Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors
Abstract
1. Introduction
2. Results
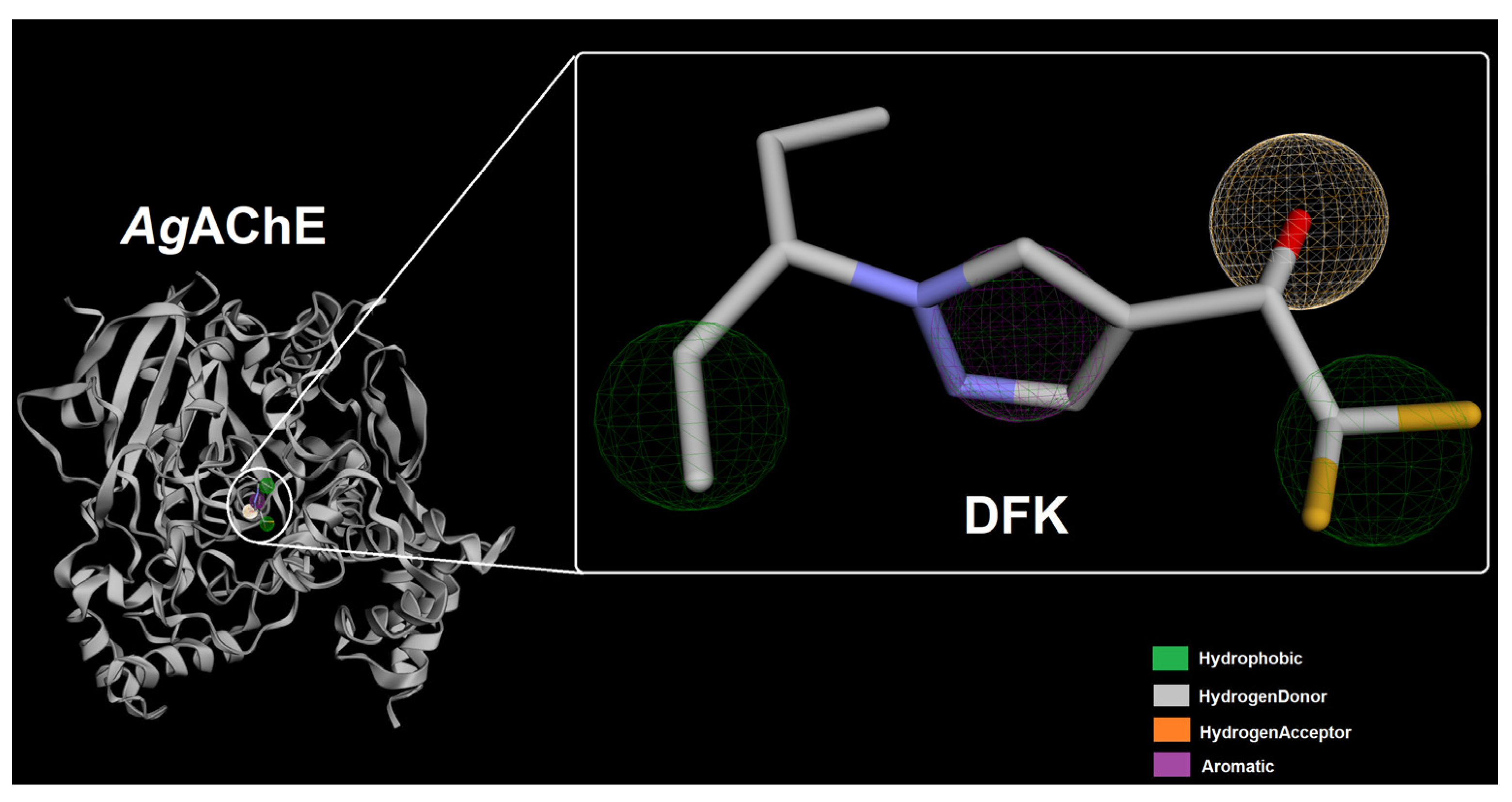
2.1. Ligand-Based Pharmacophore Modeling and Virtual Screening of PubChem Databse
2.2. ADMET Properties
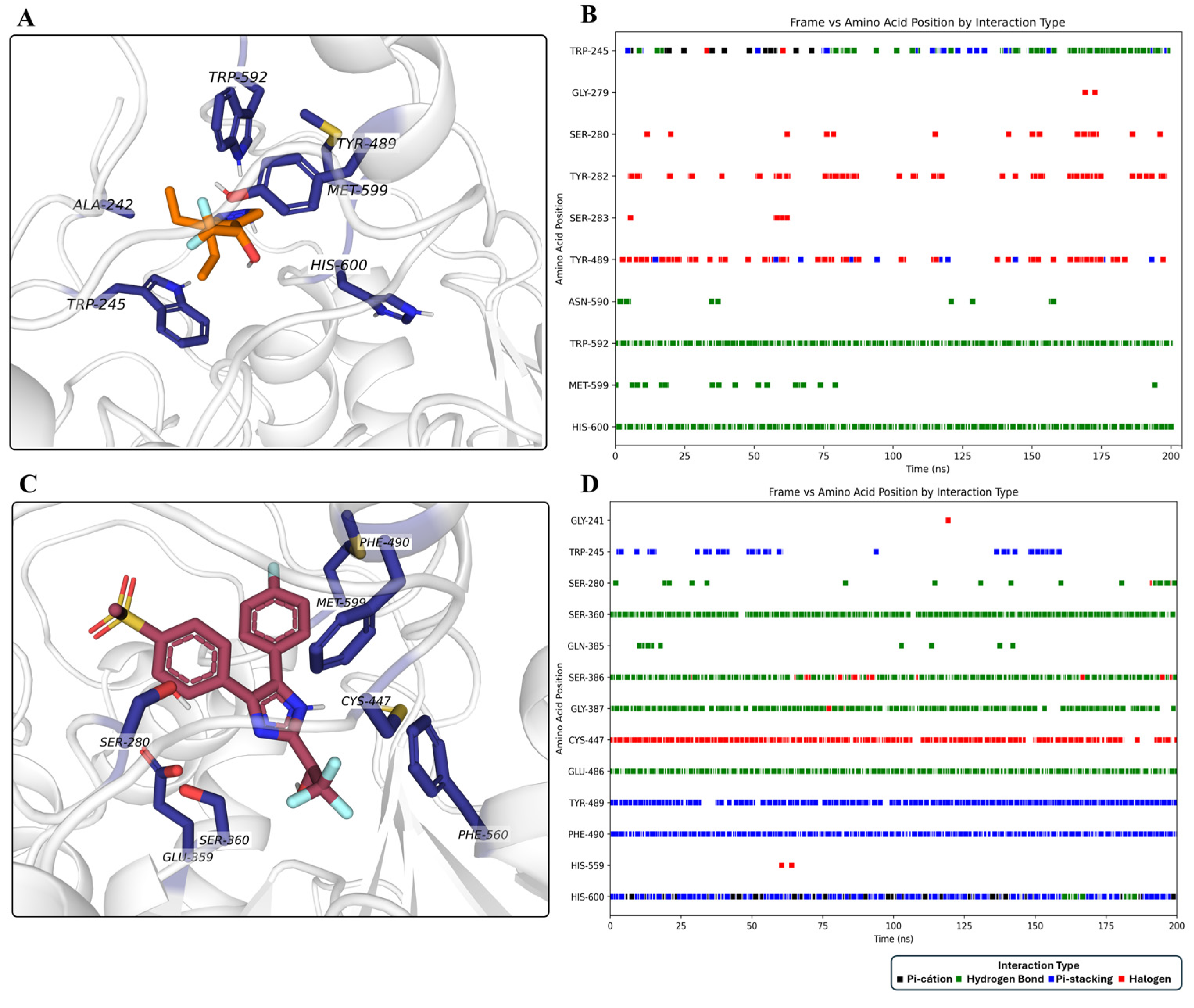
2.3. Molecular Dynamics Simulations of AgAChE-Ligands Complexes
3. Discussion
4. Materials and Methods
4.1. Ligand-Based Pharmacophore Modeling and Virtual Screening
4.2. Molecular Docking
4.3. In Silico ADMET Prediction
4.4. Molecular Dynamics Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AgAChE | Anopheles gambiae Acetylcholinesterase |
| DFK | Difluoromethyl ketone |
| ADMET | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| WHO | World Health Organization |
| OP | Organophosphate |
| ACh | Acetylcholine |
| AChR | ACh receptor |
| hAChE | human Acetylcholinesterase |
| RMSD | Root Mean Square Deviation |
| NVT | Constant set of particle numbers, volume, and temperature |
| NPT | Constant set of particle number, pressure, and temperature |
| PME | Particle Mesh Ewald |
| RMSF | Root Mean Square Fluctuation |
| MD | Molecular dynamics |
| PLIP | Protein-Ligand Interaction Profiler |
| MW | Molecular weight |
| RB | Rotatable bonds |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| HP | Hepatotoxicity |
| SS | Skin sensitization |
| MM/PBSA | Molecular Mechanics Poisson–Boltzmann Surface Area |
References
- Camerino, E.; Wong, D.M.; Tong, F.; Körber, F.; Gross, A.D.; Islam, R.; Viayna, E.; Mutunga, J.M.; Li, J.; Totrov, M.M.; et al. Difluoromethyl ketones: Potent inhibitors of wild type and carbamate-insensitive G119S mutant Anopheles gambiae acetylcholinesterase. Bioorg. Med. Chem. Lett. 2015, 25, 4405–4411. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Malaria Report 2022; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Caputo, B.; Nwakanma, D.; Jawara, M.; Adiamoh, M.; Dia, I.; Konate, L.; Petrarca, V.; Conway, D.J.; Della Torre, A. Anopheles gambiae complex along the Gambia river, with particular reference to the molecular forms of An. gambiae s.s. Malar. J. 2008, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Khani, S.; Ansari, M.; Shahlaei, M. Atomistic details on the mechanism of organophosphates resistance in insects: Insights from homology modeling, docking and molecular dynamic simulation. J. Mol. Liq. 2019, 276, 59–66. [Google Scholar] [CrossRef]
- van den Berg, H.; da Silva Bezerra, H.S.; Al-Eryani, S.; Chanda, E.; Nagpal, B.N.; Knox, T.B.; Velayudhan, R.; Yadav, R.S. Recent trends in global insecticide use for disease vector control and potential implications for resistance management. Sci. Rep. 2021, 11, 23867. [Google Scholar] [CrossRef]
- Ranson, H.; Lissenden, N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016, 32, 187–196. [Google Scholar] [CrossRef]
- Stalin, A.; Reegan, A.D.; Gandhi, M.R.; Saravanan, R.R.; Balakrishna, K.; Hesham, A.E.-L.; Ignacimuthu, S.; Zhang, Y. Mosquitocidal efficacy of embelin and its derivatives against Aedes aegypti L. and Culex quinquefasciatus Say. (Diptera: Culicidae) and computational analysis of acetylcholinesterase 1 (AChE1) inhibition. Comput. Biol. Med. 2022, 146, 105535. [Google Scholar] [CrossRef]
- Cheung, J.; Mahmood, A.; Kalathur, R.; Liu, L.; Carlier, P.R. Structure of the G119S Mutant Acetylcholinesterase of the Malaria Vector Anopheles gambiae Reveals Basis of Insecticide Resistance. Structure 2018, 26, 130–136.e132. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. Chemmedchem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Rosado-Solano, D.N.; Barón-Rodríguez, M.A.; Sanabria Florez, P.L.; Luna-Parada, L.K.; Puerto-Galvis, C.E.; Zorro-González, A.F.; Kouznetsov, V.V.; Vargas-Méndez, L.Y. Synthesis, Biological Evaluation and in Silico Computational Studies of 7-Chloro-4-(1 H-1,2,3-triazol-1-yl)quinoline Derivatives: Search for New Controlling Agents against Spodoptera frugiperda (Lepidoptera: Noctuidae) Larvae. J. Agric. Food Chem. 2019, 67, 9210–9219. [Google Scholar] [CrossRef] [PubMed]
- Hamid-Adiamoh, M.; Jabang, A.M.J.; Opondo, K.O.; Ndiath, M.O.; Assogba, B.S.; Amambua-Ngwa, A. Distribution of Anopheles gambiae thioester-containing protein 1 alleles along malaria transmission gradients in The Gambia. Malar. J. 2023, 22, 89. [Google Scholar] [CrossRef]
- Mawejje, H.D.; Weetman, D.; Epstein, A.; Lynd, A.; Opigo, J.; Maiteki-Sebuguzi, C.; Lines, J.; Kamya, M.R.; Rosenthal, P.J.; Donnelly, M.J. Characterizing pyrethroid resistance and mechanisms in Anopheles gambiae (ss) and Anopheles arabiensis from 11 districts in Uganda. Curr. Res. Parasitol. Vector-Borne Dis. 2023, 3, 100106. [Google Scholar] [CrossRef]
- Tchouakui, M.; Assatse, T.; Tazokong, H.R.; Oruni, A.; Menze, B.D.; Nguiffo-Nguete, D.; Mugenzi, L.M.J.; Kayondo, J.; Watsenga, F.; Mzilahowa, T. Detection of a reduced susceptibility to chlorfenapyr in the malaria vector Anopheles gambiae contrasts with full susceptibility in Anopheles funestus across Africa. Sci. Rep. 2023, 13, 2363. [Google Scholar] [CrossRef]
- Brodbeck, U.; Schweikert, K.; Gentinetta, R.; Rottenberg, M. Fluorinated aldehydes and ketones acting as quasi-substrate inhibitors of acetylcholinesterase. Biochim. Et Biophys. Acta (BBA)-Enzymol. 1979, 567, 357–369. [Google Scholar] [CrossRef]
- Wong, D.M.; Li, J.; Chen, Q.-H.; Han, Q.; Mutunga, J.M.; Wysinski, A.; Anderson, T.D.; Ding, H.; Carpenetti, T.L.; Verma, A. Select small core structure carbamates exhibit high contact toxicity to “carbamate-resistant” strain malaria mosquitoes, Anopheles gambiae (Akron). PLoS ONE 2012, 7, e46712. [Google Scholar] [CrossRef]
- Mahfuz, A.; Khan, M.A.; Biswas, S.; Afrose, S.; Mahmud, S.; Bahadur, N.M.; Ahmed, F. In search of novel inhibitors of anti-cancer drug target fibroblast growth factor receptors: Insights from virtual screening, molecular docking, and molecular dynamics. Arab. J. Chem. 2022, 15, 103882. [Google Scholar] [CrossRef]
- Daoui, O.; Mazoir, N.; Bakhouch, M.; Salah, M.; Benharref, A.; Gonzalez-Coloma, A.; Elkhattabi, S.; Yazidi, M.E.; Chtita, S. 3D-QSAR, ADME-Tox, and molecular docking of semisynthetic triterpene derivatives as antibacterial and insecticide agents. Struct. Chem. 2022, 33, 1063–1084. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.; Guy, R. Predicting Skin Permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Yelamanda Rao, K.; Jeelan Basha, S.; Monika, K.; Sreelakshmi, M.; Sivakumar, I.; Mallikarjuna, G.; Yadav, R.M.; Kumar, S.; Subramanyam, R.; Damu, A.G. Synthesis and anti-Alzheimer potential of novel α-amino phosphonate derivatives and probing their molecular interaction mechanism with acetylcholinesterase. Eur. J. Med. Chem. 2023, 253, 115288. [Google Scholar] [CrossRef]
- Renault, D.; Elfiky, A.; Mohamed, A. Predicting the insecticide-driven mutations in a crop pest insect: Evidence for multiple polymorphisms of acetylcholinesterase gene with potential relevance for resistance to chemicals. Environ. Sci. Pollut. Res. 2023, 30, 18937–18955. [Google Scholar] [CrossRef] [PubMed]
- Krátký, M.; Svrčková, K.; Vu, Q.A.; Štěpánková, Š.; Vinšová, J. Hydrazones of 4-(trifluoromethyl) benzohydrazide as new inhibitors of acetyl-and butyrylcholinesterase. Molecules 2021, 26, 989. [Google Scholar] [CrossRef]
- Székács, A.; Bordás, B.; Hammock, B.D. Transition state analog enzyme inhibitors: Structure-activity relationships of trifluoromethyl ketones. In Rational Approaches to Structure, Activity, and Ecotoxicology of Agrochemicals; CRC Press: Boca Raton, FL, USA, 2024; pp. 219–249. [Google Scholar]
- Sunseri, J.; Koes, D.R. Pharmit: Interactive exploration of chemical space. Nucleic Acids Res. 2016, 44, W442–W448. [Google Scholar] [CrossRef]
- Tice, C.M. Selecting the right compounds for screening: Does Lipinski’s rule of 5 for pharmaceuticals apply to agrochemicals? Pest Manag. Sci. 2001, 57, 3–16. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; De Groot, B.L.; Grubmüller, H.; MacKerell, A.D. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2016, 14, 71–73. [Google Scholar] [CrossRef]
- Bahrami, H.; Salehabadi, H.; Nazari, Z.; Amanlou, M. Combined Virtual Screening, DFT Calculations and Molecular Dynamics Simulations to Discovery of Potent MMP-9 Inhibitors. Lett. Drug Des. Discov. 2018, 16, 892–903. [Google Scholar] [CrossRef]
- Bernetti, M.; Bussi, G. Pressure control using stochastic cell rescaling. J. Chem. Phys. 2020, 153, 114107. [Google Scholar] [CrossRef] [PubMed]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Kumar, R.; Open Source Drug Discovery, C.; Lynn, A. g_mmpbsa A GROMACS tool for high-throughput MM-PBSA calculations. J. Chem. Inf. Model. 2014, 54, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]


| ID | MW 1 | MLogP | 2 RB | 3 HBA | 4 HBD | AMES | 5 HP | 6 SS |
|---|---|---|---|---|---|---|---|---|
| DFK | 218.24 | 1.44 | 5 | 4 | 1 | No | No | Yes |
| PC6 | 414.37 | 2.32 | 5 | 8 | 2 | No | Yes | No |
| Complex | ΔGMM/PBSA | SD 1 |
|---|---|---|
| AgAChE1-DFK | −26.38 | 4.02 |
| AgAChE1-PC6 | −29.89 | 7.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, F.F.; Vilachã, J.F.; Campos, O.S.; de Paula, H. Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors. Drugs Drug Candidates 2025, 4, 41. https://doi.org/10.3390/ddc4030041
Souza FF, Vilachã JF, Campos OS, de Paula H. Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors. Drugs and Drug Candidates. 2025; 4(3):41. https://doi.org/10.3390/ddc4030041
Chicago/Turabian StyleSouza, Fernanda F., Juliana F. Vilachã, Othon S. Campos, and Heberth de Paula. 2025. "Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors" Drugs and Drug Candidates 4, no. 3: 41. https://doi.org/10.3390/ddc4030041
APA StyleSouza, F. F., Vilachã, J. F., Campos, O. S., & de Paula, H. (2025). Prediction of Novel Insecticides for Malaria Prevention: Virtual Screening and Molecular Dynamics of AgAChE Inhibitors. Drugs and Drug Candidates, 4(3), 41. https://doi.org/10.3390/ddc4030041







