- Review
Gut Microbiota-Derived Metabolites to Regulate Intramuscular Fat Deposition in Pigs
- Han Yuan,
- Lanlan Yi and
- Sumei Zhao
- + 5 authors
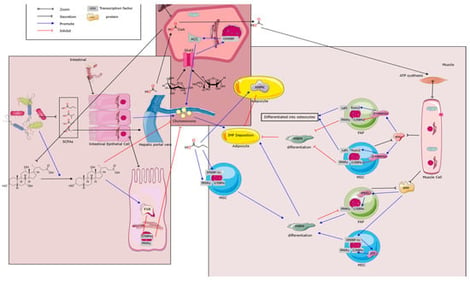
Intramuscular fat (IMF) is a crucial determinant of pork quality, influencing tenderness, flavor, and consumer preferences, yet selective breeding has reduced its levels in modern pigs. This review explores the molecular and cellular mechanisms of IMF deposition, including progenitor cell differentiation via pathways like Wnt/β-catenin and PPARγ, and advances in non-invasive detection methods such as hyperspectral imaging and Raman spectroscopy. It highlights correlations and causal links between the gut microbiota composition and IMF, established through omics analyses, fecal microbiota transplantation, and germ-free models. Key microbial metabolites, including short-chain fatty acids (SCFAs) and bile acids, modulate lipid metabolism bidirectionally via signaling receptors like GPR43, FXR, and TGR5. Future research should integrate multi-omics and develop probiotics to enhance IMF efficiency for sustainable pork production.
29 January 2026