Bioactive and Phenolic Profiles in Pinus pinaster Bark: A Comparative Study of Microwave and Ultrasound Extraction Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction
2.3. Total Phenolic Content Determination
2.4. DPPH Free Radical Scavenging Effect
2.5. ABTS Radical Cation Scavenging Effect
2.6. Oxygen Radical Absorbance Capacity
2.7. Thiobarbituric Acid Reactive Substances (TBARS) Formation Inhibition
2.8. Cellular Antioxidant Activity
2.9. Oxidative Haemolysis Inhibition Assay (OxHLIA)
2.10. Antimicrobial Activity
2.11. Antiproliferative Activity
2.12. Anti-Inflammatory Activity
2.13. Analysis of Phenolic Compounds
2.14. Statistical Analysis
3. Results and Discussion
3.1. Extraction Yield, TPC and Antioxidant Activities
3.2. Antiproliferative and Anti-Inflammatory Properties
3.3. Antibacterial Activity
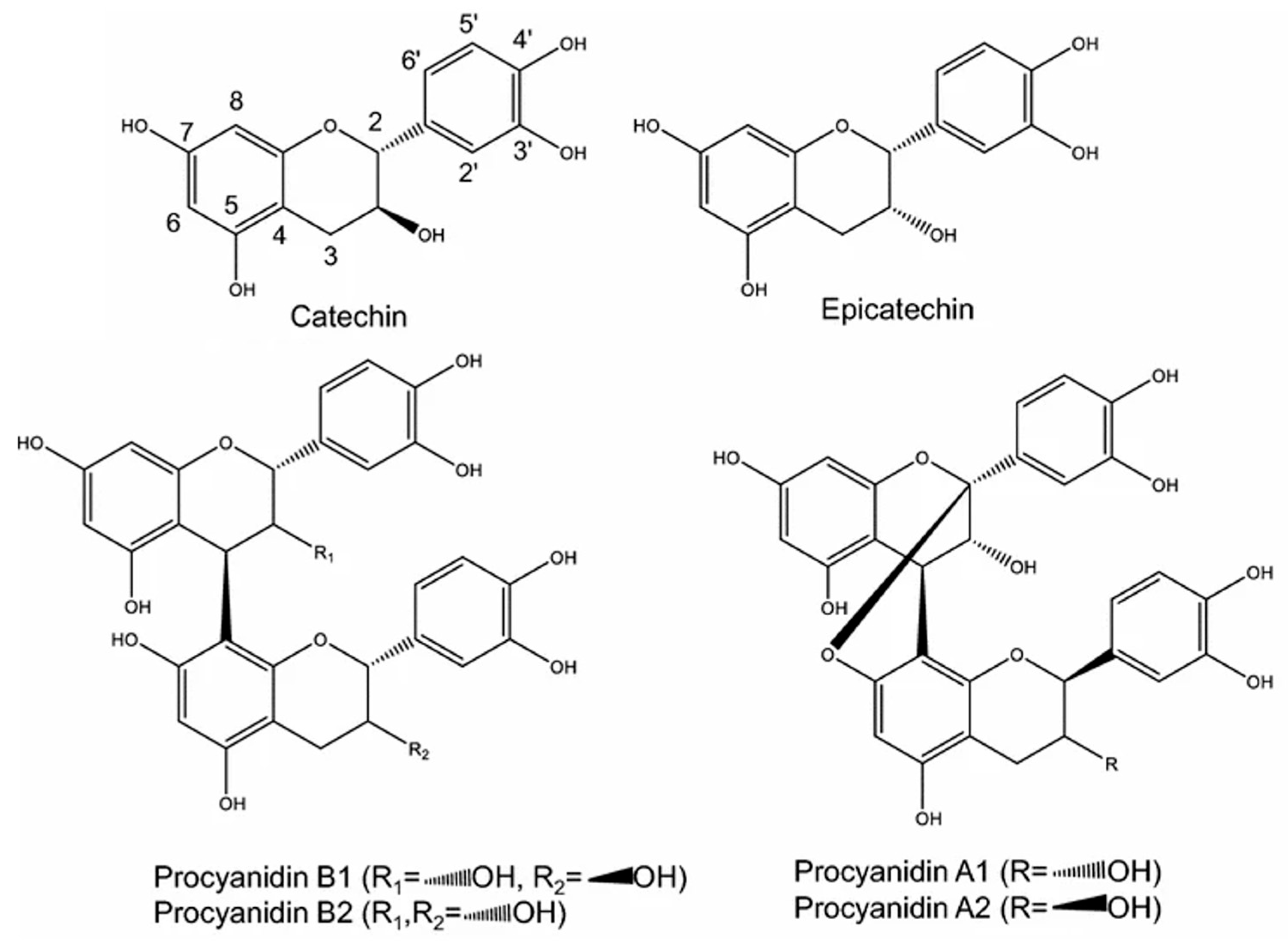
3.4. Phenolic Compound Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conde, E.; Díaz-Reinoso, B.; Moure, A.; Hemming, J.; Willför, S.; Domínguez, H.; Parajó, J.C. Extraction of Phenolic and Lipophilic Compounds from Pinus Pinaster Knots and Stemwood by Supercritical CO2. J. Supercrit. Fluids 2013, 81, 193–199. [Google Scholar] [CrossRef]
- Venkatesan, T.; Choi, Y.-W.; Kim, Y.-K. Impact of Different Extraction Solvents on Phenolic Content and Antioxidant Potential of Pinus densiflora Bark Extract. BioMed Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.S.; Jang, J.P.; Mun, S.P. Exploitation of polyphenol-rich pine barks for potent antioxidant activity. J. Wood Sci. 2007, 53, 524–528. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata Bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Barros, D.; Vieito, C.; Santos, J.; Ramos, C.; Velho, M.V. Inhibitory Effects of Pinus Pinaster Aiton Subsp. Atlantica Bark Extracts Against Known Food Pathogens. Chem. Eng. Trans. 2020, 79, 163–168. [Google Scholar] [CrossRef]
- Aspé, E.; Fernández, K. Comparison of phenolic extracts obtained of Pinus radiata bark from pulp and paper industry and sawmill industry. Maderas Cienc. Tecnol. 2011, 13, 243–252. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus Species as Prospective Reserves of Bioactive Compounds with Potential Use in Functional Food—Current State of Knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Chupin, L.; Maunu, S.L.; Reynaud, S.; Pizzi, A.; Charrier, B.; Charrier-El Bouhtoury, F. Microwave assisted extraction of maritime pine (Pinus pinaster) bark: Impact of particle size and characterization. Ind. Crops Prod. 2015, 65, 142–149. [Google Scholar] [CrossRef]
- ICNF. 6º Inventário Florestal Nacional—IFN6. Available online: https://www.agroportal.pt/6o-inventario-florestal-nacional/ (accessed on 8 May 2023).
- Ramos, P.A.B.; Pereira, C.; Gomes, A.P.; Neto, R.T.; Almeida, A.; Santos, S.A.O.; Silva, A.M.S.; Silvestre, A.J.D. Chemical Characterisation, Antioxidant and Antibacterial Activities of Pinus pinaster Ait. and Pinus pinea L. Bark Polar Extracts: Prospecting Forestry By-Products as Renewable Sources of Bioactive Compounds. Appl. Sci. 2022, 12, 784. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Santos, R.M.S.; Seabra, I.J.; Facanali, R.; Marques, M.O.M.; de Sousa, H.C. Fractioned SFE of antioxidants from maritime pine bark. J. Supercrit. Fluids 2008, 47, 37–48. [Google Scholar] [CrossRef]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. High pressure solvent extraction of maritime pine bark: Study of fractionation, solvent flow rate and solvent composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Barros, D.; Fernandes, É.; Jesus, M.; Barros, L.; Alonso-Esteban, J.I.; Pires, P.; Vaz Velho, M. The Chemical Characterisation of the Maritime Pine Bark Cultivated in Northern Portugal. Plants 2023, 12, 3940. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Carocho, M.; Barros, D.; Velho, M.V.; Heleno, S.; Barros, L. Chemical composition and industrial applications of Maritime pine (Pinus pinaster Ait.) bark and other non-wood parts. Rev. Environ. Sci. Bio/Technol. 2022, 21, 583–633. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Antimicrobial and antioxidant activities of natural extracts in vitro and in ground beef. J. Food Prot. 2004, 67, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Grün, I.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microbiol. 2007, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hameş-Kocabaş, E.E.; Çeliktaş, Ö.Y.; İşleten, M.; Sukan, F.V. Antimicrobial activity of pine bark extract and assessment of potential application in cooked red meat. GIDA 2008, 33, 123–127. [Google Scholar]
- Ruggeri, S.; Straniero, R.; Pacifico, S.; Aguzzi, A.; Virgili, F. French Marine Bark Extract Pycnogenol as a Possible Enrichment Ingredient for Yogurt. J. Dairy Sci. 2008, 91, 4484–4491. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Pazos, M.; Lois, S.; Medina, I. Contribution of Galloylation and Polymerization to the Antioxidant Activity of Polyphenols in Fish Lipid Systems. J. Agric. Food Chem. 2010, 58, 7423–7431. [Google Scholar] [CrossRef]
- Yesil Celiktas, O.; Isleten, M.; Vardar-Sukan, F.; Oyku Cetin, E. In vitro release kinetics of pine bark extract enriched orange juice and the shelf stability. Br. Food J. 2010, 112, 1063–1076. [Google Scholar] [CrossRef]
- Raitanen, J.E.; Järvenpää, E.; Korpinen, R.; Mäkinen, S.; Hellström, J.; Kilpeläinen, P.; Liimatainen, J.; Ora, A.; Tupasela, T.; Jyske, T. Tannins of Conifer Bark as Nordic Piquancy-Sustainable Preservative and Aroma? Molecules 2020, 25, 567. [Google Scholar] [CrossRef]
- Frontela-Saseta, C.; López-Nicolás, R.; González-Bermúdez, C.A.; Peso-Echarri, P.; Ros-Berruezo, G.; Martínez-Graciá, C.; Canali, R.; Virgili, F. Evaluation of Antioxidant Activity and Antiproliferative Effect of Fruit Juices Enriched with Pycnogenol® in Colon Carcinoma Cells. The Effect of In Vitro Gastrointestinal Digestion. Phytother. Res. 2011, 25, 1870–1875. [Google Scholar] [CrossRef]
- López-Nicolás, R.; González-Bermúdez, C.A.; Ros-Berruezo, G.; Frontela-Saseta, C. Influence of in vitro gastrointestinal digestion of fruit juices enriched with pine bark extract on intestinal microflora. Food Chem. 2014, 157, 14–19. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Rotar, A.; Stan, L.; Pop, C.R.; Socaci, S.; Mireşan, V.; Muste, S. Characterization of pine bud syrup and its effect on physicochemical and sensory properties of kefir. CyTA J. Food 2016, 14, 213–218. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Yoga Latha, L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TrAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L. Significance of sample preparation in developing analytical methodologies for accurate estimation of bioactive compounds in functional foods. J. Sci. Food Agric. 2006, 86, 2266–2272. [Google Scholar] [CrossRef]
- Barros, D.; Pereira-Pinto, R.; Fernandes, É.; Pires, P.; Vaz-Velho, M. Microwave-Assisted Extraction for the Sustainable Recovery and Valorization of Phenolic Compounds from Maritime Pine Bark. Sustain. Chem. 2025, 6, 26. [Google Scholar] [CrossRef]
- Duarte, H.; Gomes, V.; Aliaño-González, M.J.; Faleiro, L.; Romano, A.; Medronho, B. Ultrasound-Assisted Extraction of Polyphenols from Maritime Pine Residues with Deep Eutectic Solvents. Foods 2022, 11, 3754. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Pinelo, M.; Rubilar, M.; Sineiro, J.; Núñez, M.J. Extraction of antioxidant phenolics from almond hulls (Prunus amygdalus) and pine sawdust (Pinus pinaster). Food Chem. 2004, 85, 267–273. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Coscueta, E.R.; Reis, C.A.; Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants 2020, 9, 698. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Mármol, I.; Vieito, C.; Andreu, V.; Levert, A.; Amiot, A.; Bertrand, C.; Rodríguez-Yoldi, M.J.; Santos, J.; Vaz-Velho, M. Influence of extraction solvent on the biological properties of maritime pine bark (Pinus pinaster). Int. J. Food Stud. 2022, 11, 51–62. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement, CLSI Document M100-S22; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Rasband, W.S. ImageJ; U.S. National Institutes of Health: Bethesda, MD, USA, 1997–2018. Available online: https://imagej.net/ij/ (accessed on 5 December 2025).
- Mandim, F.; Graça, V.C.; Calhelha, R.C.; Machado, I.L.F.; Ferreira, L.F.V.; Ferreira, I.; Santos, P.F. Synthesis, Photochemical and In Vitro Cytotoxic Evaluation of New Iodinated Aminosquaraines as Potential Sensitizers for Photodynamic Therapy. Molecules 2019, 24, 863. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.M.V.; Pinela, J.; Alves, M.J.; Heleno, S.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.P.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Chemical characterization, antioxidant, anti-inflammatory and cytotoxic properties of bee venom collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Boutin, Y. The effect of extraction process on polyphenol content, triterpene composition and bioactivity of yellow birch (Betula alleghaniensis Britton) extracts. Ind. Crops Prod. 2009, 30, 297–303. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2006, 1, 7–18. [Google Scholar]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. TrAC Trends Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Tanase, C.; Erzsébet, D.; Coșarcă, S.-L.; Miklos, A.; Imre, S.; Domokos, J.; Dehelean, C. Study of the Ultrasound-assisted Extraction of Polyphenols from Beech (Fagus sylvatica L.) Bark. Bioresources 2018, 13, 2247–2267. [Google Scholar] [CrossRef]
- Paniwnyk, L.; Beaufoy, E.; Lorimer, J.P.; Mason, T.J. The extraction of rutin from flower buds of Sophora japonica. Ultrason. Sonochem. 2001, 8, 299–301. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the Biological Potential of Pinus pinaster Bark Extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef]
- Simões, R.; Pimentel, C.; Ferreira-Dias, S.; Miranda, I.; Pereira, H. Phytochemical characterization of phloem in maritime pine and stone pine in three sites in Portugal. Heliyon 2021, 7, e06718. [Google Scholar] [CrossRef]
- Sládková, A.; Benedeková, M.; Stopka, J.; Surina, I.; Haz, A.; Strižincová, P.; Kučíková, K.; Butor Skulcova, A.; Burčová, Z.; Kreps, F.; et al. Yield of Polyphenolic Substances Extracted From Spruce (Picea abies) Bark by Microwave-Assisted Extraction. Bioresources 2016, 11, 9912–9921. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate Electric Fields as a Potential Tool for Sustainable Recovery of Phenolic Compounds from Pinus pinaster Bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.; Ferreira, I.C.F.R. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Vega, E.N.; Molina, A.K.; Pereira, C.; Dias, M.I.; Heleno, S.A.; Rodrigues, P.; Fernandes, I.P.; Barreiro, M.F.; Stojković, D.; Soković, M.; et al. Anthocyanins from Rubus fruticosus L. and Morus nigra L. Applied as Food Colorants: A Natural Alternative. Plants 2021, 10, 1181. [Google Scholar] [CrossRef]
- Pilaquinga, F.; Morey, J.; Fernandez, L.; Espinoza-Montero, P.; Moncada-Basualto, M.; Pozo-Martinez, J.; Olea-Azar, C.; Bosch, R.; Meneses, L.; Debut, A.; et al. Determination of Antioxidant Activity by Oxygen Radical Absorbance Capacity (ORAC-FL), Cellular Antioxidant Activity (CAA), Electrochemical and Microbiological Analyses of Silver Nanoparticles Using the Aqueous Leaf Extract of Solanum mammosum L. Int. J. Nanomed. 2021, 16, 5879–5894. [Google Scholar] [CrossRef]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef]
- de Oliveira, A.; Moreira, T.F.M.; Pepinelli, A.L.S.; Costa, L.G.M.A.; Leal, L.E.; da Silva, T.B.V.; Gonçalves, O.H.; Porto Ineu, R.; Dias, M.I.; Barros, L.; et al. Bioactivity screening of pinhão (Araucaria Angustifolia (Bertol.) Kuntze) seed extracts: The inhibition of cholinesterases and α-amylases, and cytotoxic and anti-inflammatory activities. Food Funct. 2021, 12, 9820–9828. [Google Scholar] [CrossRef]
- Fischer, T.E.; Marcondes, A.; Zardo, D.M.; Nogueira, A.; Calhelha, R.C.; Vaz, J.A.; Barros, L.; Zielinski, A.A.F.; Alberti, A. Bioactive Activities of the Phenolic Extract from Sterile Bracts of Araucaria angustifolia. Antioxidants 2022, 11, 2431. [Google Scholar] [CrossRef]
- Kumar, S.; Brooks, M.S.-L. Use of Red Beet (Beta vulgaris L.) for Antimicrobial Applications—A Critical Review. Food Bioprocess Technol. 2018, 11, 17–42. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Gascón, S.; Jiménez-Moreno, N.; Jiménez, S.; Quero, J.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. Nutraceutical composition of three pine bark extracts and their antiproliferative effect on Caco-2 cells. J. Funct. Foods 2018, 48, 420–429. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Sun, J.; Chen, P.; Monagas, M.J.; Harnly, J.M. UHPLC-PDA-ESI/HRMSn Profiling Method to Identify and Quantify Oligomeric Proanthocyanidins in Plant Products. J. Agric. Food Chem. 2014, 62, 9387–9400. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tuo, X.; Wang, L.; Tundis, R.; Portillo, M.P.; Simal-Gandara, J.; Yu, Y.; Zou, L.; Xiao, J.; Deng, J. Bioactive procyanidins from dietary sources: The relationship between bioactivity and polymerization degree. Trends Food Sci. Technol. 2021, 111, 114–127. [Google Scholar] [CrossRef]
- Xie, C.; Wang, K.; Liu, X.; Liu, G.; Hu, Z.; Zhao, L. Characterization and bioactivity of A-type procyanidins from litchi fruitlets at different degrees of development. Food Chem. 2023, 405, 134855. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Chen, J.; McClements, D.J.; Hu, P.; Ye, X.; Liu, C.; Li, T. Protein–polyphenol interactions enhance the antioxidant capacity of phenolics: Analysis of rice glutelin–procyanidin dimer interactions. Food Funct. 2019, 10, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef] [PubMed]
| MAE | UAE | p-Value | |
|---|---|---|---|
| Extraction yield (g extract/100 g pine bark) | 11.13 ± 0.01 | 3.47 ± 0.00 | <0.001 |
| TPC (mg GAE/g DM extract) | 833 ± 80 | 514 ± 53 | 0.005 |
| DPPH (IC50, mg/mL) | 0.176 ± 0.012 | 0.243 ± 0.013 | 0.003 |
| ABTS (IC50, mg/mL) | 0.333 ± 0.004 | 0.601 ± 0.055 | 0.001 |
| ORAC (IC50, mg/mL) | 0.056 ± 0.004 | 0.057 ± 0.004 | 0.893 |
| TBARS (IC50, μg/mL) | 1.74 ± 0.11 | 0.969 ± 0.045 | 0.006 |
| CAA (% inhibition 2 mg/mL) | 41.0 ± 5.0 | 69.0 ± 4.0 | 0.002 |
| OxHLIA (IC50, µg/mL; Δt 60 min) | 14.3 ± 2.1 | 13.35 ± 0.83 | 0.491 |
| MAE | UAE | p-Value | |
|---|---|---|---|
| Cytotoxicity (GI50, μg/mL) | |||
| AGS | 185.7 ± 7.4 | 86.1 ± 6.3 | <0.001 |
| Caco2 | 228 ± 14 | 235 ± 13 | 0.539 |
| MCF-7 | 2801 ± 19 | 203.5 ± 6.7 | 0.003 |
| NCI-H460 | 183.7 ± 8.1 | 115.1 ± 3.4 | <0.001 |
| Vero | 54.4 ± 3.0 | 77.4 ± 3.6 | 0.001 |
| PLP2 | 173.9 ± 3.4 | 120.6 ± 5.5 | <0.001 |
| Anti-inflammatory activity (IC50, μg/mL) | |||
| RAW 264.7 | >400 | >400 | |
| Bacterial Species | Strain Reference | mg Extract/mL | Inhibition Halos (mm) | p-Value | |
|---|---|---|---|---|---|
| MAE | UAE | ||||
| B. cereus | NCTC 11143 | 30 | 9.64 ± 0.30 | 11.17 ± 0.28 | 0.002 |
| 50 | 11.34 ± 0.07 | 11.68 ± 0.40 | 0.821 | ||
| 65 | 11.46 ± 0.19 | 12.07 ± 0.61 | 0.331 | ||
| S. aureus | ATCC 25923 | 30 | 10.72 ± 0.34 | 10.76 ± 0.83 | 1.000 |
| 50 | 11.93 ± 0.50 | 12.53 ± 0.37 | 0.749 | ||
| 65 | 13.60 ± 0.55 | 12.31 ± 0.51 | 0.100 | ||
| C. perfringens | ATCC 13124 | 30 | 11.20 ± 0.18 | 15.88 ± 0.20 | <0.001 |
| 50 | 13.53 ± 0.57 | 16.41 ± 0.63 | <0.001 | ||
| 65 | 14.61 ± 0.28 | 18.06 ± 0.15 | <0.001 | ||
| L. monocytogenes | ATCC 13932 | 30 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 50 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| 65 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| E. coli | ATCC 25922 | 30 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 50 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| 65 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| Salmonella Enteritidis | ATCC 25928 | 30 | 0.0 ± 0.0 | 0.0 ± 0.0 | - |
| 50 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| 65 | 0.0 ± 0.0 | 0.0 ± 0.0 | - | ||
| Peak | Rt (min) | λmax (nm) | [M-H]- m/z | MS2 (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 4.24 | 281 | 577 | 559(47), 467(18), 451(100), 425(65), 407(49), 289(55) | B-type procyanidin dimer I |
| 2 | 4.86 | 280 | 289 | 245(100), 205(35), 179(22), 125(5) | (+)-Catechin |
| 3 | 5.44 | 281 | 865 | 801(31), 789(47), 779(100), 720(65), 695(41), 577(64), 575(35) | B-type procyanidin trimer I |
| 4 | 5.94 | 281 | 1153 | 865(27), 577(56), 289(49) | B-type procyanidin tetramer I |
| 5 | 6.36 | 280 | 575 | 449(50), 423(100) 407(30),289(5),287(20),285(10) | A-type procyanidin dimer I |
| 6 | 6.69 | 280 | 865 | 801(31), 789(47), 779(100), 720(65), 695(41), 577(64), 575(35) | B-type-procyanidin trimer II |
| 7 | 6.87 | 280 | 289 | 245(100), 205(35), 179(22), 125(5) | (−)-Epicatechin |
| 8 | 7.47 | 280 | 1153 | 865(27), 577(56), 289(49) | B-type procyanidin tetramer II |
| 9 | 7.93 | 280 | 1153 | 865(27), 577(56), 289(49) | B-type procyanidin tetramer III |
| 10 | 8.32 | 280 | 577 | 559(47), 467(18), 451(100), 425(65), 407(49), 289(55) | B-type procyanidin dimer II |
| 11 | 10.51 | 280 | 1151 | 1009(100), 863(89), 575(36), 289(18) | A-type procyanidin tetramer I |
| 12 | 11.54 | 282sh322 | 465 | 303(100), 285(20) | Taxifolin-7-O-hexoside |
| 13 | 12.52 | 280 | 575 | 449(50), 423(100) 407(30), 289(5), 287(20), 285(10) | A-type procyanidin dimer II |
| 14 | 14.41 | 281 | 1151 | 1009(100), 863(89), 575(36), 289(18) | A-type procyanidin tetramer II |
| 15 | 14.75 | 281 | 1151 | 1009(100), 863(89), 575(36), 289(18) | A-type procyanidin tetramer III |
| 16 | 15.23 | 280 | 863 | 575(100), 437(26), 289(4), 287(8) | A-type procyanidin trimers I |
| 17 | 26.01 | 280 | 1153 | 865(27), 577(56), 289(49) | B-type procyanidin tetramer IV |
| 18 | 29.25 | 280 | 863 | 575(100), 437(26), 289(4), 287(8) | A-type procyanidin trimer II |
| MAE | UAE | p-Value | |
|---|---|---|---|
| B-type procyanidin dimer I | 18.57 ± 0.15 | 10.95 ± 0.26 | <0.001 |
| (+)-Catechin | 48.88 ± 0.20 | 2.86 ± 0.03 | <0.001 |
| B-type procyanidin trimer I | 73.30 ± 0.31 | 26.79 ± 0.30 | <0.001 |
| B-type procyanidin tetramer I | 2.84 ± 0.02 | 8.29 ± 0.12 | <0.001 |
| A-type procyanidin dimer I | 3.52 ± 0.02 | 5.58 ± 0.04 | <0.001 |
| B-type-procyanidin trimer II | 2.43 ± 0.02 | 3.82 ± 0.01 | <0.001 |
| (−)-Epicatechin | 6.63 ± 0.03 | 3.27 ± 0.02 | <0.001 |
| B-type procyanidin tetramer II | 3.40 ± 0.03 | 1.45 ± 0.02 | <0.001 |
| B-type procyanidin tetramer III | 4.75 ± 0.03 | 3.72 ± 0.02 | <0.001 |
| B-type procyanidin dimer II | 4.75 ± 0.03 | n.d. | - |
| A-type procyanidin tetramer I | 3.41 ± 0.03 | 2.69 ± 0.02 | <0.001 |
| Taxifolin-7-O-hexoside | 0.19 ± 0.00 | 0.96 ± 0.01 | <0.001 |
| A-type procyanidin dimer II | n.d. | 0.36 ± 0.00 | - |
| A-type procyanidin tetramer II | 8.07 ± 0.30 | 4.62 ± 0.03 | <0.001 |
| A-type procyanidin tetramer III | 24.15 ± 0.15 | 4.66 ± 0.03 | <0.001 |
| A-type procyanidin trimers I | 10.29 ± 0.30 | n.d. | - |
| B-type procyanidin tetramer IV | 5.33 ± 0.07 | 1.90 ± 0.03 | <0.001 |
| A-type procyanidin trimer II | 7.48 ± 0.03 | 5.03 ± 0.02 | <0.001 |
| Total A-type procyanidins | 56.92 ± 0.83 | 22.94 ± 0.14 | <0.001 |
| Total B-type procyanidins | 117.41 ± 0.66 | 56.92 ± 0.77 | <0.001 |
| Total monomeric flavan-3-ols | 55.50 ± 0.23 | 6.13 ± 0.05 | <0.001 |
| Total flavanonols | 0.19 ± 0.00 | 0.96 ± 0.01 | <0.001 |
| Total phenolic compounds | 230.0 ± 1.7 | 86.95 ± 0.97 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Barros, D.; Alonso-Esteban, J.I.; Finimundy, T.C.; Pereira, C.; Vaz, J.A.; Pereira-Pinto, R.; Fernandes, É.; Pires, P.; Santos, J.; Barros, L.; et al. Bioactive and Phenolic Profiles in Pinus pinaster Bark: A Comparative Study of Microwave and Ultrasound Extraction Methods. ChemEngineering 2026, 10, 2. https://doi.org/10.3390/chemengineering10010002
Barros D, Alonso-Esteban JI, Finimundy TC, Pereira C, Vaz JA, Pereira-Pinto R, Fernandes É, Pires P, Santos J, Barros L, et al. Bioactive and Phenolic Profiles in Pinus pinaster Bark: A Comparative Study of Microwave and Ultrasound Extraction Methods. ChemEngineering. 2026; 10(1):2. https://doi.org/10.3390/chemengineering10010002
Chicago/Turabian StyleBarros, Diana, José Ignacio Alonso-Esteban, Tiane C. Finimundy, Carla Pereira, Josiana A. Vaz, Ricardo Pereira-Pinto, Élia Fernandes, Preciosa Pires, Joana Santos, Lillian Barros, and et al. 2026. "Bioactive and Phenolic Profiles in Pinus pinaster Bark: A Comparative Study of Microwave and Ultrasound Extraction Methods" ChemEngineering 10, no. 1: 2. https://doi.org/10.3390/chemengineering10010002
APA StyleBarros, D., Alonso-Esteban, J. I., Finimundy, T. C., Pereira, C., Vaz, J. A., Pereira-Pinto, R., Fernandes, É., Pires, P., Santos, J., Barros, L., & Vaz-Velho, M. (2026). Bioactive and Phenolic Profiles in Pinus pinaster Bark: A Comparative Study of Microwave and Ultrasound Extraction Methods. ChemEngineering, 10(1), 2. https://doi.org/10.3390/chemengineering10010002














