Prevalence of Liver Steatosis and Fibrosis Assessed by Transient Elastography in a High Cardiovascular-Risk Outpatient Cohort Including T1DM and T2DM Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Laboratory Examination
2.4. Vibration-Controlled Transient Elastography
2.5. Carotid Intima Media Thickness
2.6. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Characteristics by MASLD and Fibrosis Status
3.3. Predictors of MASLD and Fibrosis
3.4. MASLD and Fibrosis in CVD Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacke, F.; Horn, P.; Wong, V.W.-S.; Ratziu, V.; Bugianesi, E.; Francque, S.; Zelber-Sagi, S.; Valenti, L.; Roden, M.; Schick, F.; et al. EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Wong, V.W.; Ekstedt, M.; Wong, G.L.; Hagström, H. Changing epidemiology, global trends and implications for outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Osorio-Conles, Ó.; Vega-Beyhart, A.; Ibarzabal, A.; Balibrea, J.M.; Graupera, I.; Rimola, J.; Vidal, J.; de Hollanda, A. A Distinctive NAFLD Signature in Adipose Tissue from Women with Severe Obesity. Int. J. Mol. Sci. 2021, 22, 10541. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Lopez, C.; Lomonaco, R.; Orsak, B.; Finch, J.; Chang, Z.; Kochunov, V.G.; Hardies, J.; Cusi, K. Prevalence of prediabetes and diabetes and metabolic profile of patients with nonalcoholic fatty liver disease (NAFLD). Diabetes Care 2012, 35, 873–878. [Google Scholar] [CrossRef]
- Younossi, Z.; Golabi, P.; Paik, J.; Henry, A.; Van Dongen, C.; Henry, L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): A systematic review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Wong, R.J.; Aguilar, M.; Cheung, R.; Perumpail, R.B.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015, 148, 547–555. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S32–S42. [Google Scholar] [CrossRef]
- Driessen, S.; de Jong, V.D.; van Son, K.C.; Klompenhouwer, T.; Colardelle, Y.; Alings, M.; Moreno, C.; Anker, S.D.; Castro Cabezas, M.; Holleboom, A.G.; et al. A global survey of health care workers’ awareness of non-alcoholic fatty liver disease: The AwareNASH survey. United Eur. Gastroenterol. J. 2023, 11, 654–662. [Google Scholar] [CrossRef]
- Targher, G.; Byrne, C.D.; Tilg, H. MASLD: A systemic metabolic disorder with cardiovascular and malignant complications. Gut 2024, 73, 691–702. [Google Scholar] [CrossRef]
- Driessen, S.; Francque, S.M.; Anker, S.D.; Cabezas, M.C.; Grobbee, D.E.; Tushuizen, M.E.; Holleboom, A.G. Metabolic dysfunction associated steatotic liver disease and the heart. Hepatology 2023, 82, 487–503. [Google Scholar] [CrossRef]
- Zhou, X.D.; Targher, G.; Byrne, C.D.; Somers, V.; Kim, S.U.; Chahal, C.A.A.; Wong, V.W.; Cai, J.; Shapiro, M.D.; Eslam, M.; et al. An international multidisciplinary consensus statement on MAFLD and the risk of CVD. Hepatol. Int. 2023, 17, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Duell, P.B.; Welty, F.K.; Miller, M.; Chait, A.; Hammond, G.; Ahmad, Z.; Cohen, D.E.; Horton, J.D.; Pressman, G.S.; Toth, P.P. Nonalcoholic Fatty Liver Disease and Cardiovascular Risk: A Scientific Statement From the American Heart Association. Arter. Thromb. Vasc. Biol. 2022, 42, e168–e185. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, G.; Huang, H. Correlation between metabolic dysfunction-associated steatotic liver disease and subclinical coronary atherosclerosis in eastern China. Diabetol. Metab. Syndr. 2025, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Aller, R.; Fernández-Rodríguez, C.; Iacono, O.L.; Bañares, R.; Abad, J.; Carrión, J.A.; García-Monzón, C.; Caballería, J.; Berenguer, M.; Rodríguez-Perálvarez, M.; et al. Consensus document. Management of non-alcoholic fatty liver disease (NAFLD). Clinical practice guideline. Gastroenterol. Hepatol. 2018, 41, 328–349. [Google Scholar] [CrossRef]
- Lee, J.; Vali, Y.; Boursier, J.; Spijker, R.; Anstee, Q.M.; Bossuyt, P.M.; Zafarmand, M.H. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: A systematic review. Liver Int. 2021, 41, 261–270. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; de Avila, L.; Paik, J.M.; Srishord, M.; Fukui, N.; Qiu, Y.; Burns, L.; Afendy, A.; Nader, F. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol. 2019, 71, 793–801. [Google Scholar] [CrossRef]
- Brouwers, M.; Simons, N.; Kooi, M.E.; de Ritter, R.; van Dongen, M.; Eussen, S.; Bekers, O.; Kooman, J.; van Greevenbroek, M.M.J.; van der Kallen, C.J.H.; et al. Intrahepatic lipid content is independently associated with soluble E-selectin levels: The Maastricht study. Dig. Liver Dis. 2022, 54, 1038–1043. [Google Scholar] [CrossRef]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Boursier, J.; Zarski, J.P.; de Ledinghen, V.; Rousselet, M.C.; Sturm, N.; Lebail, B.; Fouchard-Hubert, I.; Gallois, Y.; Oberti, F.; Bertrais, S.; et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology 2013, 57, 1182–1191. [Google Scholar] [CrossRef]
- van Breukelen-van der Stoep, D.F.; van Zeben, D.; Klop, B.; van de Geijn, G.J.; Janssen, H.J.; Hazes, M.J.; Birnie, E.; van der Meulen, N.; De Vries, M.A.; Cabezas, M.C. Association of Cardiovascular Risk Factors with Carotid Intima Media Thickness in Patients with Rheumatoid Arthritis with Low Disease Activity Compared to Controls: A Cross-Sectional Study. PLoS ONE 2015, 10, e0140844. [Google Scholar] [CrossRef] [PubMed]
- Ciardullo, S.; Perseghin, G. Prevalence of elevated liver stiffness in patients with type 1 and type 2 diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2022, 190, 109981. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, R.; Godinez Leiva, E.; Bril, F.; Shrestha, S.; Mansour, L.; Budd, J.; Portillo Romero, J.; Schmidt, S.; Chang, K.L.; Samraj, G.; et al. Advanced Liver Fibrosis Is Common in Patients With Type 2 Diabetes Followed in the Outpatient Setting: The Need for Systematic Screening. Diabetes Care 2021, 44, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis-Noort, E.C.; Berk, K.A.; Neggers, S.; Lely, A.J.V. The Fascinating Interplay between Growth Hormone, Insulin-Like Growth Factor-1, and Insulin. Endocrinol. Metab. 2024, 39, 83–89. [Google Scholar] [CrossRef]
- de Vries, M.; Westerink, J.; El-Morabit, F.; Kaasjager, H.; de Valk, H.W. Prevalence of non-alcoholic fatty liver disease (NAFLD) and its association with surrogate markers of insulin resistance in patients with type 1 diabetes. Diabetes Res. Clin. Pract. 2022, 186, 109827. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients With Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024, 22, 1999–2010. [Google Scholar] [CrossRef]
- Roulot, D.; Costes, J.L.; Buyck, J.F.; Warzocha, U.; Gambier, N.; Czernichow, S.; Le Clesiau, H.; Beaugrand, M. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011, 60, 977–984. [Google Scholar] [CrossRef]
- Caballería, L.; Pera, G.; Arteaga, I.; Rodríguez, L.; Alumà, A.; Morillas, R.M.; de la Ossa, N.; Díaz, A.; Expósito, C.; Miranda, D.; et al. High Prevalence of Liver Fibrosis Among European Adults With Unknown Liver Disease: A Population-Based Study. Clin. Gastroenterol. Hepatol. 2018, 16, 1138–1145.e5. [Google Scholar] [CrossRef]
- Koehler, E.M.; Plompen, E.P.; Schouten, J.N.; Hansen, B.E.; Darwish Murad, S.; Taimr, P.; Leebeek, F.W.; Hofman, A.; Stricker, B.H.; Castera, L.; et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology 2016, 63, 138–147. [Google Scholar] [CrossRef]
- Kim, D.; Cholankeril, G.; Loomba, R.; Ahmed, A. Prevalence of Fatty Liver Disease and Fibrosis Detected by Transient Elastography in Adults in the United States, 2017–2018. Clin. Gastroenterol. Hepatol. 2021, 19, 1499–1501.e1492. [Google Scholar] [CrossRef]
- Fabrellas, N.; Alemany, M.; Urquizu, M.; Bartres, C.; Pera, G.; Juvé, E.; Rodríguez, L.; Torán, P.; Caballería, L. Using transient elastography to detect chronic liver diseases in a primary care nurse consultancy. Nurs. Res. 2013, 62, 450–454. [Google Scholar] [CrossRef]
- Trifan, A.; Stratina, E.; Nastasa, R.; Rotaru, A.; Stafie, R.; Zenovia, S.; Huiban, L.; Sfarti, C.; Cojocariu, C.; Cuciureanu, T.; et al. Simultaneously Screening for Liver Steatosis and Fibrosis in Romanian Type 2 Diabetes Mellitus Patients Using Vibration-Controlled Transient Elastography with Controlled Attenuation Parameter. Diagnostics 2022, 12, 1753. [Google Scholar] [CrossRef]
- Mikolasevic, I.; Rahelic, D.; Turk-Wensween, T.; Ruzic, A.; Domislovic, V.; Hauser, G.; Matic, T.; Radic-Kristo, D.; Krznaric, Z.; Radic, M.; et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicenter study. Diabetes Res. Clin. Pract. 2021, 177, 108884. [Google Scholar] [CrossRef]
- van den Berg, E.H.; Amini, M.; Schreuder, T.C.; Dullaart, R.P.; Faber, K.N.; Alizadeh, B.Z.; Blokzijl, H. Prevalence and determinants of non-alcoholic fatty liver disease in lifelines: A large Dutch population cohort. PLoS ONE 2017, 12, e0171502. [Google Scholar] [CrossRef] [PubMed]
- Koehler, E.M.; Schouten, J.N.; Hansen, B.E.; van Rooij, F.J.; Hofman, A.; Stricker, B.H.; Janssen, H.L. Prevalence and risk factors of non-alcoholic fatty liver disease in the elderly: Results from the Rotterdam study. J. Hepatol. 2012, 57, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Harrison, S.A.; Hajji, Y.; Magnanensi, J.; Petit, S.; Majd, Z.; Delecroix, E.; Rosenquist, C.; Hum, D.; Staels, B.; et al. NIS2+(TM) as a screening tool to optimize patient selection in metabolic dysfunction-associated steatohepatitis clinical trials. J. Hepatol. 2024, 80, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Stefanakis, K.; Mingrone, G.; George, J.; Mantzoros, C.S. Accurate non-invasive detection of MASH with fibrosis F2-F3 using a lightweight machine learning model with minimal clinical and metabolomic variables. Metabolism 2025, 163, 156082. [Google Scholar] [CrossRef]
- McPherson, S.; Dyson, J.K.; Jopson, L.; Masson, S.; Patel, P.; Anstee, Q.M. Letter: Beyond advanced fibrosis-The critical need for assessing NITs performance in identifying F2-F3 fibrosis. Authors’ reply. Aliment. Pharmacol. Ther. 2024, 60, 976–977. [Google Scholar] [CrossRef]
- Vereniging, N.V.v.G.-e.N.I.V.N.L. Richtlijn MASLD/MASH; 2024. Available online: https://richtlijnendatabase.nl/richtlijn/richtlijn_masld_mash/startpagina_masld_mash.html (accessed on 1 September 2025).
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef]
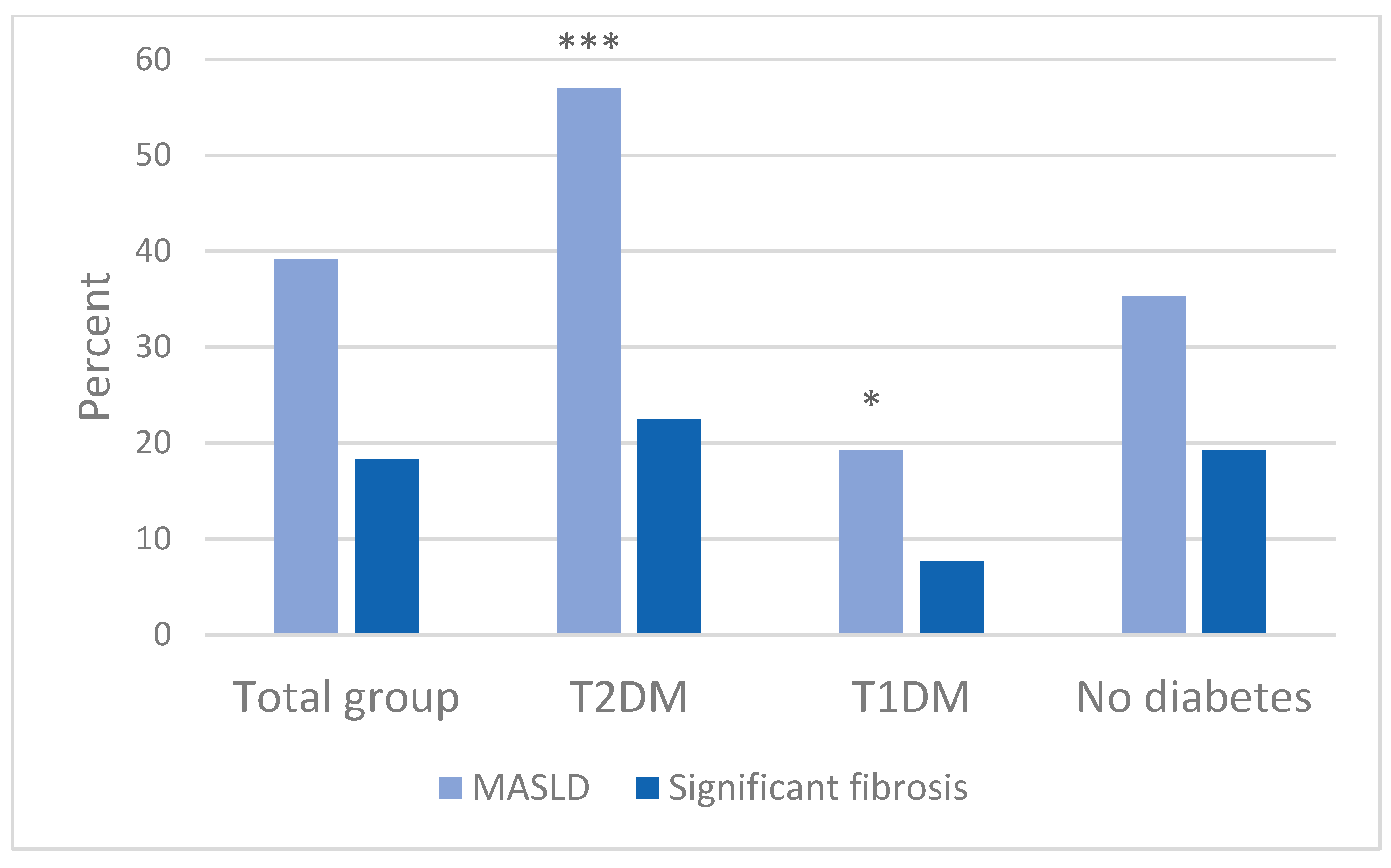
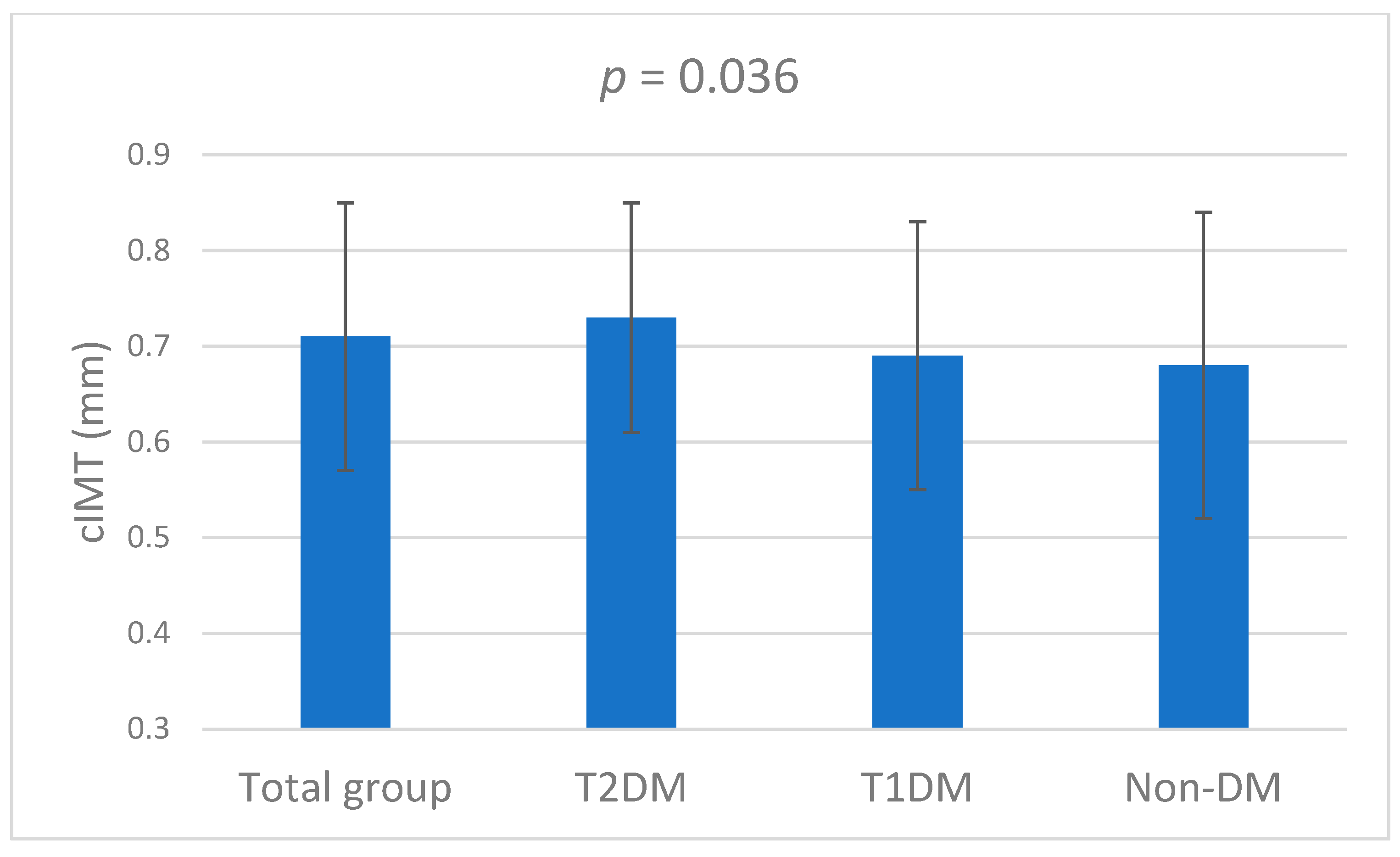
| Characteristics | Total (N = 475) | T2DM (N = 142) | T1DM (N = 78) | Non-DM (N = 255) | p-Value |
|---|---|---|---|---|---|
| Age (years) | 53 ± 14 | 59 ± 12 *** | 52 ± 12 | 50 ± 14 | <0.001 |
| M/F | 252/223 | 86/56 | 39/39 | 127/128 | 0.101 |
| BMI (kg/m2) | 29.8 ± 6.7 | 30.4 ± 6.4 | 27.1 ± 5.0 *** | 30.6 ± 7.3 | <0.001 |
| cIMT (mm) | 0.71 ± 0.14 | 0.73 ± 0.12 | 0.69 ± 0.14 | 0.68 ± 0.16 | 0.036 |
| Total cholesterol (mmol/L) | 4.6 (3.8–5.5) | 4.1 (3.7–5.3) | 4.3 (3.7–5.0) | 5.1 (4.5–5.7) | 0.573 |
| HDL-C (mmol/L) | 1.3 ± 0.39 | 1.2 ± 0.32 | 1.5 ± 0.42 *** | 1.3 ± 0.37 | <0.001 |
| LDL-C (mmol/L) | 2.5 ± 0.92 | 2.3 ± 0.93 *** | 2.3 ± 0.75 *** | 3.0 ± 0.80 | <0.001 |
| Triglycerides (mmol/L) | 1.6 (1.0–2.4) | 2.1 (1.3–3.1) * | 1.1 (0.8–1.7) ** | 1.5 (1.1–2.3) | <0.001 |
| Glucose (mmol/L) | 6.2 (5.4–7.9) | 10.0 (7.4–14) *** | 8.5 (5.6–11) ** | 5.8 (5.1–6.4) | <0.001 |
| HbA1c (mmol/mol) | 50.9 ± 15 | 59.0 ± 13 *** | 57.3 ± 12 *** | 36.4 ± 5.0 | <0.001 |
| Apo B (g/L) | 1.05 ± 0.27 | 1.05 ± 0.38 | 0.82 ± 0.27 | 1.07 ± 0.20 | 0.330 |
| Apo AI (g/L) | 1.54 ± 0.26 | 1.49 ± 0.24 | 1.39 ± NA | 1.59 ± 0.27 | 0.490 |
| CRP (mg/L) | 4.0 (2.0–7.0) | 3.0 (2.0–6.0) | 8.0 (4.0–25) | 4.0 (2.0–7.0) | 0.176 |
| Hemoglobin (mmol/L) | 8.7 ± 1.1 | 8.7 ± 1.1 | 8.2 ± 1.0 * | 8.8 ± 1.1 | 0.057 |
| Thrombocytes (109/L) | 258 ± 87 | 253 ± 93 | 305 ± 114 | 255 ± 82 | 0.099 |
| GGT (U/L) | 58 (31–106) | 71 (38–177) | 23 (14–140) | 58 (29–97) | 0.082 |
| ALT (U/L) | 38 (27–52) | 38 (28–60) | 22 (18–39) | 39 (28–51) | 0.158 |
| AST (U/L) | 39 (24–67) | 38 (25–72) | 29 (14–36) | 41 (23–67) | 0.131 |
| Creatinine (μmol/L) | 74 (65–87) | 77 (67–96) ** | 76 (66–92) | 72 (65–81) | 0.006 |
| Albumin (g/L) | 44 (41–46) | 44 (42–47) | 46 (40–46) | 44 (41–46) | 0.850 |
| eGFR (mL/min/1.73m2) | 80 (68–88) | 77 (59–86) *** | 78 (70–87) | 82 (75–91) | <0.001 |
| FIB-4 | 1.86 ± 1.50 | 1.94 ± 1.46 | 1.73 ± 1.12 | 1.85 ± 1.62 | 0.789 |
| CAP (dB/m) | 263 ± 59 | 282 ± 59 *** | 238 ± 52 * | 259 ± 58 | <0.001 |
| LSM (kPa) | 6.1 ± 3.0 | 6.2 ± 2.8 | 5.4 ± 1.8 * | 6.3 ± 3.3 | 0.039 |
| Characteristics | Total (N = 475) | T2DM (N = 142) | T1DM (N = 78) | Non-DM (N = 255) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MASLD (N = 186) | No MASLD (N = 289) | p-Value | MASLD (N = 81) | No MASLD (N = 61) | p-Value | MASLD (N = 15) | No MASLD (N = 63) | p-Value | MASLD (N = 90) | No MASLD (N = 165) | p-Value | |
| Age (years) | 52 ± 14 | 54 ± 13 | 0.068 | 58 ± 12 | 61 ± 11 | 0.092 | 56 ± 11 | 51 ± 12 | 0.197 | 45 ± 14 | 52 ± 13 | <0.001 |
| M/F | 100/86 | 152/137 | 0.803 | 49/32 | 37/24 | 0.984 | 4/11 | 35/28 | 0.044 | 47/43 | 80/50 | 0.568 |
| BMI (kg/m2) | 33.1 ± 6.4 | 27.6 ± 6.0 | <0.001 | 32.3 ± 6.2 | 27.8 ± 5.9 | <0.001 | 31.9 ± 4.2 | 25.9 ± 4.4 | <0.001 | 34.3 ± 6.8 | 28.3 ± 6.7 | <0.001 |
| cIMT (mm) | 0.72 ± 0.12 | 0.70 ± 0.14 | 0.409 | 0.73 ± 0.12 | 0.74 ± 0.13 | 0.643 | 0.71 ± 0.12 | 0.68 ± 0.14 | 0.459 | 0.69 ± 0.15 | 0.68 ± 0.17 | 0.975 |
| Total cholesterol (mmol/L) | 5.5 ± 7.0 | 4.6 ± 1.2 | 0.129 | 6.0 ± 9.7 | 4.1 ± 1.2 | 0.207 | 4.5 ± 1.3 | 4.4 ± 0.91 | 0.696 | 5.1 ± 0.95 | 5.1 ± 1.2 | 0.958 |
| HDL-C (mmol/L) | 1.2 ± 0.36 | 1.3 ± 0.42 | 0.231 | 1.2 ± 0.31 | 1.1 ± 0.33 | 0.942 | 1.6 ± 0.39 | 1.5 ± 0.44 | 0.579 | 1.3 ± 0.35 | 1.3 ± 0.39 | 0.960 |
| LDL-C (mmol/L) | 2.6 ± 0.90 | 2.5 ± 0.93 | 0.155 | 2.4 ± 1.0 | 2.0 ± 0.87 | 0.042 | 2.4 ± 0.87 | 2.3 ± 0.72 | 0.634 | 3.0 ± 0.70 | 3.1 ± 0.87 | 0.850 |
| Triglycerides (mmol/L) | 2.2 ± 1.3 | 1.8 ± 1.3 | 0.035 | 2.5 ± 1.5 | 2.2 ± 1.6 | 0.324 | 1.1 ± 0.52 | 1.3 ± 0.74 | 0.455 | 2.0 ± 1.1 | 1.9 ± 1.4 | 0.611 |
| Glucose (mmol/L) | 7.1 ± 2.7 | 7.6 ± 3.6 | 0.441 | 9.8 ± 3.4 | 11.5 ± 4.6 | 0.222 | 8.1 ± 2.7 | 8.8 ± 3.7 | 0.806 | 5.9 ± 0.93 | 5.9 ± 1.1 | 0.911 |
| HbA1c (mmol/mol) | 51 ± 14 | 51 ± 15 | 0.900 | 59 ± 13 | 60 ± 12 | 0.680 | 56 ± 7.8 | 58 ± 13 | 0.612 | 37 ± 4.7 | 36 ± 5.3 | 0.365 |
| ApoB (g/L) | 1.1 ± 0.30 | 1.0 ± 0.23 | 0.209 | 1.1 ± 0.39 | 0.90 ± 0.31 | 0.207 | 0.87 ± 0.36 | 0.74 ± NA | NA | 1.09 ± 0.21 | 1.05 ± 0.20 | 0.543 |
| Apo AI (g/L) | 1.6 ± 0.23 | 1.5 ± 0.27 | 0.726 | 1.6 ± 0.28 | 1.4± 0.17 | 0.169 | NA | 1.4 ± NA | NA | 1.5 ± 0.12 | 1.6 ± 0.30 | 0.680 |
| CRP (mg/L) | 7.4 ± 9.3 | 8.0 ± 16 | 0.781 | 4.2 ± 4.1 | 4.7 ± 3.5 | 0.686 | 25.0 ± NA | 10.2 ± 10.1 | NA | 8.6 ± 11 | 8.7 ± 19 | 0.970 |
| Hemoglobin (mmol/L) | 8.8 ± 1.2 | 8.7 ± 1.1 | 0.480 | 8.7 ± 1.1 | 8.8 ± 1.0 | 0.537 | 8.5 ± 0.57 | 8.1 ± 1.1 | 0.633 | 8.9 ± 1.2 | 8.7 ± 1.1 | 0.436 |
| Thrombocytes (109/L) | 266 ± 83 | 252 ± 90 | 0.230 | 264 ± 79 | 238 ± 110 | 0.283 | 296 ± 216 | 306 ± 106 | 0.910 | 266 ± 83 | 248 ± 81 | 0.194 |
| GGT (U/L) | 82.4 ± 76 | 106 ± 141 | 0.190 | 102 ± 98 | 173 ± 188 | 0.159 | NA | 66.0 ± 73.9 | NA | 73.9 ± 62.5 | 94.7 ± 130 | 0.202 |
| ALT (U/L) | 55 ± 37 | 40 ± 26 | 0.003 | 56 ± 37 | 45 ± 35 | 0.240 | NA | 31 ± 21 | NA | 53 ± 38 | 40 ± 24 | 0.010 |
| AST (U/L) | 65 ± 51 | 42 ± 30 | <0.001 | 68 ± 63 | 44 ± 40 | 0.077 | 26 ± 13 | 38 ± 34 | 0.624 | 65 ± 43 | 41 ± 26 | <0.001 |
| Creatinine (μmol/L) | 79.9 ± 21 | 81.3 ± 42 | 0.693 | 85.0 ± 24 | 91.7 ± 77 | 0.486 | 81.5 ± 20 | 81.5 ± 23 | 0.995 | 73.9 ± 15 | 76.0 ± 16 | 0.371 |
| Albumin (g/L) | 44.1 ± 3.3 | 46.7 ± 41 | 0.557 | 44.7 ± 3.2 | 42.3 ± 6.3 | 0.126 | 41.0 ± 1.4 | 43.8 ± 3.7 | 0.343 | 44.0 ± 3.3 | 48.1 ± 47 | 0.505 |
| eGFR (mL/min/1.73m2) | 76.1 ± 15.3 | 75.6 ± 15.3 | 0.802 | 70.7 ± 16.7 | 71.8 ± 18.0 | 0.758 | 74.2 ± 13.9 | 74.7 ± 15.6 | 0.917 | 83.3 ± 10.5 | 78.4 ± 12.6 | 0.064 |
| FIB-4 | 1.68 ± 1.3 | 1.98 ± 1.6 | 0.139 | 1.82 ± 1.2 | 2.10 ± 1.7 | 0.444 | 1.40 ± 0.56 | 1.79 ± 1.2 | 0.439 | 1.60 ± 1.5 | 2.01 ± 1.7 | 0.182 |
| CAP (dB/m) | 323 ± 30 | 223 ± 36 | <0.001 | 325 ± 32 | 227 ± 37 | <0.001 | 318 ± 31 | 219 ± 35 | <0.001 | 323 ± 29 | 224 ± 36 | <0.001 |
| LSM (kPa) | 6.7 ± 3.0 | 5.8 ± 2.9 | <0.001 | 7.0 ± 3.0 | 5.1 ± 2.2 | <0.001 | 5.8 ± 2.4 | 5.3 ± 1.6 | 0.436 | 6.6 ± 3.1 | 6.2 ± 3.4 | 0.329 |
| Characteristics | Total (N = 475) | T2DM (N = 142) | T1DM (N = 78) | Non-DM (N = 255) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibrosis (N = 87) | No Fibrosis (N = 388) | p-Value | Fibrosis (N = 32) | No Fibrosis (N = 110) | p-Value | Fibrosis (N = 6) | No Fibrosis (N = 72) | p-Value | Fibrosis (N = 49) | No Fibrosis (N = 206) | p-Value | |
| Age (years) | 54 ± 13 | 53 ± 14 | 0.453 | 56 ± 13 | 60 ± 11 | 0.124 | 56 ± 6.1 | 52 ± 12 | 0.144 | 52 ± 14 | 49 ± 14 | 0.187 |
| M/F | 54/33 | 198/190 | 0.062 | 21/11 | 65/45 | 0.506 | 3/3 | 36/36 | 1.000 | 30/19 | 97/109 | 0.075 |
| BMI (kg/m2) | 31.9 ± 7.6 | 29.5 ± 6.5 | 0.023 | 34.2 ± 7.5 | 29.4 ± 5.8 | 0.004 | 31.7 ± 6.0 | 26.7 ± 4.7 | 0.017 | 29.7 ± 7.5 | 30.7 ± 7.3 | 0.523 |
| cIMT (mm) | 0.76 ± 0.11 | 0.70 ± 0.14 | 0.120 | 0.76 ± 0.10 | 0.73 ± 0.13 | 0.565 | 0.77 ± 0.16 | 0.68 ± 0.13 | 0.178 | 0.76 ± NA | 0.68 ± 0.16 | NA |
| Total cholesterol (mmol/L) | 5.0 ± 1.6 | 5.0 ± 5.0 | 0.989 | 5.0 ± 8.1 | 5.2 ± 1.7 | 0.907 | 4.2 ± 0.81 | 4.4 ± 1.0 | 0.728 | 5.2 ± 1.6 | 5.1 ± 1.0 | 0.891 |
| HDL-C (mmol/L) | 1.2 ± 0.48 | 1.3 ± 0.38 | 0.134 | 1.1 ± 0.37 | 1.2 ± 0.31 | 0.748 | 1.5 ± 0.18 | 1.5 ± 0.44 | 0.989 | 1.1 ± 0.66 | 1.3 ± 0.31 | 0.506 |
| LDL-C (mmol/L) | 2.7 ± 1.2 | 2.5 ± 0.87 | 0.575 | 2.6 ± 1.3 | 2.2 ± 0.84 | 0.257 | 2.0 ± 0.55 | 2.3 ± 0.76 | 0.382 | 3.1 ± 1.2 | 3.0 ± 0.74 | 0.762 |
| Triglycerides (mmol/L) | 2.3 ± 1.2 | 1.9 ± 1.4 | 0.168 | 2.6 ± 1.2 | 2.3 ± 1.6 | 0.425 | 1.6 ± 0.78 | 1.3 ± 0.70 | 0.360 | 2.0 ± 1.1 | 1.9 ± 1.3 | 0.905 |
| Glucose (mmol/L) | 8.0 ± 3.0 | 7.3 ± 3.3 | 0.317 | 10.3 ± 3.4 | 10.9 ± 4.3 | 0.691 | 9.3 ± 1.1 | 8.5 ± 3.8 | 0.800 | 6.0 ± 0.76 | 5.9 ± 1.1 | 0.787 |
| HbA1c (mmol/mol) | 52 ± 15 | 51 ± 15 | 0.656 | 60 ± 16 | 59 ± 12 | 0.690 | 57 ± 6.5 | 57 ± 13 | 0.997 | 38 ± 4.2 | 36 ± 5.1 | 0.079 |
| Apo B (g/L) | 1.1 ± 0.32 | 1.0 ± 0.27 | 0.454 | 1.2 ± 0.38 | 1.0 ± 0.38 | 0.399 | 0.61 ± NA | 0.93 ± 0.27 | NA | 1.2 ± 0.17 | 1.1 ± 0.20 | 0.351 |
| Apo AI (g/L) | 1.4 ± NA | 1.5 ± 0.26 | NA | 1.4 ± NA | 1.5 ± 0.25 | NA | NA | 1.4 ± NA | NA | NA | 1.6 ± 0.27 | NA |
| CRP (mg/L) | 6.8 ± 8.6 | 8.0 ± 15 | 0.639 | 3.9 ± 2.2 | 4.6 ± 4.3 | 0.623 | 8.0 ± NA | 13 ± 12 | NA | 7.9 ± 10 | 8.9 ± 17 | 0.786 |
| Hemoglobin (mmol/L) | 8.8 ± 1.1 | 8.7 ± 1.1 | 0.866 | 8.7 ± 0.98 | 8.7 ± 1.1 | 0.987 | 8.5 ± 0.57 | 8.1 ± 1.1 | 0.633 | 8.8 ± 1.2 | 8.8 ± 1.1 | 0.925 |
| Thrombocytes (109/L) | 240 ± 87 | 263 ± 87 | 0.085 | 240 ± 90 | 259 ± 95 | 0.469 | 193 ± 70.7 | 321 ± 111 | 0.143 | 243 ± 88 | 258 ± 80 | 0.308 |
| GGT (U/L) | 96.8 ± 80.2 | 96.4 ± 131 | 0.983 | 117 ± 89 | 143 ± 175 | 0.537 | 98 ± NA | 58 ± 83 | NA | 86.3 ± 76.0 | 87.3 ± 118 | 0.967 |
| ALT (U/L) | 57 ± 45 | 42 ± 24 | 0.021 | 67 ± 47 | 43 ± 26 | 0.052 | 82 ± NA | 25 ± 9.6 | NA | 51 ± 45 | 42 ± 23 | 0.277 |
| AST (U/L) | 65 ± 56 | 48 ± 35 | 0.025 | 91 ± 80 | 45 ± 37 | 0.018 | 66 ± 43 | 31 ± 28 | 0.156 | 51 ± 36 | 50 ± 35 | 0.793 |
| Creatinine (μmol/L) | 76.6 ± 17 | 81.6 ± 38 | 0.283 | 81.5 ± 19 | 89.7 ± 60 | 0.490 | 81.0 ± 27 | 81.5 ± 22 | 0.958 | 72.2 ± 11 | 76.0 ± 16 | 0.087 |
| Albumin (g/L) | 52.4 ± 61 | 43.4 ± 4.1 | 0.315 | 44.3 ± 2.9 | 43.4 ± 5.5 | 0.552 | 43.0 ± 4.2 | 43.3 ± 3.7 | 0.912 | 57.0 ± 76 | 43.3 ± 3.7 | 0.334 |
| eGFR (mL/min/1.73m2) | 75.5 ± 12.5 | 75.8 ± 15.7 | 0.901 | 71.7 ± 14.7 | 71.2 ± 17.8 | 0.973 | 67.3 ± 11.9 | 75.1 ± 15.1 | 0.391 | 80.5 ± 8.7 | 80.2 ± 12.7 | 0.916 |
| FIB-4 | 1.79 ± 1.3 | 1.88 ± 1.5 | 0.738 | 1.62 ± 1.1 | 2.08 ± 1.6 | 0.240 | 2.34 ± NA | 1.72 ± 1.1 | NA | 1.90 ± 1.5 | 1.83 ± 1.7 | 0.840 |
| CAP (dB/m) | 283 ± 62 | 258 ± 58 | <0.001 | 310 ± 46 | 275 ± 60 | <0.001 | 286 ± 62 | 234 ± 50 | 0.019 | 266 ± 66 | 257 ± 56 | 0.308 |
| LSM (kPa) | 10.8 ± 3.6 | 5.1 ± 1.4 | <0.001 | 10.5 ± 2.1 | 5.0 ± 1.5 | <0.001 | 9.8 ± 1.3 | 5.0 ± 1.2 | <0.001 | 11.1 ± 4.5 | 5.2 ± 1.4 | <0.001 |
| Group | Variable | B (SE) | Exp(B) | 95% CI | p-Value |
|---|---|---|---|---|---|
| Total cohort | Constant | −4.745 (0.849) | 0.009 | <0.001 | |
| BMI (kg/m2) | 0.125 (0.026) | 1.133 | 1.077–1.192 | <0.001 | |
| Triglycerides (mmol/L) | 0.403 (0.174) | 1.496 | 1.064–2.102 | 0.020 | |
| T2DM | Constant | −3.491 (1.403) | 0.030 | 0.013 | |
| BMI (kg/m2) | 0.125 (0.026) | 1.132 | 1.033–1.240 | 0.008 | |
| T1DM | Constant | −7.001 (2.143) | 0.001 | 0.001 | |
| BMI (kg/m2) | 0.202 (0.071) | 1.223 | 1.064–1.406 | 0.005 | |
| Non-diabetics | Constant | −5.152 (1.513) | 0.006 | <0.001 | |
| BMI (kg/m2) | 0.111 (0.038) | 1.117 | 1.037–1.203 | 0.004 |
| Group | Variable | B (SE) | Exp(B) | 95% CI | p-Value |
|---|---|---|---|---|---|
| Total cohort | Constant | −4.337 (1.047) | 0.013 | <0.001 | |
| Triglycerides (mmol/L) | 0.422 (0.208) | 1.524 | 1.015–2.289 | 0.042 | |
| T2DM | Constant | −4.746 (1.544) | 0.009 | 0.002 | |
| BMI (kg/m2) | 0.104 (0.047) | 1.110 | 1.012–1.217 | 0.027 | |
| T1DM | Constant | −8.904 (3.238) | 0.000 | 0.006 | |
| BMI (kg/m2) | 0.212 (0.097) | 1.236 | 1.022–1.494 | 0.029 | |
| Non-diabetics | Constant | −3.020 (0.724) | 0.049 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saidi, A.N.; Theel, W.B.; Grobbee, D.E.; van der Lely, A.-J.; Dirksmeier-Harinck, F.; Alings, M.; van der Zwan-van Beek, E.; Rauh, S.P.; Rasheed, M.; Castro Cabezas, M. Prevalence of Liver Steatosis and Fibrosis Assessed by Transient Elastography in a High Cardiovascular-Risk Outpatient Cohort Including T1DM and T2DM Patients. Diabetology 2025, 6, 129. https://doi.org/10.3390/diabetology6110129
Saidi AN, Theel WB, Grobbee DE, van der Lely A-J, Dirksmeier-Harinck F, Alings M, van der Zwan-van Beek E, Rauh SP, Rasheed M, Castro Cabezas M. Prevalence of Liver Steatosis and Fibrosis Assessed by Transient Elastography in a High Cardiovascular-Risk Outpatient Cohort Including T1DM and T2DM Patients. Diabetology. 2025; 6(11):129. https://doi.org/10.3390/diabetology6110129
Chicago/Turabian StyleSaidi, Alina N., Willy B. Theel, Diederick E. Grobbee, Aart-Jan van der Lely, Femme Dirksmeier-Harinck, Marco Alings, Ellen van der Zwan-van Beek, Simone P. Rauh, Moniba Rasheed, and Manuel Castro Cabezas. 2025. "Prevalence of Liver Steatosis and Fibrosis Assessed by Transient Elastography in a High Cardiovascular-Risk Outpatient Cohort Including T1DM and T2DM Patients" Diabetology 6, no. 11: 129. https://doi.org/10.3390/diabetology6110129
APA StyleSaidi, A. N., Theel, W. B., Grobbee, D. E., van der Lely, A.-J., Dirksmeier-Harinck, F., Alings, M., van der Zwan-van Beek, E., Rauh, S. P., Rasheed, M., & Castro Cabezas, M. (2025). Prevalence of Liver Steatosis and Fibrosis Assessed by Transient Elastography in a High Cardiovascular-Risk Outpatient Cohort Including T1DM and T2DM Patients. Diabetology, 6(11), 129. https://doi.org/10.3390/diabetology6110129







