Journal Description
Drugs and Drug Candidates
Drugs and Drug Candidates
is an international, peer-reviewed, open access journal on drug discovery, development, and knowledge, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 15 days after submission; acceptance to publication is undertaken in 4.7 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
- Drugs and Drug Candidates is a companion journal of Pharmaceuticals.
- Journal Clusters-Pharmaceutical Science: Scientia Pharmaceutica, Marine Drugs, Pharmaceuticals, Pharmaceutics, Pharmacy, Future Pharmacology, Pharmacoepidemiology, Drugs and Drug Candidates and Journal of Pharmaceutical and BioTech Industry.
Latest Articles
Molecular Hybridization of Naphthoquinones as Selective Inhibitors of Shikimate Kinase: A Promising Strategy Against Mycobacterium tuberculosis
Drugs Drug Candidates 2025, 4(4), 59; https://doi.org/10.3390/ddc4040059 - 18 Dec 2025
Abstract
Background: Tuberculosis (TB) remains a critical global health concern, exacerbated by the emergence of multidrug-resistant and extensively drug-resistant strains of Mycobacterium tuberculosis. In the search for novel therapeutic agents, naphthoquinones have garnered interest due to their diverse mechanisms of action and potent
[...] Read more.
Background: Tuberculosis (TB) remains a critical global health concern, exacerbated by the emergence of multidrug-resistant and extensively drug-resistant strains of Mycobacterium tuberculosis. In the search for novel therapeutic agents, naphthoquinones have garnered interest due to their diverse mechanisms of action and potent antimycobacterial activity. In this study, we report the design, synthesis, and biological evaluation of a novel series of eleven naphthoquinone-based derivatives (compounds 22–32), developed through a molecular hybridization strategy targeting shikimate kinase (Mtb-SK) an essential enzyme present exclusively in M. tuberculosis. Methods: The compounds were synthesized via a straightforward and efficient synthetic route, and preliminary screening identified five molecules with significant anti-TB activity. Notably, compound 26, 4-(4-ethoxyphenyl) amino) Naphthalene-1,2-dione, exhibited a minimum inhibitory concentration (MIC) of 21.33 µM, comparable to ethambutol and substantially more potent than pyrazinamide. Results: Molecular docking studies indicated that all active compounds interact favorably within the shikimate binding pocket of Mtb-SK, following the proposed mechanism of action. Additionally, ongoing cytotoxicity assays in HepG2 cells aim to assess the selectivity of these derivatives. Conclusions: These findings support the potential of this new class of naphthoquinones as promising scaffolds for the development of anti-TB agents, contributing to the growing body of research focused on new chemotherapeutic options against tuberculosis.
Full article
(This article belongs to the Collection Anti-Parasite Drug Discovery)
►
Show Figures
Open AccessArticle
Cytotoxic and Antiproliferative Effects of Chlorella vulgaris Lectin on Colon Cancer Cells
by
Vivianne Lays Ribeiro Cavalcanti, Maria Carla Santana de Arruda, Thalya Natasha da Silva Santos, Daniela de Araújo Viana Marques, Romero Marcos Pedrosa Brandão Costa, Luiza Rayanna Amorim de Lima, Ana Lúcia Figueiredo Porto and Raquel Pedrosa Bezerra
Drugs Drug Candidates 2025, 4(4), 58; https://doi.org/10.3390/ddc4040058 - 18 Dec 2025
Abstract
Background/Objectives: Colon cancer is the third most common type of cancer in the world, characterized by a high risk of metastasis, resistance to various drugs, and late diagnosis. In addition, the drugs used for treatment are associated with serious neurological damage, causing acute
[...] Read more.
Background/Objectives: Colon cancer is the third most common type of cancer in the world, characterized by a high risk of metastasis, resistance to various drugs, and late diagnosis. In addition, the drugs used for treatment are associated with serious neurological damage, causing acute and chronic pain and compromising the patient’s quality of life. Meanwhile, lectins are proteins capable of exerting cytotoxic action on cells from various tumors in a selective manner, without exerting significant toxicity on healthy cells. Despite this, studies on the potential of lectins obtained from microalgae are still scarce in the literature. In this sense, the objective of this study was to evaluate the antitumor activity of lectin isolated from the microalgae Chlorella vulgaris (CvL) on colorectal cancer cells, HT-29. Methods: The purified lectin was tested for cytotoxicity using MTT colorimetric methods, in addition to clonogenicity, cell cycle, apoptosis, and necrosis tests, analyzed by flow cytometry. Results: The assays demonstrated that the lectin was able to induce cell death in the HT-29 tumor line by approximately 83.75% with an IC50 value of 21.5 µg/mL−1, reduced colony formation by more than 90%, was able to regulate the cell cycle by apoptosis, and did not present significant necrosis. These results show that microalgae lectins have the potential to be exploited in the control of neoplastic cells.
Full article
(This article belongs to the Section Drug Candidates from Natural Sources)
►▼
Show Figures

Graphical abstract
Open AccessEditorial
Drugs and Drug Candidates: Constantly Progressing
by
Jean Jacques Vanden Eynde
Drugs Drug Candidates 2025, 4(4), 57; https://doi.org/10.3390/ddc4040057 - 18 Dec 2025
Abstract
►▼
Show Figures
As we are closing Volume 4 of Drugs and Drug Candidates (DDC), this gives me the opportunity to share the journal’s latest developments [...]
Full article
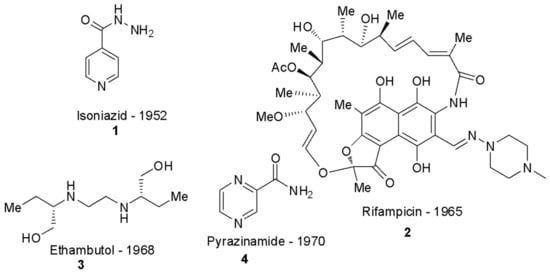
Figure 1
Open AccessArticle
Repositioning Imipramine for Antiparasitic Effects Against Giardia lamblia
by
Xareni Zinereth Herrera-Valero, Sendar Daniel Nery-Flores, Filiberto Gutiérrez-Gutiérrez, Lizeth Guadalupe Campos-Múzquiz, Sandra Cecilia Esparza-González, Raúl Rodríguez-Herrera and Lissethe Palomo-Ligas
Drugs Drug Candidates 2025, 4(4), 56; https://doi.org/10.3390/ddc4040056 - 16 Dec 2025
Abstract
Background/Objectives: Giardia lamblia is an intestinal protozoan responsible for giardiasis, a globally prevalent parasitic disease. Current therapeutic options, including nitroimidazoles and benzimidazoles, have increasing treatment failures due to resistance, adverse reactions, and patient non-compliance. Drug repositioning offers a cost-effective strategy for identifying
[...] Read more.
Background/Objectives: Giardia lamblia is an intestinal protozoan responsible for giardiasis, a globally prevalent parasitic disease. Current therapeutic options, including nitroimidazoles and benzimidazoles, have increasing treatment failures due to resistance, adverse reactions, and patient non-compliance. Drug repositioning offers a cost-effective strategy for identifying new antigiardial agents. This study aimed to evaluate the in vitro antiparasitic effects and possible mechanisms of action of the tricyclic antidepressant imipramine against G. lamblia trophozoites. Methods: Trophozoites were exposed to increasing concentrations of imipramine (25–125 µM). Growth inhibition and adhesion capacity were quantified using cell counts. Apoptosis- or necrosis-like death was evaluated through Annexin V/PI staining. The expression and distribution of α-tubulin and lipid rafts were analyzed by immunofluorescence microscopy. Finally, the effect of the drug on encystment efficiency was assessed in vitro. Results: Imipramine inhibited G. lamblia trophozoite growth in a concentration-dependent manner, with an IC50 of 42.31 µM at 48 h. The drug significantly reduced adhesion capacity (>90% at 125 µM) and induced apoptosis-like cell death, as evidenced by Annexin V positivity. Immunofluorescence revealed disruption of α-tubulin distribution and lipid raft organization, accompanied by morphological rounding. Moreover, encystment efficiency decreased in a concentration-dependent mode, suggesting interference in the differentiation process. Conclusions: This investigation describes, for the first time, the antigiardial potential of imipramine, which alters cytoskeletal organization, membrane microdomains, and differentiation pathways, ultimately leading to apoptosis-like cell death. These findings position this compound as a promising lead structure and support further exploration of tricyclic antidepressants as scaffolds for the development and optimization of new antiparasitic agents, as well as future studies on their molecular targets and in vivo efficacy.
Full article
(This article belongs to the Collection Anti-Parasite Drug Discovery)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Thalidomide-Based PROTACs: A Viable Strategy Against Trypanosomatids?
by
Romina Manarin, Gianfranco Frattini, Victoria L. Alonso, Victoria Boselli, Giselle R. Bedogni, Elvio Rodríguez Araya, Diego M. Moreno and Esteban Serra
Drugs Drug Candidates 2025, 4(4), 55; https://doi.org/10.3390/ddc4040055 - 10 Dec 2025
Abstract
Background: In recent years, compounds known as Proteolysis Targeted Chimeras (PROTACs) have revitalized the field of bioactive molecule design. These compounds promote proteolysis of therapeutic targets by recruiting them to ubiquitin ligases. One of the most commonly used classes of compounds in the
[...] Read more.
Background: In recent years, compounds known as Proteolysis Targeted Chimeras (PROTACs) have revitalized the field of bioactive molecule design. These compounds promote proteolysis of therapeutic targets by recruiting them to ubiquitin ligases. One of the most commonly used classes of compounds in the synthesis of PROTACs are immunomodulatory imides (IMiDs), such as thalidomide (TLD), which interact with the E3 ligase CRL4CRBN via the CULT domain of the cereblon protein (CRBN). This domain has been identified in proteins across various phylogenetic groups, including trypanosomatids, leading to the hypothesis that IMiD-derived PROTACs should be active in these organisms. Methods: The trypanocidal activity of the PROTAC dBET1 and its separated components (JQ1 and TLD) were assayed using a T. cruzi strain expressing β-glalactosidase. Potential CRL4-E3L complexes from humans and trypanosomatids were assembled in silico with MultimerMapper. The IMiD-binding site of HsCRBN and its trypanosomatid homologs were analyzed using molecular dynamics and docking simulations. Results: We demonstrate that the compound dBET1 does not function as a PROTAC in Trypanosoma cruzi. In silico structural analysis of CRL4-E3L complex orthologs revealed that the trypanosomal CULT-containing protein is not part of such a complex. Molecular dynamics simulations showed that the pocket of this CULT domain is smaller than that of mammalian CRBN and cannot accommodate IMiDs within. Conclusions: We underscore the importance of functional and structural validation in drug discovery, particularly when extrapolating mechanisms between evolutionarily distant species. While PROTACs hold promise in human therapeutics, our work advocates for re-evaluating the rationale behind thalidomide-based PROTACs in trypanosomatid research.
Full article
(This article belongs to the Collection Anti-Parasite Drug Discovery)
►▼
Show Figures
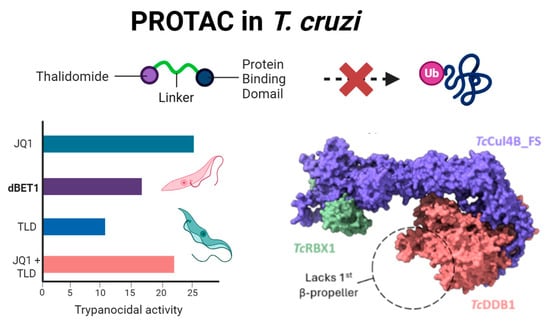
Graphical abstract
Open AccessArticle
Aqueous Leaf Extracts of Bauhinia cheilantha (Bong.) Steud.: Phytochemical Profile, Antioxidant Activity and In Vitro Safety Evaluation
by
Palloma Lima de Oliveira, José Rafael da Silva Araújo, Camila Marinho da Silva, Kyria Cilene de Andrade Bortoleti, Silvany de Sousa Araújo, Márcia Vanusa da Silva, Dráulio Costa da Silva, Marcos dos Santos Lima, Ana Paula de Oliveira and Ana Christina Brasileiro-Vidal
Drugs Drug Candidates 2025, 4(4), 54; https://doi.org/10.3390/ddc4040054 - 8 Dec 2025
Abstract
Background/Objectives: Bauhinia cheilantha Bong. Steud. (Leguminosae; “pata-de-vaca”) is traditionally used in folk medicine for its antidiabetic, anti-inflammatory, and sedative properties. This study aimed to evaluate aqueous leaf extracts of B. cheilantha, non-delipidated and delipidated, regarding their phytochemical composition, phenolic profile, antioxidant potential,
[...] Read more.
Background/Objectives: Bauhinia cheilantha Bong. Steud. (Leguminosae; “pata-de-vaca”) is traditionally used in folk medicine for its antidiabetic, anti-inflammatory, and sedative properties. This study aimed to evaluate aqueous leaf extracts of B. cheilantha, non-delipidated and delipidated, regarding their phytochemical composition, phenolic profile, antioxidant potential, and cytotoxic, genotoxic, and antigenotoxic effects. Methods: Phytochemical screening was performed by TLC, and phenolic compounds were determined by HPLC. Antioxidant activity was assessed using DPPH, ABTS, and phosphomolybdenum assays. Cytotoxicity, genotoxicity, and antigenotoxicity were evaluated in L929 murine fibroblast cells using MTT and cytokinesis-block micronucleus (CBMN) assays. Results: Both extracts contained anthocyanins, phenolics, lignans, saponins, and hydrolyzable tannins. The delipidated extract showed higher total phenolic content (17.54 mg/kg) than the non-delipidated (13.76 mg/kg). Major constituents included kaempferol 3-glucoside, quercetin, hesperidin, naringenin, and t-cinnamic acid. Antioxidant assays revealed EC50 values of 25.84, 13.60, and 66.09 µg/mL for the non-delipidated extract, and 26.19, 16.34, and 52.78 µg/mL for the delipidated extract in the DPPH, ABTS, and phosphomolybdenum assays, respectively. No cytotoxicity was observed, except at 1600 µg/mL for the non-delipidated extract and 800–1600 µg/mL for the delipidated extract. Genotoxicity occurred only at 400 µg/mL. Antigenotoxic evaluation showed that the non-delipidated extract (100 µg/mL) reduced methyl methanesulfonate-induced chromosomal damage in simultaneous and post-treatment conditions, while the delipidated extract was only effective for post-treatment. Conclusions: Aqueous extracts of B. cheilantha exhibit antioxidant and antigenotoxic properties. At active concentrations, they were non-cytotoxic and non-genotoxic. The non-delipidated extract, in particular, showed the strongest genome-protective potential, supporting its traditional use and highlighting its relevance in the development of natural therapeutic agents.
Full article
(This article belongs to the Section Drug Candidates from Natural Sources)
►▼
Show Figures
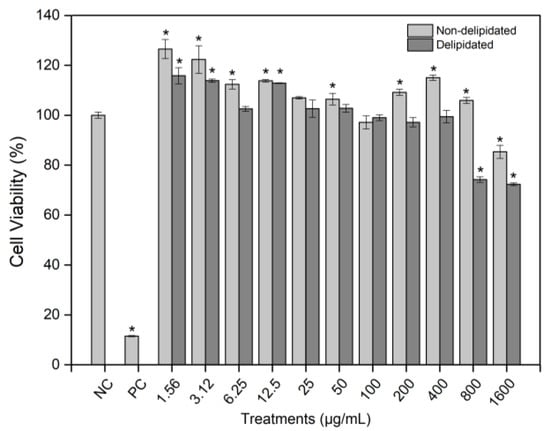
Figure 1
Open AccessArticle
Synthetic Derivatives of Vinpocetine as Antiproliferative Agents
by
Mihira Gutti, Melanie Tsui, Stella Yang, Selina Xi, Jennifer Luo, Arshia Desarkar, Yining Xie, Mirabelle Feng, Udbhav Avadhani, Shloka Raghavan, Elena Brierley-Green, Erika Yu and Edward Njoo
Drugs Drug Candidates 2025, 4(4), 53; https://doi.org/10.3390/ddc4040053 - 28 Nov 2025
Abstract
Background/Objectives: Vincamine is an indole alkaloid initially isolated from plants of the Vinca genus and has previously been demonstrated to have antioxidant, hypoglycemic, and hypolipidemic activities. Vinpocetine, a synthetic derivative of vincamine with an enhanced pharmacological profile, has demonstrated promising antiproliferative properties. While
[...] Read more.
Background/Objectives: Vincamine is an indole alkaloid initially isolated from plants of the Vinca genus and has previously been demonstrated to have antioxidant, hypoglycemic, and hypolipidemic activities. Vinpocetine, a synthetic derivative of vincamine with an enhanced pharmacological profile, has demonstrated promising antiproliferative properties. While previously reported vinpocetine derivatives have undergone extensive investigation for their pharmacological properties, the role of the E-ring ethyl ester in the antiproliferative properties of compounds with this scaffold has not yet been fully described. Methods: Here, the antiproliferative activity of two vinpocetine analogs with modifications at the E-ring was evaluated through cell viability and LDH assays, and their mechanism of action was investigated through cell cycle analysis, apoptosis detection, and reporter assays for Wnt-1, NF-κB, and STAT3 signaling. Results: Cell viability assays revealed that reduction of the ethyl ester to an alcohol exhibited strong dose-dependent antiproliferative activity across five mammalian cell lines, but did not induce significant markers of apoptosis or necrotic death as determined by FITC/Annexin V and cell cycle flow cytometry, respectively. Through label-free cell imaging, we found the antiproliferative activity of vinpocetine alcohol to be correlated with a decrease in membrane integrity in treated cells. We further observe that both analogs exhibit dose-dependent modulation of TCF/LEF, NF-kB, and STAT3 reporter cells, which appears to be coupled with trends in antiproliferative activity. Conclusions: Altogether, this work demonstrates the potential for E-ring modifications of vinpocetine as antiproliferative agents.
Full article
(This article belongs to the Section Preclinical Research)
►▼
Show Figures

Graphical abstract
Open AccessArticle
A Silver (I) Complex-Loaded Lipid Nanoemulsion: A New Approach Against Candida albicans Biofilms
by
Giovanna Capaldi Fortunato, Gabriel Davi Marena, Débora Eduarda Soares Silva, Tamara Renata Machado Ribeiro, Cristiano Gallina Moreira, Flávia Aparecida Resende Nogueira, Leonardo Delello Di Filippo, Marlus Chorilli, Adelino Vieira de Godoy Netto and Taís Maria Bauab
Drugs Drug Candidates 2025, 4(4), 52; https://doi.org/10.3390/ddc4040052 - 15 Nov 2025
Abstract
Introduction: The increasing prevalence of fungal infections, particularly those caused by Candida albicans, presents a significant clinical challenge due to the emergence of drug-resistant strains. Silver (I) coordination complexes show promise as antifungal agents; however, their poor water solubility limits clinical
[...] Read more.
Introduction: The increasing prevalence of fungal infections, particularly those caused by Candida albicans, presents a significant clinical challenge due to the emergence of drug-resistant strains. Silver (I) coordination complexes show promise as antifungal agents; however, their poor water solubility limits clinical application. Methods: In this study, we developed and characterized a lipid nanoemulsion (Ag-LN) to enhance the delivery and activity of a silver (I) complex. Results: The formulation exhibited nanoscale size, spherical morphology, and stability for up to 60 days. Ag-LN showed potent antifungal effects, preventing biofilm formation and eradicating mature biofilms. Importantly, nanoencapsulation preserved antifungal activity while reducing mutagenic potential and acute toxicity compared with the free compound. Conclusions: These findings support Ag-LN as a promising antifungal platform for future preclinical studies.
Full article
(This article belongs to the Collection Bioinorganic Chemistry in Drug Discovery)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Integration of In Vitro Glucose Utilization, Metabolomics and Network Pharmacology Strategy to Explore Antidiabetic Mechanisms of Gunnera perpensa and Erythrina zeyheri Extracts
by
Oyinlola Oluwunmi Olaokun
Drugs Drug Candidates 2025, 4(4), 51; https://doi.org/10.3390/ddc4040051 - 14 Nov 2025
Abstract
Background: Type 2 diabetes mellitus (T2DM) is a complex metabolic disease requiring multi-targeted therapeutic strategies. Gunnera perpensa and Erythrina zeyheri are traditionally used in diabetes management, but their mechanisms remain poorly understood. Methods: This study used in vitro, metabolomics, and network
[...] Read more.
Background: Type 2 diabetes mellitus (T2DM) is a complex metabolic disease requiring multi-targeted therapeutic strategies. Gunnera perpensa and Erythrina zeyheri are traditionally used in diabetes management, but their mechanisms remain poorly understood. Methods: This study used in vitro, metabolomics, and network pharmacology approaches to elucidate their antidiabetic potential. Leaf extracts were screened for glucose utilization in C2C12 cells, and cytotoxicity in Vero cells. Metabolites profiled via GC×GC-TOF-MS and those retrieved from Phytochemical Interaction Database were evaluated for drug-likeness and target prediction using SwissADME and SwissTargetPrediction. Diabetes-related targets were obtained from databases, and overlapping targets were used to construct interaction networks using Cytoscape and STRING. Functional enrichment analyses were conducted via DAVID for GO and KEGG pathways. Results: G. perpensa acetone and methanol extracts enhanced superior glucose utilization (IC50 = 78.5 and 94.8 µg/mL, respectively), with low cytotoxicity (LC50 > 600 µg/mL). Key compounds including arabinose, identified from both plants, showed multi-target binding potential against STAT3, PIK3RI and JAK2. Enrichment analyses revealed pathways related to insulin signaling, inflammation, and glucose metabolism. Conclusions: This study supports the therapeutic relevance of phytochemical synergy in the traditional use of both plants and demonstrated systems-level approaches for elucidating complex drug–target interactions in T2DM.
Full article
(This article belongs to the Section Drug Candidates from Natural Sources)
►▼
Show Figures

Figure 1
Open AccessArticle
In Silico Evaluation of Structural Consequences in the Human CYP3A4 Caused by Molnupiravir-Induced Mutations During COVID-19 Treatment
by
Madhumita Aggunna, Chiranjeevi V. M. Ganteti, Keerthi R. Bhukya, Meghana Mathangi, Joyjethin Neelam, Aswitha Gurrala, Bavana Grandhi, Noahjeevan Vejendla, Sriharshini Mathangi, Swarnalatha Gudapati and Ravikiran S. Yedidi
Drugs Drug Candidates 2025, 4(4), 50; https://doi.org/10.3390/ddc4040050 - 11 Nov 2025
Abstract
Background/Objectives: Molnupiravir (MOV) and nirmatrelvir (NMV) are antiviral drugs that were FDA-approved under the emergency use authorization (EUA) for coronavirus disease-2019 (COVID-19) treatment. MOV and NMV target the viral RNA-dependent RNA polymerase and main protease, respectively. Paxlovid is a combination of NMV and
[...] Read more.
Background/Objectives: Molnupiravir (MOV) and nirmatrelvir (NMV) are antiviral drugs that were FDA-approved under the emergency use authorization (EUA) for coronavirus disease-2019 (COVID-19) treatment. MOV and NMV target the viral RNA-dependent RNA polymerase and main protease, respectively. Paxlovid is a combination of NMV and ritonavir (RTV), an inhibitor of the human cytochrome P450-3A4 (hCYP3A4). In this study, the structural consequences in the hCYP3A4 caused by MOV-induced mutations (MIM) were evaluated using in silico tools. Methods: MOV-induced mutations (MIM) were inserted into all the possible hotspots in the active site region of the hCYP3A4 gene, and mutant protein models were built. Structural changes in the heme-porphyrin ring of hCYP3A4 were analyzed in the presence and absence of substrates/inhibitors, including RTV. Molecular dynamics (MD) simulations were performed to analyze the effect of MIM-induced structural changes in hCYP3A4 on drug binding. Results: MD simulations confirm that MIMs, R375G and R440G in hCYP3A4 severely affect the heme-porphyrin ring stability by causing a tilt that in turn affects RTV binding, suggesting a possible inefficiency in the function of hCYP3A4. Similar results were seen for amlodipine, atorvastatin, sildenafil and warfarin, which are substrates of hCYP3A4. Conclusions: The current in silico studies indicate that hCYP3A4 containing MIMs can create complications in the treatment of COVID-19 patients, particularly with co-morbidities due to its functional inefficiency. Hence, clinicians must be vigilant when using MOV in combination with other drugs. Further in vitro studies focused on hCYP3A4 containing MIMs are currently in progress to support our current in silico findings.
Full article
(This article belongs to the Special Issue Fighting SARS-CoV-2 and Related Viruses)
►▼
Show Figures

Figure 1
Open AccessArticle
Leveraging Gene Expression Data for Drug Repurposing in Schizophrenia: A Signature Reversion Approach
by
Maria Chalkioti, Thomas Papikinos, Marios G. Krokidis, Panagiotis Vlamos and Themis P. Exarchos
Drugs Drug Candidates 2025, 4(4), 49; https://doi.org/10.3390/ddc4040049 - 11 Nov 2025
Abstract
Background/Objectives: Despite continuous pharmacological advances, the treatment of schizophrenia remains challenging, and suboptimal outcomes are still too frequent. There are currently limited new approved drugs without resistance. Methods: For this reason, drug repurposing presents a promising solution for identifying existing drugs
[...] Read more.
Background/Objectives: Despite continuous pharmacological advances, the treatment of schizophrenia remains challenging, and suboptimal outcomes are still too frequent. There are currently limited new approved drugs without resistance. Methods: For this reason, drug repurposing presents a promising solution for identifying existing drugs with therapeutic effects for schizophrenia. In this study, we provide a workflow of signature-based drug repurposing methodology. We initially utilized a dataset from Gene Expression Omnibus which consists of RNA sequence data from blood-derived leukocyte samples from individuals with schizophrenia and control subjects, and conducted an analysis. Results: This analysis identified 1205 statistically significant differentially expressed genes, of which 150 upregulated and 150 downregulated genes were used in the CMap and L1000CDS2 tools. Then, each database generated a list of potential compounds that could reverse the disease’s signature and potentially have therapeutic effects for schizophrenia. Subsequently, the compounds associated with the disease, as identified in the research, were chemically clustered, and then their modes of action were predicted. In the last stage, we conducted a literature review to evaluate the relationship of these modes of action with the disease. Conclusions: This systematic analysis provided a list of potential drugs for schizophrenia treatment so that their efficacy can be evaluated in the wet-lab experiments, which is the next stage of drug repurposing.
Full article
(This article belongs to the Section In Silico Approaches in Drug Discovery)
►▼
Show Figures

Figure 1
Open AccessReview
Cyclophosphamide: Old Drug with Great Future
by
Georg Voelcker
Drugs Drug Candidates 2025, 4(4), 48; https://doi.org/10.3390/ddc4040048 - 3 Nov 2025
Abstract
This paper does not describe the results of a systematic search for the mechanism of action of cyclophosphamide and the consequences and possible indications arising from this mechanism. Rather, it describes a puzzle in which our own results, with some of them being
[...] Read more.
This paper does not describe the results of a systematic search for the mechanism of action of cyclophosphamide and the consequences and possible indications arising from this mechanism. Rather, it describes a puzzle in which our own results, with some of them being very old, were re-evaluated with the latest biochemical knowledge and supplemented by results from the scientific literature. The mechanism of action of cyclophosphamide, which has been indispensable in clinical practice for 60 years, was unknown until recently simply because biochemical knowledge was lacking and because results from in vitro experiments were uncritically extrapolated to in vivo conditions. In vitro, the DNA alkylating metabolite phosphoramide mustard (PAM) is formed from the CP metabolite aldophosphamide (ALD) by phosphate and bicarbonate ion-catalyzed β-elimination of acrolein; in vivo, however, ALD is cleaved by phosphoesterases or DNA polymerase δ and ε, which are associated with 3′-5′ exonucleases, into the complementary metabolites PAM and 3-hydroxypropanal (HPA). The following describes the mechanism of action of CP, namely the complementary interaction of alkylating PAM and apoptosis-enhancing HPA, and it is shown that by optimizing the complementary effects of PAM and HPA, the antitumor efficacy in the P388 mouse tumor model can be increased by more than ten thousand-fold. Further experiments show that by optimizing the interaction of DNA alkylation and enhancing the resulting apoptosis by HPA, the formation of resistant metastases can be prevented and low-toxicity chemotherapy can be achieved.
Full article
(This article belongs to the Section Marketed Drugs)
►▼
Show Figures

Figure 1
Open AccessArticle
Discovery and Characterization of 7,8-Dihydropyrido[4,3-d]pyrimidines as SARS-CoV-2 Entry Inhibitors
by
Sean P. Bradley, Jazmin M. Galván Achi, Laura Cooper, Malaika D. Argade, Han Cheng, Ryan Bott, Christian A. Zielinski, Arsen M. Gaisin, Luke T. Jesikiewicz, José A. Villegas, Hyun Lee, Kiira Ratia, Norton P. Peet, Lijun Rong and Irina N. Gaisina
Drugs Drug Candidates 2025, 4(4), 47; https://doi.org/10.3390/ddc4040047 - 29 Oct 2025
Abstract
Background/Objectives: We have established a robust, cell-based high-throughput screening platform capable of identifying SARS-CoV-2 entry inhibitors within a BSL-2 facility. Methods: Using a curated compound library, we conducted a screening campaign that led to the discovery of potent viral entry inhibitors
[...] Read more.
Background/Objectives: We have established a robust, cell-based high-throughput screening platform capable of identifying SARS-CoV-2 entry inhibitors within a BSL-2 facility. Methods: Using a curated compound library, we conducted a screening campaign that led to the discovery of potent viral entry inhibitors active in both pseudoviral and infectious SARS-CoV-2 inhibition assays. Results: Among those, Hit-1 exhibited submicromolar antiviral activity across all tested SARS-CoV-2 strains, including the highly transmissible Omicron subvariants. Biophysical binding assays confirmed that Hit-1 and related compounds directly engage the prefusion-stabilized SARS-CoV-2 spike proteins of both authentic WA1/2020 and Omicron viral strains. To elucidate potential binding orientations and interactions of the hit compounds with the SARS-CoV-2 spike protein, molecular docking studies were performed targeting two putative binding sites. Conclusions: Preliminary structure–activity relationship studies identified a promising subset of drug-like 7,8-dihydropyrido[4,3-d]pyrimidine-based inhibitors with potential for further development as novel therapeutic agents aimed at blocking viral entry and thereby preventing or mitigating SARS-CoV-2 infection. Among these, compound 13 stands out due to its superior in vitro potency and favorable pharmacokinetic properties, positioning it as a strong candidate for in vivo efficacy evaluation.
Full article
(This article belongs to the Special Issue Fighting SARS-CoV-2 and Related Viruses)
►▼
Show Figures

Figure 1
Open AccessCorrection
Correction: Tabatabaei et al. SARS-CoV-2 and Coronaviruses: Understanding Transmission, Impact, and Strategies for Prevention and Treatment. Drugs Drug Candidates 2025, 4, 5
by
Seyede Nafise Tabatabaei, Zahra Keykhaee, Saghi Nooraei, Mohammad Amin Ayati, Mohammad Behzadmand, Saba Azimi, Fatemeh Eskati and Gholamreza Ahmadian
Drugs Drug Candidates 2025, 4(4), 46; https://doi.org/10.3390/ddc4040046 - 23 Oct 2025
Abstract
In the published manuscript [...]
Full article
Open AccessArticle
Efficient Enrichment of Total Flavonoids and Antibacterial Activity of the Ethyl Acetate Fraction of Croton blanchetianus Baill. (Euphorbiaceae) Leaves
by
Pedro Artur Ferreira Marinho, Wêndeo Kennedy Costa, Maria Tereza dos Santos Correia, Wliana Alves Viturino da Silva, Magda Rhayanny Assunção Ferreira, Luiz Alberto Lira Soares, José Jailson Lima Bezerra and Alisson Macário de Oliveira
Drugs Drug Candidates 2025, 4(4), 45; https://doi.org/10.3390/ddc4040045 - 18 Oct 2025
Abstract
Background/Objectives: This study investigated the flavonoid enrichment and antimicrobial activity of the ethyl acetate fraction (EAF) obtained from Croton blanchetianus (Euphorbiaceae) leaves against Staphylococcus aureus, including the methicillin-resistant strains (MRSA) that were isolated, as well as its possible mechanism of action.
[...] Read more.
Background/Objectives: This study investigated the flavonoid enrichment and antimicrobial activity of the ethyl acetate fraction (EAF) obtained from Croton blanchetianus (Euphorbiaceae) leaves against Staphylococcus aureus, including the methicillin-resistant strains (MRSA) that were isolated, as well as its possible mechanism of action. Methods: Croton blanchetianus leaves were extracted with ethanol:water (50%), then the extract was spray-dried and partitioned (8×) with ethyl acetate. Phytochemical analysis was performed using thin layer chromatography (TLC), while antibacterial activity was conducted using minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) methods. Results: Chemical profiling (TLC) confirmed multiple flavonoid bands and the presence of hyperoside; the total flavonoid content in the EAF reached 25.3% (≈2.28× the spray-dried extract and 6.65× the aqueous fraction). The MIC and MBC assays against S. aureus ATCC 29213 and six clinical isolates showed an MIC of 4–32 μg/mL and an MBC of 16–64 μg/mL for EAF. The combination of EAF with chloramphenicol showed a complete synergistic effect for S. aureus ATCC 29213 and S. aureus UFPEDA 705, a partial effect for S. aureus UFPEDA-659 and S. aureus UFPEDA-671, antagonistic effect for S. aureus UFPEDA 731 and S. aureus UFPEDA 802, and no effect for S. aureus UFPEDA-691. Growth curves indicated time- and concentration-dependent inhibition. Membrane integrity assays revealed K+ efflux and release of DNA/RNA and proteins, suggesting bacterial membrane destabilization as a likely mechanism. Conclusions: The flavonoid-rich fraction of C. blanchetianus exhibits potent anti-S. aureus activity, including MRSA. Furthermore, it was observed that EAF has a synergistic effect with chloramphenicol and acts through membrane damage, making it a candidate for a phytoderived adjuvant in antimicrobial therapies.
Full article
(This article belongs to the Section Drug Candidates from Natural Sources)
►▼
Show Figures

Figure 1
Open AccessReview
Glycyrrhizin (Glycyrrhizic Acid)—Pharmacological Applications and Associated Molecular Mechanisms
by
Deepak Kumar Semwal, Ankit Kumar, Ruchi Badoni Semwal, Nand Kishor Dadhich, Ashutosh Chauhan and Vineet Kumar
Drugs Drug Candidates 2025, 4(4), 44; https://doi.org/10.3390/ddc4040044 - 30 Sep 2025
Cited by 1
Abstract
Background/Objectives: Natural products, especially plant metabolites, play a crucial role in drug development and are widely used in medicine, cosmetics, and nutrition. The present review aims to provide a comprehensive overview of the pharmacological profile of Glycyrrhizin (GL), with a specific focus on
[...] Read more.
Background/Objectives: Natural products, especially plant metabolites, play a crucial role in drug development and are widely used in medicine, cosmetics, and nutrition. The present review aims to provide a comprehensive overview of the pharmacological profile of Glycyrrhizin (GL), with a specific focus on its molecular targets. Methods: Scientific literature was thoroughly retrieved from reputable databases, including Scopus, Web of Science, and PubMed, up to 30 July 2025. The keywords “glycyrrhizin” and “glycyrrhizic acid” were used to identify relevant references, with a focus on pharmacological applications. Studies on synthetic analogs, non-English publications, non-pharmacological applications, and GL containing crude extracts were largely excluded. Results: Glycyrrhizin, the major bioactive constituent of Glycyrrhiza glabra, exhibits diverse pharmacological activities, including anti-inflammatory, antiviral, hepatoprotective, antitumor, neuroprotective, and immunomodulatory effects. These actions are primarily mediated through the inhibition of high-mobility group box 1 (HMGB1) and the modulation of key signaling pathways, including nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), and various cytokine networks. As a result of its therapeutic potential, GL-based formulations, including Stronger Neo-Minophagen C, and GL-rich extracts of G. glabra are commercially available as pharmaceutical preparations and food additives. Conclusions: Despite its therapeutic potential, the clinical application of GL is limited by poor oral bioavailability, metabolic variability, and adverse effects such as pseudoaldosteronism. Hence, careful consideration of pharmacokinetics and safety is essential for translating its therapeutic potential into clinical practice.
Full article
(This article belongs to the Section Drug Candidates from Natural Sources)
►▼
Show Figures

Graphical abstract
Open AccessCorrection
Correction: Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4
by
Noureddine Chaachouay
Drugs Drug Candidates 2025, 4(3), 43; https://doi.org/10.3390/ddc4030043 - 22 Sep 2025
Abstract
In the published publication [...]
Full article
Open AccessArticle
Design of a First-in-Class homoPROTAC to Induce ICP0 Degradation in Human Herpes Simplex Virus 1
by
Leyla Salimova, Ali Sahin, Ozge Ardicli, Fatima Hacer Kurtoglu Babayev, Zeynep Betul Sari, Muhammed Emin Sari, Muhammet Guzel Kurtoglu, Sena Ardicli and Huseyn Babayev
Drugs Drug Candidates 2025, 4(3), 42; https://doi.org/10.3390/ddc4030042 - 8 Sep 2025
Abstract
Background/Objectives: Human Herpes Simplex Virus 1 (HSV-1) is a common pathogen that establishes lifelong latent infections. The emergence of drug resistance necessitates novel therapeutic strategies. This study introduces a novel antiviral approach: a bivalent degrader designed to induce the degradation of an
[...] Read more.
Background/Objectives: Human Herpes Simplex Virus 1 (HSV-1) is a common pathogen that establishes lifelong latent infections. The emergence of drug resistance necessitates novel therapeutic strategies. This study introduces a novel antiviral approach: a bivalent degrader designed to induce the degradation of an essential protein. Methods: A structural model of ICP0, generated via the Chai-1 AI platform, was analyzed with fpocket, P2Rank, and KVFinder to identify a superior allosteric target site. An iterative de novo design workflow with CReM-dock then yielded a lead scaffold based on its predicted affinity and drug-like properties. This selected “warhead” was used to rationally design the final bivalent degrader, ICP0-deg-01, for the ICP0 dimer model. Results: The generative process yielded a lead chemical scaffold that was selected based on its predicted binding affinity and favorable drug-like properties. This scaffold was used to rationally design a single candidate bivalent degrader, ICP0-deg-01. Our structural model predicts that ICP0-deg-01 can successfully bridge two ICP0 protomers, forming an energetically favorable ternary complex. Conclusions: This work provides a computational proof-of-concept for a novel class of anti-herpetic agents and identifies a lead candidate for future molecular dynamics simulations and experimental validation.
Full article
(This article belongs to the Section In Silico Approaches in Drug Discovery)
►▼
Show Figures

Figure 1
Open AccessArticle
Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors
by
Fernanda F. Souza, Juliana F. Vilachã, Othon S. Campos and Heberth de Paula
Drugs Drug Candidates 2025, 4(3), 41; https://doi.org/10.3390/ddc4030041 - 1 Sep 2025
Abstract
Background/Objectives: Malaria is a prominent vector-borne disease, with a high mortality rate, particularly in children under five years old. Despite the use of various insecticides for its control, the emergence of resistant mosquitoes poses a significant public health threat. Acetylcholinesterase (AChE) is
[...] Read more.
Background/Objectives: Malaria is a prominent vector-borne disease, with a high mortality rate, particularly in children under five years old. Despite the use of various insecticides for its control, the emergence of resistant mosquitoes poses a significant public health threat. Acetylcholinesterase (AChE) is a crucial enzyme in nerve transmission and a primary target for insecticide development due to its role in preventing repeated nerve impulses. Recent studies have identified difluoromethyl ketone (DFK) as a potent inhibitor of both sensitive and resistant Anopheles gambiae acetylcholinesterase (AgAChE). This study aimed to identify novel AgAChE inhibitors that could be explored for malaria prevention. Methods: We performed a virtual screening on the PubChem database using a pharmacophore model from difluoromethyl ketone-inhibited AgAChE’s crystal structure. The most promising compound was then subjected to molecular docking and dynamics studies with AgAChE to confirm initial findings. ADMET and agrochemical likeness (ag-like) properties were also analyzed to assess its potential as an agrochemical agent. Results: PubChem18463786 was identified as the most suitable compound from the virtual screening. Molecular docking and molecular dynamics studies confirmed its strong interaction with AgAChE. The ADMET and ag-like analyses indicated that PubChem18463786 possesses physicochemical properties suggesting a high probability of non-absorption in humans and meets the criteria for agrochemical similarity. Conclusions: Our findings suggest that PubChem18463786 is a potential AgAChE inhibitor candidate. After validation through in vitro and in vivo experiments, it could be exploited for malaria prevention and serve as a lead compound for the synthesis of new, more effective, and selective agrochemical agents.
Full article
(This article belongs to the Section In Silico Approaches in Drug Discovery)
►▼
Show Figures

Figure 1
Open AccessReview
Drugs, Mother, and Child—An Integrative Review of Substance-Related Obstetric Challenges and Long-Term Offspring Effects
by
Atziri Alejandra Jiménez-Fernández, Joceline Alejandra Grajeda-Perez, Sofía de la Paz García-Alcázar, Mariana Gabriela Luis-Díaz, Francisco Javier Granada-Chavez, Emiliano Peña-Durán, Jesus Jonathan García-Galindo and Daniel Osmar Suárez-Rico
Drugs Drug Candidates 2025, 4(3), 40; https://doi.org/10.3390/ddc4030040 - 25 Aug 2025
Abstract
Substance use during pregnancy is an increasingly important yet under-recognized threat to maternal and child health. This narrative review synthesizes the current evidence available on the epidemiology, pathophysiology, clinical management, and policy landscape of prenatal exposure to alcohol, tobacco, opioids, benzodiazepines, cocaine, cannabis,
[...] Read more.
Substance use during pregnancy is an increasingly important yet under-recognized threat to maternal and child health. This narrative review synthesizes the current evidence available on the epidemiology, pathophysiology, clinical management, and policy landscape of prenatal exposure to alcohol, tobacco, opioids, benzodiazepines, cocaine, cannabis, methamphetamines, and other synthetic drugs. All major psychoactive substances readily cross the placenta and can remain detectable in breast milk, leading to a shared cascade of obstetric complications (hypertensive disorders, placental abruption, pre-term labor), fetal consequences (growth restriction, structural malformations), and neonatal morbidities such as neonatal abstinence syndrome and sudden infant death. Mechanistically, trans-placental diffusion, oxidative stress, inflammatory signaling, and placental vascular dysfunction converge to disrupt critical neuro- and cardiovascular developmental windows. Early identification hinges on the combined use of validated screening questionnaires (4 P’s Plus, CRAFFT, T-ACE, AUDIT-C, TWEAK) and matrix-specific biomarkers (PEth, EtG, FAEE, CDT), while effective treatment requires integrated obstetric, addiction, and mental health services. Medication for opioid use disorders, particularly buprenorphine, alone or with naloxone, confers superior neonatal outcomes compared to methadone and underscores the value of harm-reducing non-punitive care models. Public-health strategies, such as Mexico’s “first 1 000 days” framework, wrap-around clinics, and home-visiting programs, demonstrate the potential of multisectoral interventions, but are hampered by structural inequities and punitive legislation that deter care-seeking. Research gaps persist in polysubstance exposure, culturally tailored therapies, and long-term neurodevelopmental trajectories. Multigenerational, omics-enabled cohorts, and digital longitudinal-care platforms represent promising avenues for closing these gaps and informing truly preventive perinatal health policies.
Full article
(This article belongs to the Section Clinical Research)
►▼
Show Figures

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
DDC
Therapeutic Protease and Peptidase Inhibitors
Guest Editor: François MarceauDeadline: 30 April 2026
Special Issue in
DDC
Antioxidant Drug Candidates: Mechanistic and Computational Insights into Free Radical Scavenging and Redox Modulation
Guest Editor: Žiko B. MilanovićDeadline: 20 June 2026
Special Issue in
DDC
Microbes and Medicines
Guest Editors: Paul Hyman, Jennifer BennettDeadline: 31 July 2026
Topical Collections
Topical Collection in
DDC
Bioinorganic Chemistry in Drug Discovery
Collection Editors: Tanja Soldatović, Snežana Jovanović-Stević
Topical Collection in
DDC
Chirality in Drugs and Drug Candidates
Collection Editors: Carla Fernandes, Maria Emília De Sousa
Topical Collection in
DDC
Heterocycles in Drug Discovery
Collection Editors: Thierry Besson, Nicolas Primas, Jean Jacques Vanden Eynde
![Discovery and Characterization of 7,8-Dihydropyrido[4,3-<em>d</em>]pyrimidines as SARS-CoV-2 Entry Inhibitors](https://pub.mdpi-res.com/title_story/title_story_17642282701971.jpg?1765786175)