- Article
Valorization of Waste Cooking Oils into Antimicrobial Soaps with Honey, Propolis, and Essential Oils
- Mirel Glevitzky,
- Gabriela-Alina Dumitrel and
- Mihaela Laura Vică
- + 3 authors
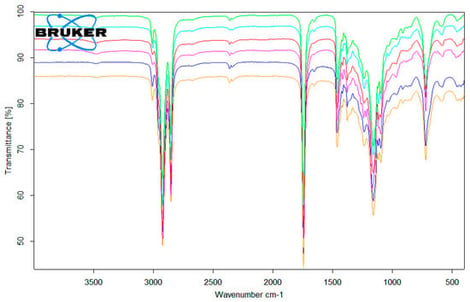
The valorization of waste cooking oils (WCOs) provides a strategy to reduce environmental impact while converting residues from the food industry into valuable products. This study developed and characterized antimicrobial soaps from purified WCOs (sunflower, palm, and pumpkin oils) enriched with natural bioactive ingredients. WCOs were purified by filtration, treatment with 10% NaCl, and bleaching with 3% H2O2, followed by cold saponification with NaOH. Twelve soap formulations were prepared, including six enriched with bee products (propolis, poly-floral honey, linden, acacia, honeydew, and sunflower) and six enriched with essential oils (EOs) (clove, rosemary, mace, nutmeg, white pepper, and juniper). The WCOs, natural bioactive ingredients, and soaps were characterized using physico-chemical methods (FTIR, GC-FID, phenols, flavonoids, etc.), while their antibacterial activity was determined against two microbial strains: Staphylococcus aureus and Escherichia coli. The antimicrobial activity of soaps is related to their alkaline pH, while the addition of honey, propolis, or EOs contributes to additional antimicrobial effects. Among honey- and propolis-enriched soaps, those with propolis produced the largest inhibition zones (up to 8.67 mm for S. aureus and 7.0 mm for E. coli). EO-based soaps exhibited higher activity, with rosemary EO-based soap showing the largest zones (up to 9.5 mm for S. aureus and 7.5 mm for E. coli). These data support the potential of enriched soaps containing honey, propolis, and EOs for antimicrobial applications, highlighting their value as a sustainable alternative in the valorization of WCOs.
11 February 2026