- Article
Evaluating Halloysite-Rich Kaolin/Biopolymer Composites for Enhanced Carbon Capture—A Study of Isotherms and Mechanisms
- Siavash Davoodi,
- Bhabananda Biswas and
- Ravi Naidu
- + 2 authors
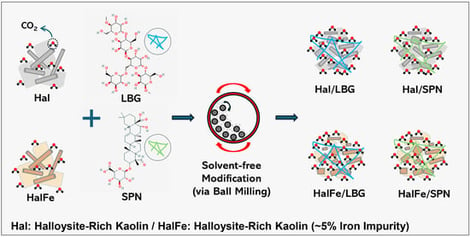
Anthropogenic CO2 emissions have accelerated climate change, prompting the need for effective capture technologies. Adsorption using clay-based sorbents offers an eco-friendly alternative, although performance often requires enhancement. This study explored mechanochemical modification of two halloysite-rich kaolin clay samples—iron-poor (Hal) and iron-rich (HalFe)—using locust bean gum and quillaja saponin and compared their CO2 uptake with the calcined counterparts (CHal, CHalFe). All samples were characterized using standard techniques, and their CO2 uptake was measured volumetrically across 0.1–20 bar and 15–35 °C. Modified sorbents showed enhanced mesoporosity and binding sites, increasing CO2 uptake by up to 26% at 20 bar (11.85 mg/g) and 125% at 1 bar (2.25 mg/g). Calcination, however, reduced surface area and sorption capacity. Isosteric heat values remained within the physisorption range, as supported by FTIR, XRF, and XPS, which showed no bulk carbonate formation. These sorbents show lower CO2 uptakes than conventional ones. Yet their low costs, abundance, biocompatibility, and solvent-free synthesis indicate strong potential for large-scale applications, especially for low-pressure implementations such as landfills. Further detailed studies on kinetics, thermodynamics, and sorbent regeneration are needed. Spent sorbents can potentially be repurposed for subsequent use in other applications, e.g., water treatment, construction materials, thereby minimizing waste production and supporting circular economy principles.
3 February 2026