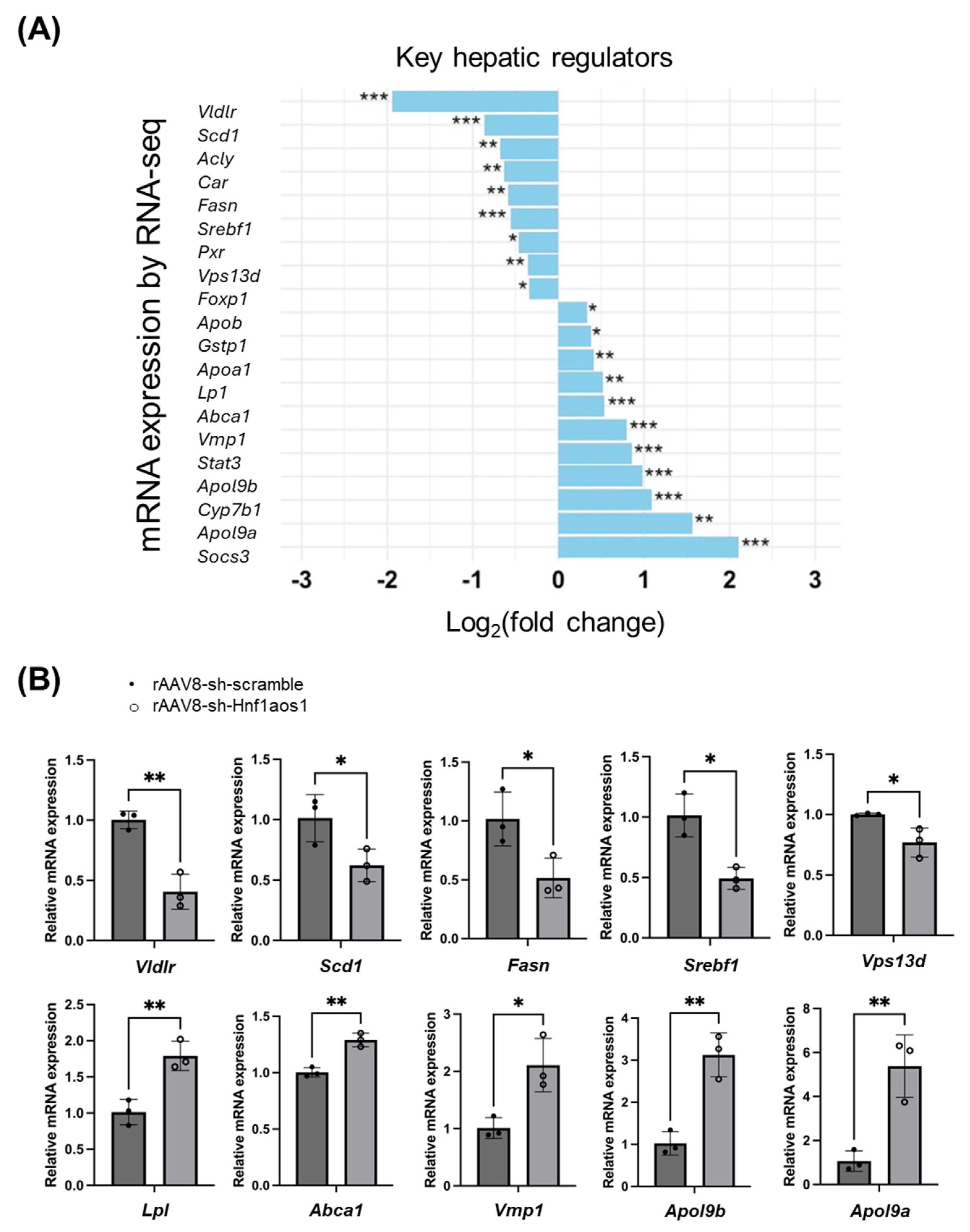
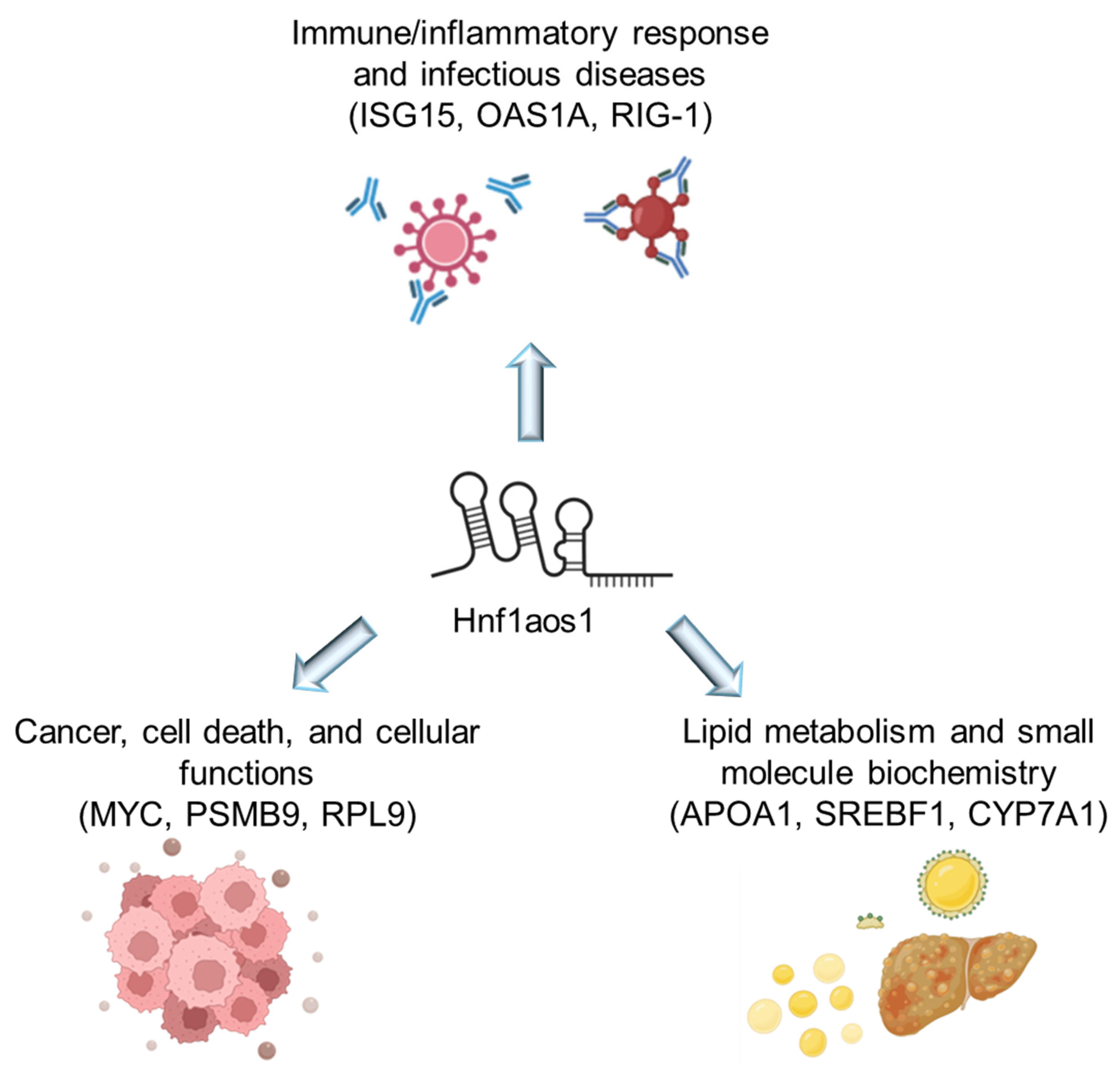
2.2. The Knockdown of Hnf1aos1 Induces Widespread Transcriptional Changes in Liver Metabolism and Immune Pathways
2.3. Hnf1aos1 Knockdown Induces Widespread Proteomic Changes in Liver Metabolism and Immune Pathways
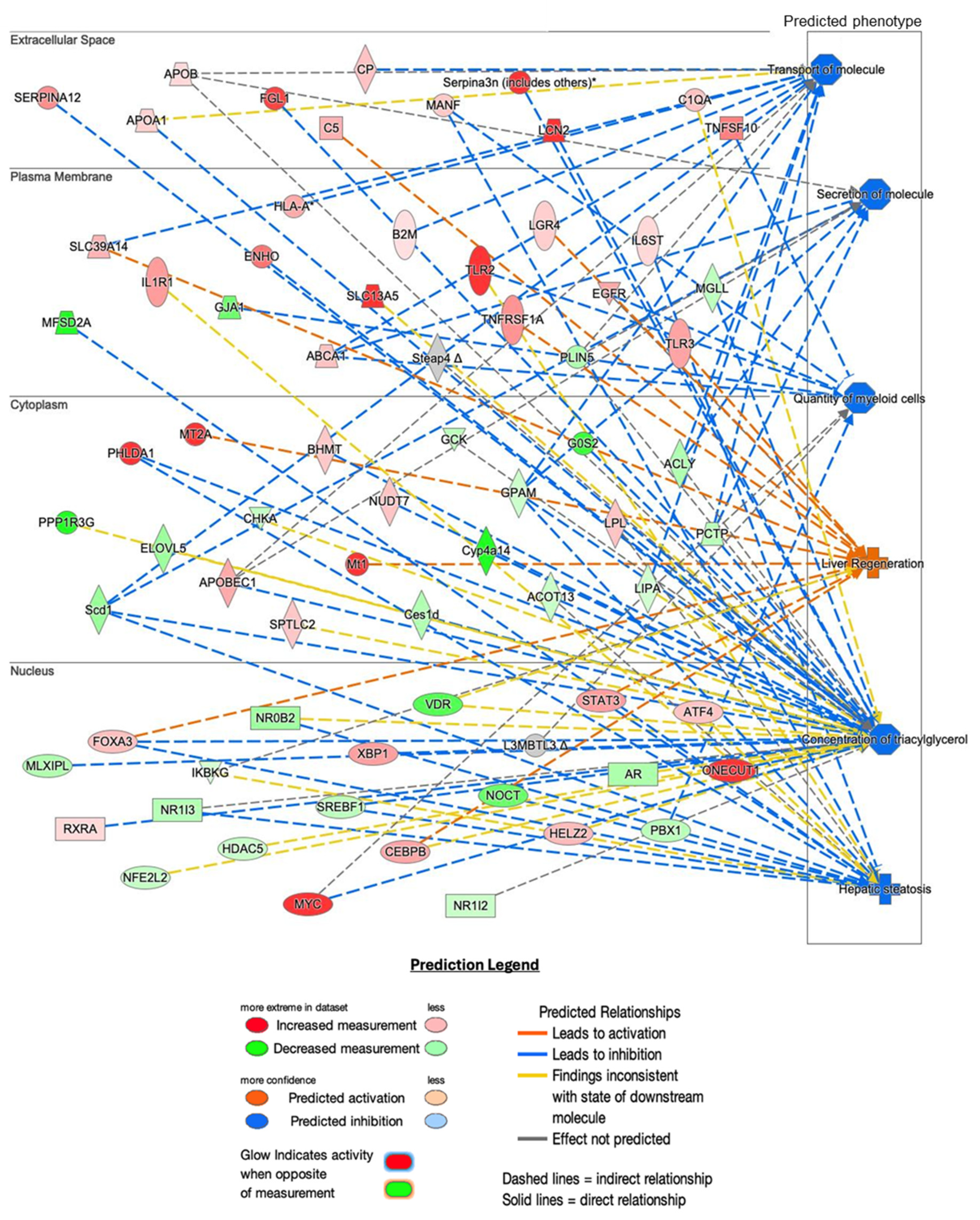
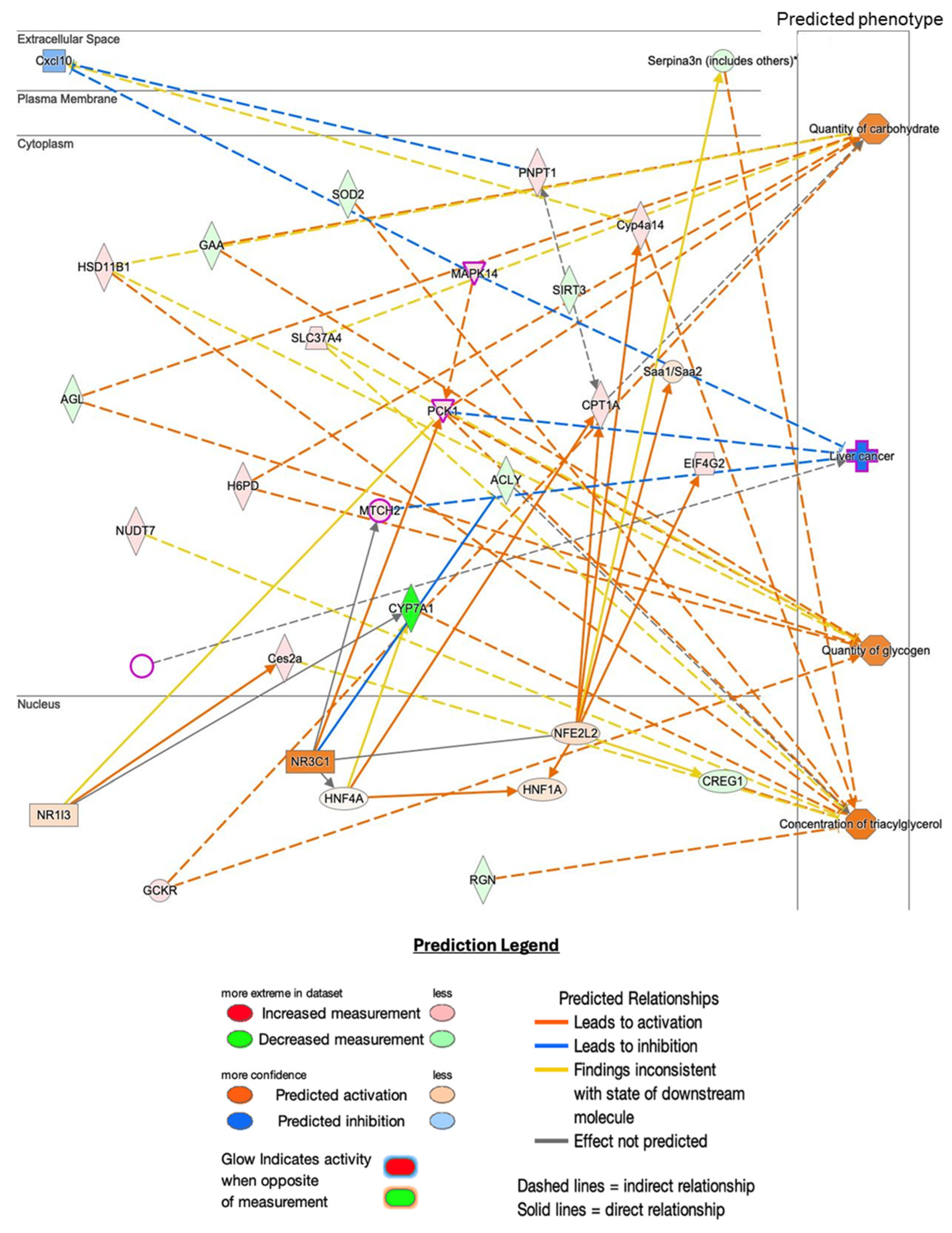
2.4. Shared Networks Between mRNA and Protein Expression Analysis
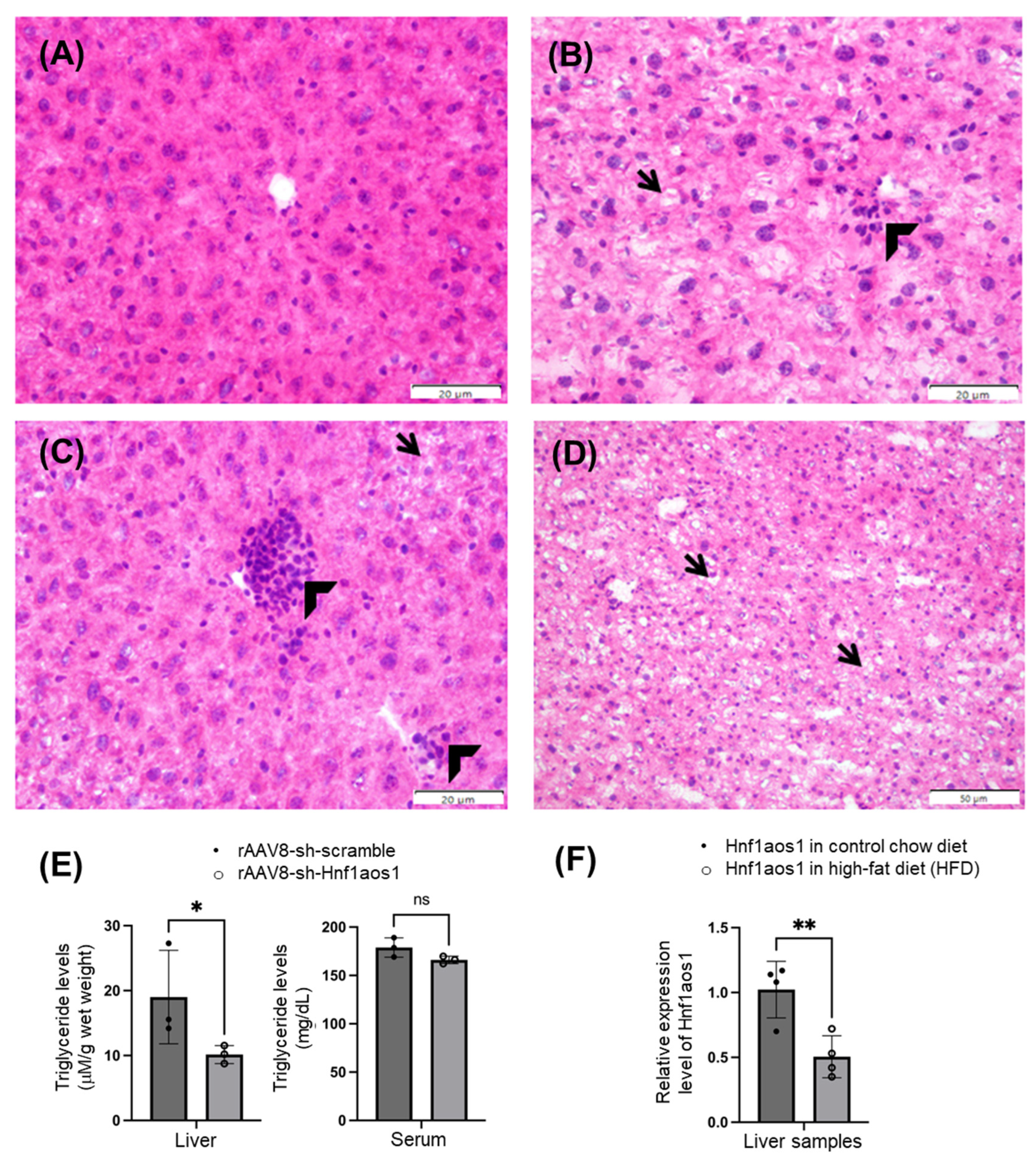
2.5. Histopathological Analysis and Predicted Metabolic Impacts on Liver Health
3. Discussion
3.1. Hnf1aos1 Knockdown Disrupts Hepatic Homeostasis
3.2. Multi-Omics Analysis Reveals Key Regulatory Changes
3.3. Implications for Liver Diseases: Shared Pathways and Impact on Metabolic Dysfunction-Associated Steototic Liver Disease (MASLD)
3.4. The Shared Pathways Analysis Revealed Several Key Areas Affected by Hnf1aos1 Knockdown
3.5. Future Directions
4. Materials and Methods
4.1. Cell Culture, Treatment, and Transient Transfection
4.2. Viral Plasmids and Virus Production for Animal Treatment
4.3. Quantification of Gene Expression in In Vitro Cells and In Vivo Mice via RT-qPCR
4.4. Transcriptomic Analysis via RNA-Seq
4.5. Untargeted Protein Identification and Label-Free Quantification via Tandem Mass Spectrometry
4.6. H&E Staining
4.7. Quantification of TG
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mahpour, A.; Mullen, A.C. Our Emerging Understanding of the Roles of Long Non-Coding RNAs in Normal Liver Function, Disease, and Malignancy. JHEP Rep. 2021, 3, 100177. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long Non-Coding RNAs: From Disease Code to Drug Role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Hezroni, H.; Koppstein, D.; Schwartz, M.G.; Avrutin, A.; Bartel, D.P.; Ulitsky, I. Principles of Long Noncoding RNA Evolution Derived from Direct Comparison of Transcriptomes in 17 Species. Cell Rep. 2015, 11, 1110–1122. [Google Scholar] [CrossRef] [PubMed]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Sarropoulos, I.; Marin, R.; Cardoso-Moreira, M.; Kaessmann, H. Developmental Dynamics of LncRNAs across Mammalian Organs and Species. Nature 2019, 571, 510–514. [Google Scholar] [CrossRef]
- Johnsson, P.; Lipovich, L.; Grandér, D.; Morris, K.V. Evolutionary Conservation of Long Non-Coding RNAs; Sequence, Structure, Function. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1063–1071. [Google Scholar] [CrossRef]
- Beucher, A.; Miguel-Escalada, I.; Balboa, D.; De Vas, M.G.; Maestro, M.A.; Garcia-Hurtado, J.; Bernal, A.; Gonzalez-Franco, R.; Vargiu, P.; Heyn, H.; et al. The HASTER LncRNA Promoter Is a Cis-Acting Transcriptional Stabilizer of HNF1A. Nat. Cell Biol. 2022, 24, 1528–1540. [Google Scholar] [CrossRef]
- Chen, L.; Bao, Y.; Jiang, S.; Zhong, X. The Roles of Long Noncoding RNAs HNF1α-AS1 and HNF4α-AS1 in Drug Metabolism and Human Diseases. Noncoding RNA 2020, 6, 24. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, L.; Liu, J.; Chen, S.; Liu, G.; Nie, Y.; Wang, P.; Yang, W.; Chen, L.; Zhong, X.; et al. The HNF1α-Regulated LncRNA HNF1α-AS1 Is Involved in the Regulation of Cytochrome P450 Expression in Human Liver Tissues and Huh7 Cells. J. Pharmacol. Exp. Ther. 2019, 368, 353–362. [Google Scholar] [CrossRef]
- Ding, C.-H.; Yin, C.; Chen, S.-J.; Wen, L.-Z.; Ding, K.; Lei, S.-J.; Liu, J.-P.; Wang, J.; Chen, K.; Jiang, H.; et al. The HNF1α-Regulated LncRNA HNF1A-AS1 Reverses the Malignancy of Hepatocellular Carcinoma by Enhancing the Phosphatase Activity of SHP-1. Mol. Cancer 2018, 17, 63. [Google Scholar] [CrossRef]
- Qian, H.; Deng, X.; Huang, Z.-W.; Wei, J.; Ding, C.-H.; Feng, R.-X.; Zeng, X.; Chen, Y.-X.; Ding, J.; Qiu, L.; et al. An HNF1α-Regulated Feedback Circuit Modulates Hepatic Fibrogenesis via the Crosstalk between Hepatocytes and Hepatic Stellate Cells. Cell Res. 2015, 25, 930–945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, J.; Luo, J.; Liu, C.; Zhu, L. Regulatory Mechanisms and Potential Medical Applications of HNF1A-AS1 in Cancers. Am. J. Transl. Res. 2022, 14, 4154–4168. [Google Scholar] [PubMed]
- Wang, Y.; Wang, P.; Wang, Q.; Chen, S.; Wang, X.; Zhong, X.; Hu, W.; Thorne, R.F.; Han, S.; Wu, M.; et al. The Long Noncoding RNA HNF1A-AS1 with Dual Functions in the Regulation of Cytochrome P450 3A4. Biochem. Pharmacol. 2024, 220, 116016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, Y.; Li, L.; He, Y.; Zheng, D.; Yu, P.; Yu, L.; Tang, L.; Wang, Y.; Wang, Z. EZH2 RIP-Seq Identifies Tissue-Specific Long Non-Coding RNAs. Curr. Gene Ther. 2018, 18, 275–285. [Google Scholar] [CrossRef]
- Sepehri, Z.; Banerjee, A.; Vizeacoumar, F.S.; Freywald, A.; Vizeacoumar, F.J.; Dolinsky, V.W.; Davie, J.R. Differential Expression of HNF1A and HNF1A-AS1 in Colon Cancer Cells. IUBMB Life 2022, 74, 496–507. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Liu, L.; Yang, W.; Zhang, Q. HNF1A-AS1 Regulates Cell Migration, Invasion and Glycolysis via Modulating MiR-124/MYO6 in Colorectal Cancer Cells. Onco Targets Ther. 2020, 13, 1507–1518. [Google Scholar] [CrossRef]
- Wang, X.; Hassan, W.; Zhao, J.; Bakht, S.; Nie, Y.; Wang, Y.; Pang, Q.; Huang, Z. The Impact of Hepatocyte Nuclear Factor-1α on Liver Malignancies and Cell Stemness with Metabolic Consequences. Stem Cell Res. Ther. 2019, 10, 315. [Google Scholar] [CrossRef]
- Ni, Q.; Ding, K.; Wang, K.; He, J.; Yin, C.; Shi, J.; Zhang, X.; Xie, W.; Shi, Y. Deletion of HNF1 α in Hepatocytes Results in Fatty Liver-related Hepatocellular Carcinoma in Mice. FEBS Lett. 2017, 591, 1947–1957. [Google Scholar] [CrossRef]
- Jin, J.; Nguyen, L.T.G.; Wassef, A.; Sadek, R.; Schmitt, T.M.; Guo, G.L.; Rasmussen, T.P.; Zhong, X. Identification and Functional Characterization of Alternative Transcripts of LncRNA HNF1A-AS1 and Their Impacts on Cell Growth, Differentiation, Liver Diseases, and in Response to Drug Induction. Noncoding RNA 2024, 10, 28. [Google Scholar] [CrossRef]
- Bao, Y.; Phan, M.; Zhu, J.; Ma, X.; Manautou, J.E.; Zhong, X. Alterations of Cytochrome P450–Mediated Drug Metabolism during Liver Repair and Regeneration after Acetaminophen-Induced Liver Injury in Mice. Drug Metab. Dispos. 2022, 50, 694–703. [Google Scholar] [CrossRef]
- Wang, C.; Mou, L.; Chai, H.-X.; Wang, F.; Yin, Y.-Z.; Zhang, X.-Y. Long Non-Coding RNA HNF1A-AS1 Promotes Hepatocellular Carcinoma Cell Proliferation by Repressing NKD1 and P21 Expression. Biomed. Pharmacother. 2017, 89, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Li, Y.; Kim, H.; Eum, S.; Park, K.; Lee, C.-H. The Role of EZH1 and EZH2 in Development and Cancer. BMB Rep. 2022, 55, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Liu, H.; Shi, X.; Yao, Y.; Yang, W.; Song, Y. The Long Non-Coding RNA HNF1A-AS1 Regulates Proliferation and Metastasis in Lung Adenocarcinoma. Oncotarget 2015, 6, 9160–9172. [Google Scholar] [CrossRef]
- Liu, H.-T.; Liu, S.; Liu, L.; Ma, R.-R.; Gao, P. EGR1-Mediated Transcription of LncRNA-HNF1A-AS1 Promotes Cell-Cycle Progression in Gastric Cancer. Cancer Res. 2018, 78, 5877–5890. [Google Scholar] [CrossRef]
- Zhao, R.; Guo, X.; Zhang, G.; Liu, S.; Ma, R.; Wang, M.; Chen, S.; Zhu, W.; Liu, Y.; Gao, P.; et al. CMYC-Initiated HNF1A-AS1 Overexpression Maintains the Stemness of Gastric Cancer Cells. Cell Death Dis. 2024, 15, 288. [Google Scholar] [CrossRef]
- Xu, C.; Lu, Y.; Zheng, X.; Feng, X.; Yang, X.; Timm, J.; Wu, J.; Wang, B.; Lu, M.; Yang, D.; et al. TLR2 Expression in Peripheral CD4+ T Cells Promotes Th17 Response and Is Associated with Disease Aggravation of Hepatitis B Virus-Related Acute-On-Chronic Liver Failure. Front. Immunol. 2017, 8, 1609. [Google Scholar] [CrossRef]
- Cousins, M.M.; Morris, E.; Maurino, C.; Devasia, T.P.; Karnak, D.; Ray, D.; Parikh, N.D.; Owen, D.; Ten Haken, R.K.; Schipper, M.J.; et al. TNFR1 and the TNFα Axis as a Targetable Mediator of Liver Injury from Stereotactic Body Radiation Therapy. Transl. Oncol. 2021, 14, 100950. [Google Scholar] [CrossRef]
- Tran, M.; Wu, J.; Wang, L.; Shin, D.-J. A Potential Role for SerpinA3N in Acetaminophen-Induced Hepatotoxicity. Mol. Pharmacol. 2021, 99, 277–285. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Zhang, X.; Su, W. Cytochrome P450 Omega-Hydroxylase 4a14 Attenuates Cholestatic Liver Fibrosis. Front. Physiol. 2021, 12, 688259. [Google Scholar] [CrossRef]
- Wan, T.; Chen, Z.; Li, J.; Yuan, X.; Zheng, M.; Qin, L.; Zhang, L.; Hou, T.; Liu, C.; Li, R. AMPK Agonist AICAR Ameliorates Maternal Hepatic Lipid Metabolism Disorder, Inflammation, and Fibrosis Caused by PM2.5 Exposure during Pregnancy. Sci. Rep. 2025, 15, 8689. [Google Scholar] [CrossRef]
- Rex, V.; Stempel, M.; Halle, S.; Brinkmann, M.M. The Two Faces of Oligoadenylate Synthetase-like: Effective Antiviral Protein and Negative Regulator of Innate Immunity. Curr. Opin. Virol. 2023, 60, 101329. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Kang, J.-S.; Hwang, Y.S.; Kim, Y.-J. Oligoadenylate Synthase-like (OASL) Proteins: Dual Functions and Associations with Diseases. Exp. Mol. Med. 2015, 47, e144. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.N.; Harioudh, M.K.; Shao, L.; Perez, J.; Ghosh, A. The Many Faces of Oligoadenylate Synthetases. J. Interf. Cytokine Res. 2023, 43, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, X.; Ding, Y.; Liu, X.; Diggle, K.; Kisseleva, T.; Brenner, D.A. SREBP Regulation of Lipid Metabolism in Liver Disease, and Therapeutic Strategies. Biomedicines 2023, 11, 3280. [Google Scholar] [CrossRef]
- Lounis, M.A.; Bergeron, K.-F.; Burhans, M.S.; Ntambi, J.M.; Mounier, C. Oleate Activates SREBP-1 Signaling Activity in SCD1-Deficient Hepatocytes. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E710–E720. [Google Scholar] [CrossRef]
- Heeren, J.; Scheja, L. Metabolic-Associated Fatty Liver Disease and Lipoprotein Metabolism. Mol. Metab. 2021, 50, 101238. [Google Scholar] [CrossRef]
- Lyssenko, N.N.; Haider, N.; Picataggi, A.; Cipollari, E.; Jiao, W.; Phillips, M.C.; Rader, D.J.; Chavali, V.R.M. Directional ABCA1-Mediated Cholesterol Efflux and ApoB-Lipoprotein Secretion in the Retinal Pigment Epithelium. J. Lipid Res. 2018, 59, 1927–1939. [Google Scholar] [CrossRef]
- Rakateli, L.; Huchzermeier, R.; van der Vorst, E.P.C. AhR, PXR and CAR: From Xenobiotic Receptors to Metabolic Sensors. Cells 2023, 12, 2752. [Google Scholar] [CrossRef]
- Poudel, S.; Huber, A.D.; Chen, T. Regulation of Nuclear Receptors PXR and CAR by Small Molecules and Signal Crosstalk: Roles in Drug Metabolism and Beyond. Drug Metab. Dispos. 2023, 51, 228–236. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling in Liver Disease and Therapy. Liver Res. 2017, 1, 3–9. [Google Scholar] [CrossRef]
- Li, T.; Apte, U. Bile Acid Metabolism and Signaling in Cholestasis, Inflammation, and Cancer. Adv. Pharmacol. 2015, 74, 263–302. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.A.; Kersten, S.; Qi, L. Lipoprotein Lipase and Its Regulators: An Unfolding Story. Trends Endocrinol. Metab. 2021, 32, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, H.; Wang, P.; Wang, J.; Zou, L. The Roles of SOCS3 and STAT3 in Bacterial Infection and Inflammatory Diseases. Scand. J. Immunol. 2018, 88, e12727. [Google Scholar] [CrossRef] [PubMed]
- Lynch, D.M.; Forrester, B.; Webb, T.; Ciulli, A. Unravelling the Druggability and Immunological Roles of the SOCS-Family Proteins. Front. Immunol. 2024, 15, 1449397. [Google Scholar] [CrossRef]
- Morelli, M.; Madonna, S.; Albanesi, C. SOCS1 and SOCS3 as Key Checkpoint Molecules in the Immune Responses Associated to Skin Inflammation and Malignant Transformation. Front. Immunol. 2024, 15, 1393799. [Google Scholar] [CrossRef]
- Dziurdzik, S.K.; Conibear, E. The Vps13 Family of Lipid Transporters and Its Role at Membrane Contact Sites. Int. J. Mol. Sci. 2021, 22, 2905. [Google Scholar] [CrossRef]
- Shen, J.L.; Fortier, T.M.; Zhao, Y.G.; Wang, R.; Burmeister, M.; Baehrecke, E.H. Vmp1, Vps13D, and Marf/Mfn2 Function in a Conserved Pathway to Regulate Mitochondria and ER Contact in Development and Disease. Curr. Biol. 2021, 31, 3028–3039.e7. [Google Scholar] [CrossRef]
- Barritt, S.A.; DuBois-Coyne, S.E.; Dibble, C.C. Coenzyme A Biosynthesis: Mechanisms of Regulation, Function and Disease. Nat. Metab. 2024, 6, 1008–1023. [Google Scholar] [CrossRef]
- Shi, L.; Tu, B.P. Acetyl-CoA and the Regulation of Metabolism: Mechanisms and Consequences. Curr. Opin. Cell Biol. 2015, 33, 125–131. [Google Scholar] [CrossRef]
- Alnasser, S.M. The Role of Glutathione S-Transferases in Human Disease Pathogenesis and Their Current Inhibitors. Genes. Dis. 2024, 12, 101482. [Google Scholar] [CrossRef]
- Semova, I.; Biddinger, S.B. Triglycerides in Nonalcoholic Fatty Liver Disease: Guilty Until Proven Innocent. Trends Pharmacol. Sci. 2021, 42, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A. Hepatic Lipotoxicity and the Pathogenesis of Nonalcoholic Steatohepatitis. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the Gut-Liver Axis in NASH Pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.; Wu, C.; Huang, W.; Lo, Y.M.; Lin, S.; Wu, J.S.; Huang, W.; Shen, S. Alleviative Effects of A-lipoic Acid on Muscle Atrophy via the Modulation of TNF-α/JNK and PI3K/AKT Pathways in High-fat Diet and Streptozotocin-induced Type 2 Diabetic Rats. Food Sci. Nutr. 2023, 11, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qu, N.; Wang, X.; Chen, L.; Liu, M. The Function of Long Non-Coding RNA in Non-Alcoholic Fatty Liver Disease. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102095. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol Metabolism and the Pathogenesis of Non-Alcoholic Steatohepatitis. Prog. Lipid Res. 2013, 52, 175–191. [Google Scholar] [CrossRef]
- Zhao, C.; Dahlman-Wright, K. Liver X Receptor in Cholesterol Metabolism. J. Endocrinol. 2010, 204, 233–240. [Google Scholar] [CrossRef]
- Rao, G.; Peng, X.; Li, X.; An, K.; He, H.; Fu, X.; Li, S.; An, Z. Unmasking the Enigma of Lipid Metabolism in Metabolic Dysfunction-Associated Steatotic Liver Disease: From Mechanism to the Clinic. Front. Med. 2023, 10, 1294267. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Hnf1aos1 | GACTGTCCCCAAACTCCTCA | TTCCCTGAGATTATCGCCCG |
| Fasn | CACAGTGCTCAAAGGACATGCC | CACCAGGTGTAGTGCCTTCCTC |
| Lpl | GCGTAGCAGGAAGTCTGACCAA | AGCGTCATCAGGAGAAAGGCG |
| Shp-1 | TGGTTTCACCGGGACCTCAGC | AGTAAGGCTGCCGCAGGTAGA |
| Apol9a | GTGGATAGGATTGCCAGCAAG | AGAGGGGTTCCTTTCAGACTG |
| Apol9b | GTAGCTAGGATTGTCAACAAGA | CAGAGGGGTTCCTTCCAGCC |
| Abca1 | GGAGCCTTTGTGGAACTCTTCC | CGCTCTCTTCAGCCACTTTGAG |
| Vmp1 | GTTGGTCTTGGAACAGGACTGC | ATCTGGTCAGGATAGGGTGGCT |
| Vps13d | AAAGTGACCCTCCAGATTCCT | CGCTTTCTTACGCTCCCTCT |
| Vldlr | ACGGCAGCGATGAGGTCAACTG | CAGAGCCATCAACACAGTCTCG |
| Srebf1 | CGACTACATCCGCTTCTTGCAG | CCTCCATAGACACATCTGTGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armanios, B.; Jin, J.; Kolmel, H.; Laddha, A.P.; Mishra, N.; Manautou, J.E.; Zhong, X.-B. Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice. Non-Coding RNA 2025, 11, 52. https://doi.org/10.3390/ncrna11040052
Armanios B, Jin J, Kolmel H, Laddha AP, Mishra N, Manautou JE, Zhong X-B. Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice. Non-Coding RNA. 2025; 11(4):52. https://doi.org/10.3390/ncrna11040052
Chicago/Turabian StyleArmanios, Beshoy, Jing Jin, Holly Kolmel, Ankit P. Laddha, Neha Mishra, Jose E. Manautou, and Xiao-Bo Zhong. 2025. "Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice" Non-Coding RNA 11, no. 4: 52. https://doi.org/10.3390/ncrna11040052
APA StyleArmanios, B., Jin, J., Kolmel, H., Laddha, A. P., Mishra, N., Manautou, J. E., & Zhong, X.-B. (2025). Unraveling the Regulatory Impact of LncRNA Hnf1aos1 on Hepatic Homeostasis in Mice. Non-Coding RNA, 11(4), 52. https://doi.org/10.3390/ncrna11040052







