MnO2-Supported Pd Nanocatalyst for Efficient Electrochemical Reduction of 2,4-Dichlorobenzoic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Pd/MnO2 Catalyst
2.3. Electrochemical Experiments
2.4. Analysis Methods
2.5. Theoretical Calculation Methods
3. Results
3.1. Optimization and Characterization of Electrochemical Catalysts
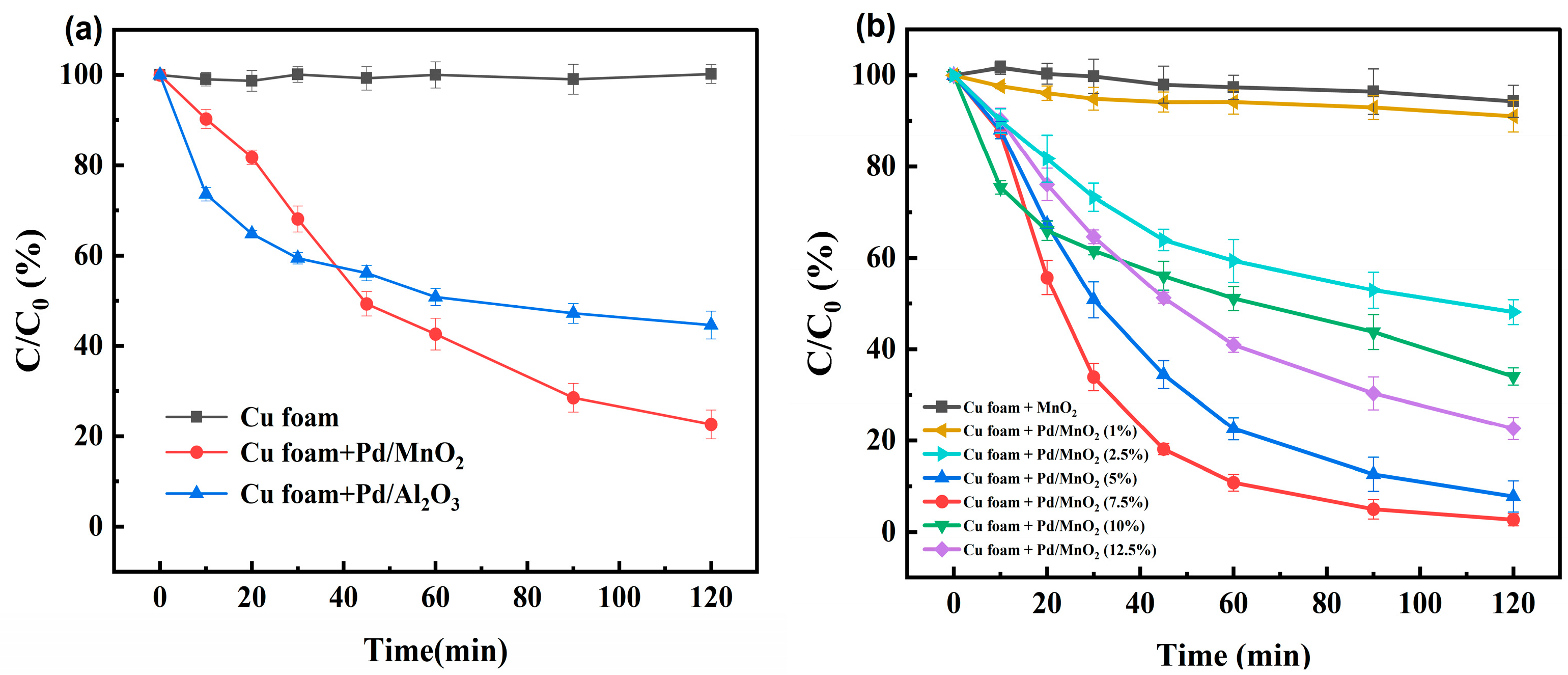
3.1.1. Comparison of Different Catalyst Materials
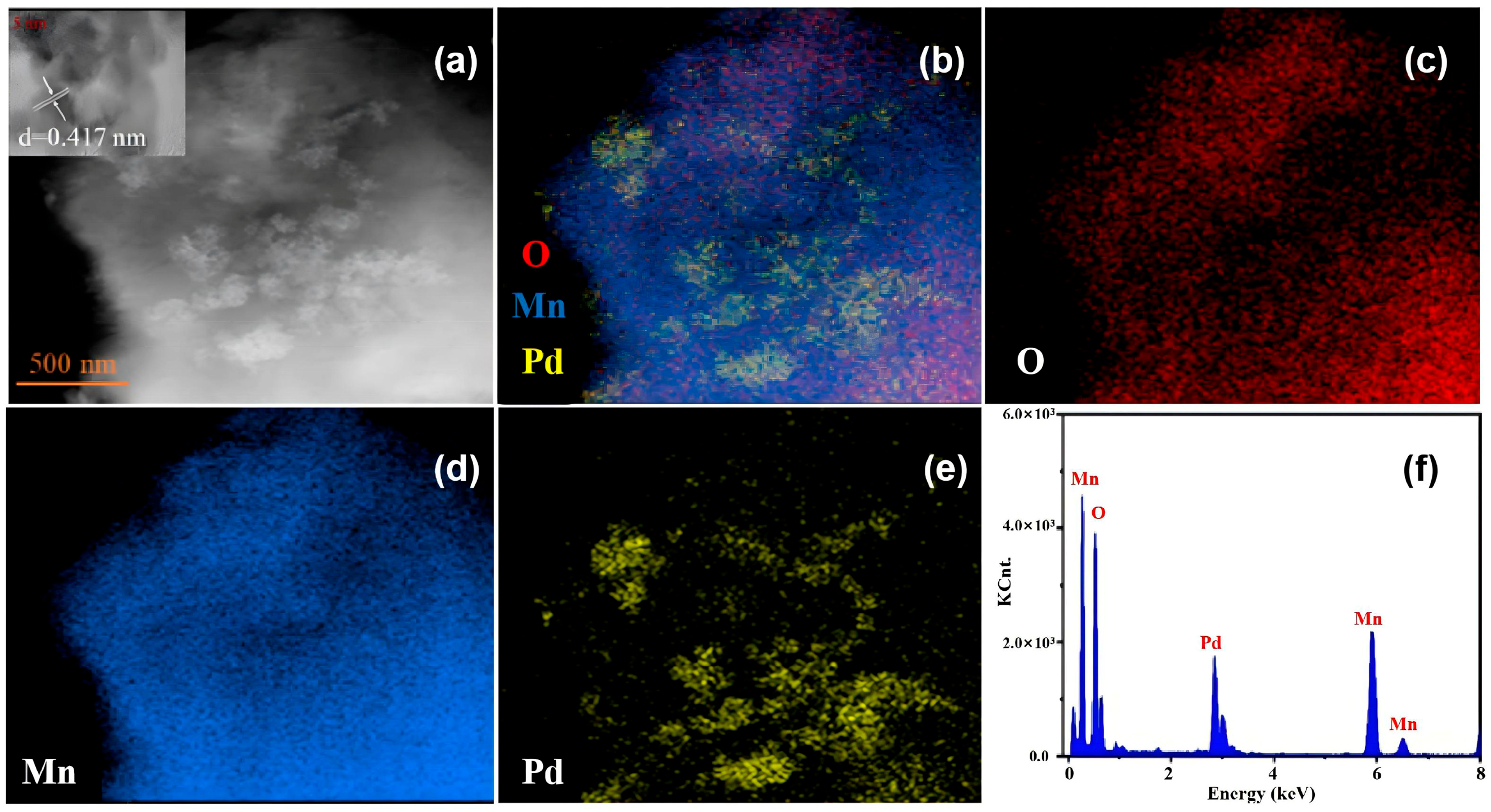
3.1.2. Morphological Characterization of Pd/MnO2 Catalyst
3.2. Influencing Factors of Electrochemical Reduction
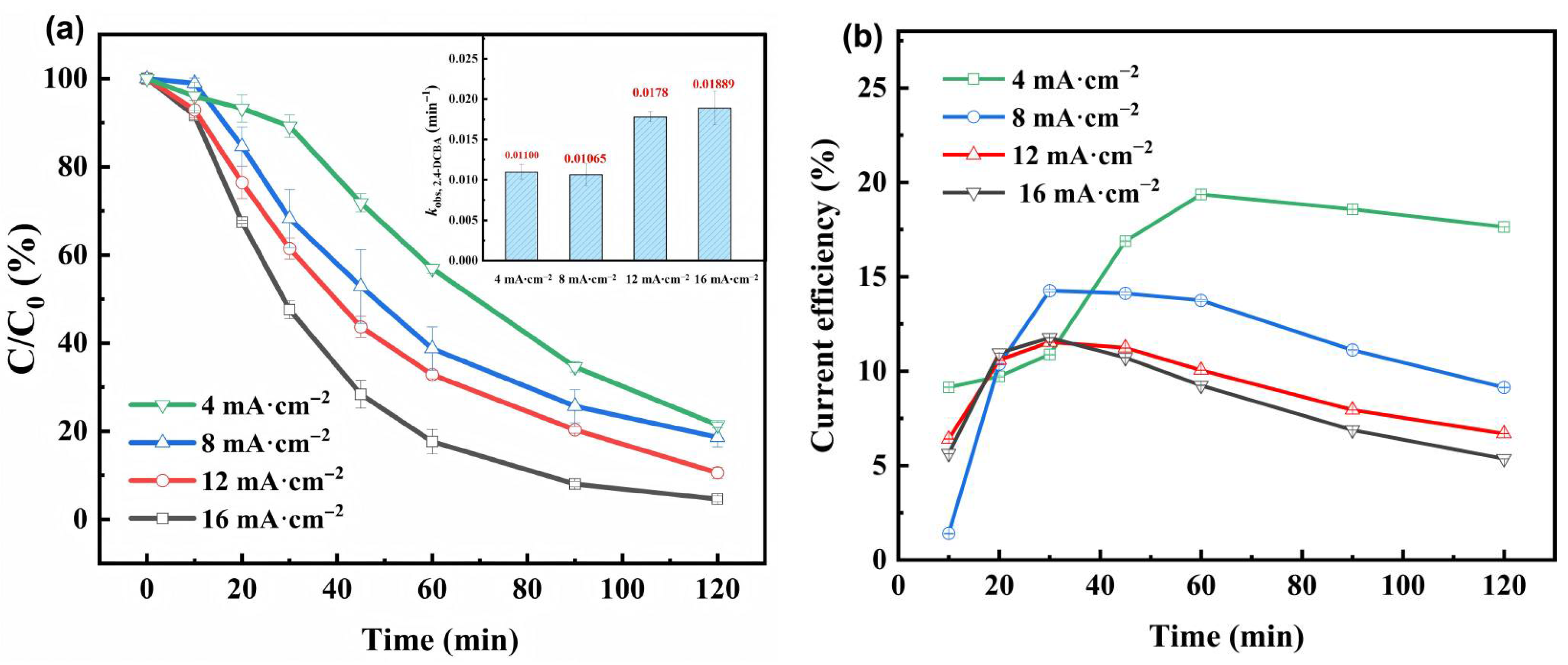
3.2.1. Effects of Current Density
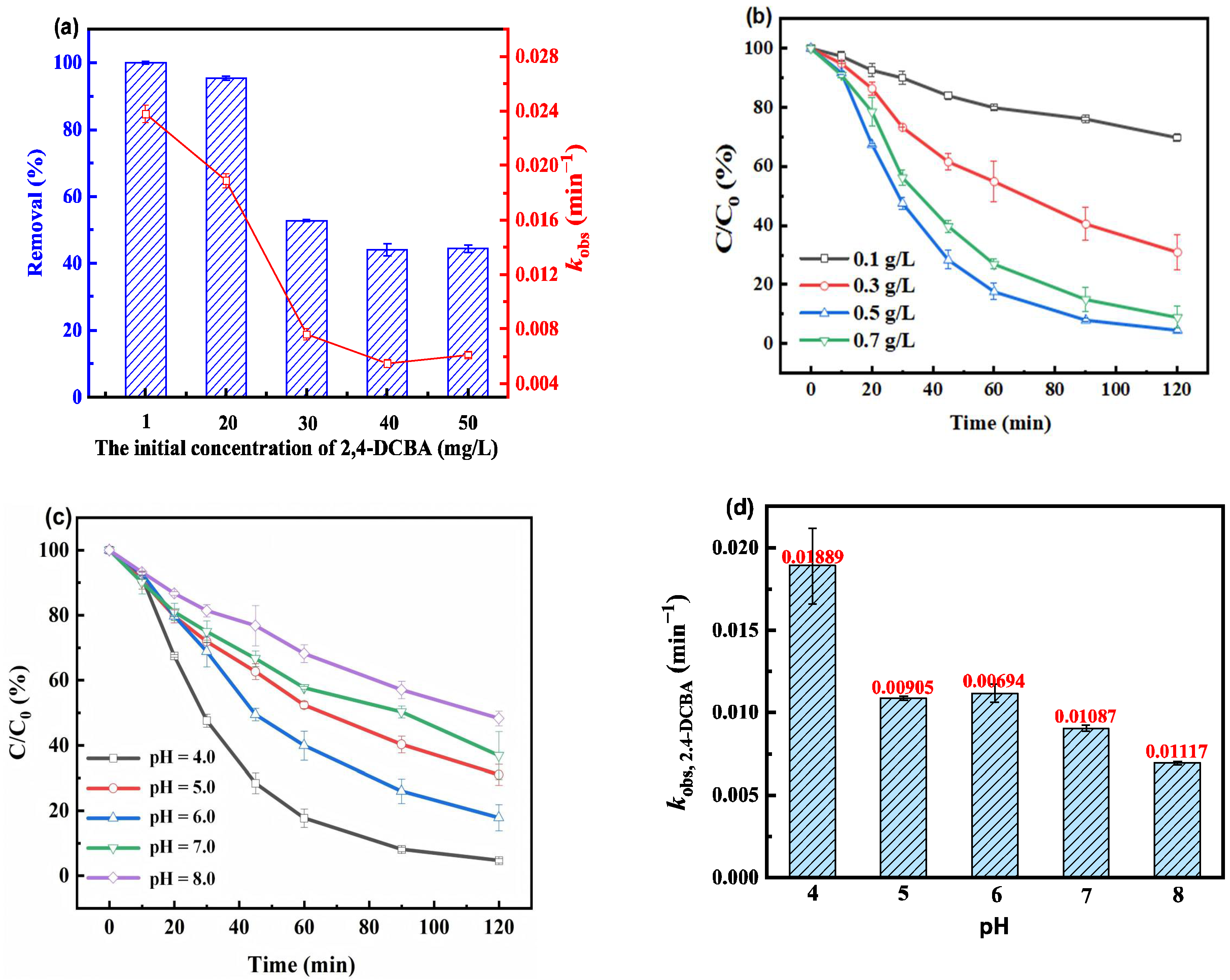
3.2.2. Solution Influencing Factors of the Electrochemical System
3.3. Mechanisms of Electrocatalytic Reduction by Pd/MnO2 Catalyst
3.3.1. Electrochemical Characterization Analysis
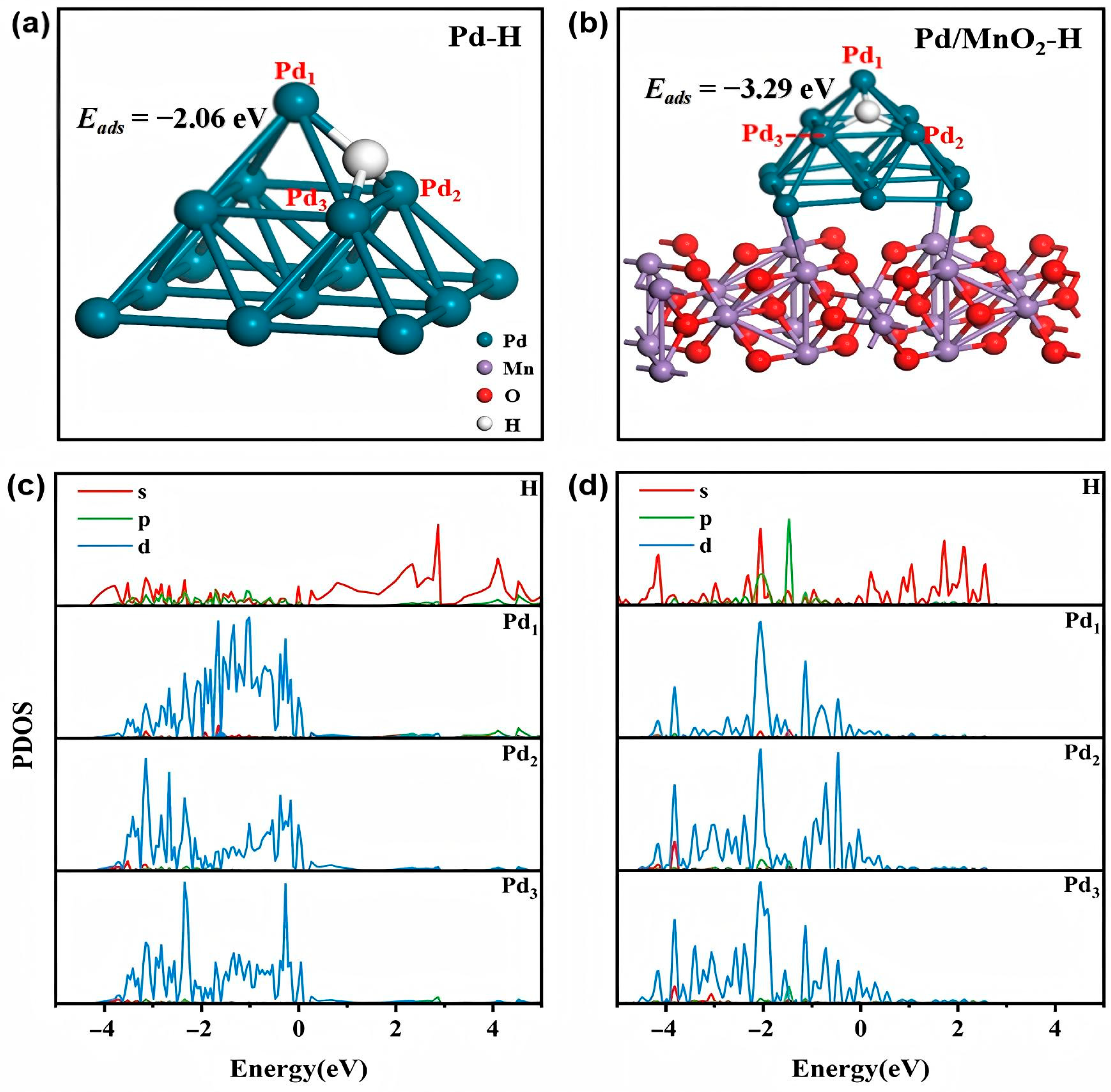
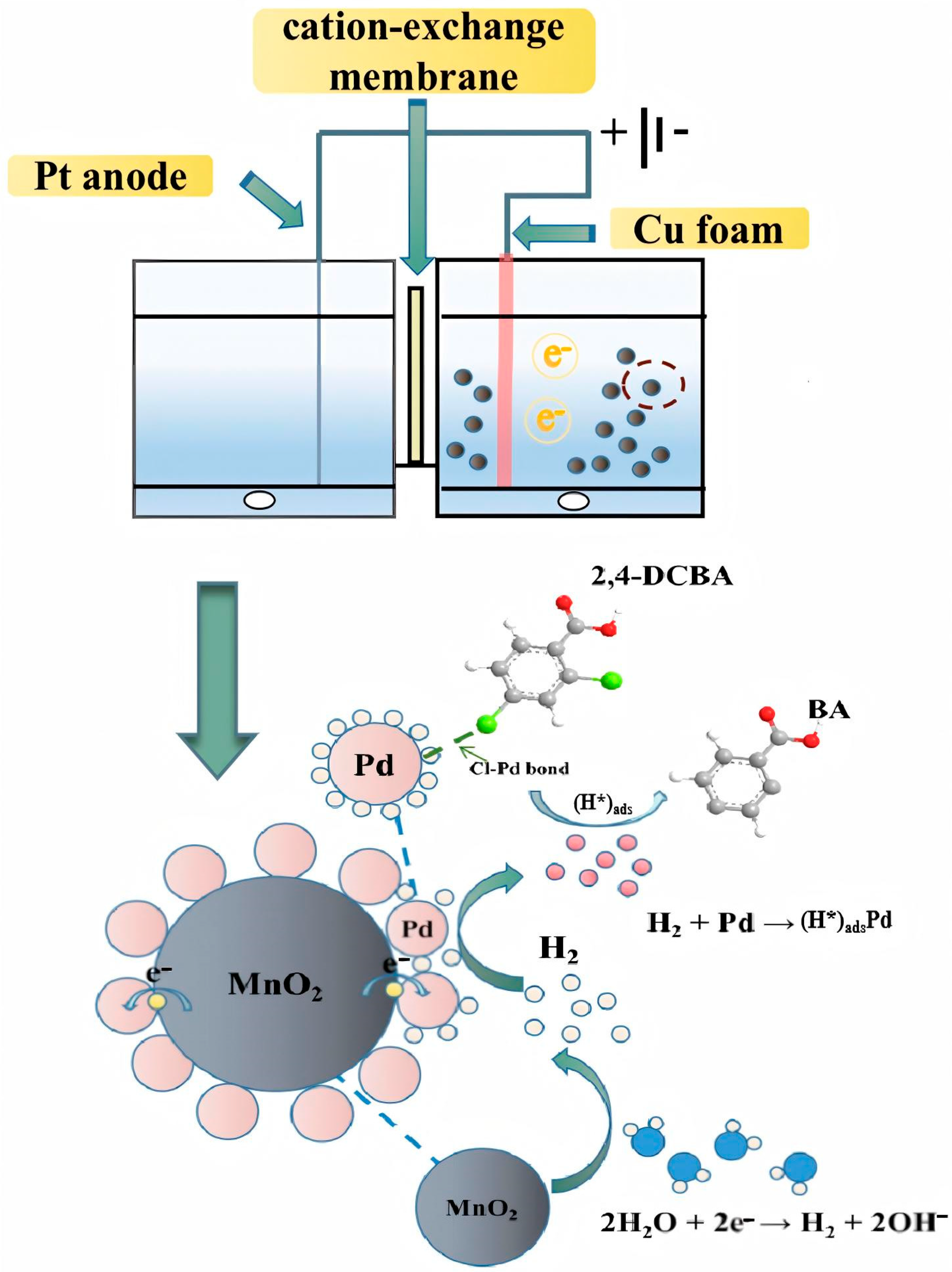
3.3.2. Electrocatalytic Mechanisms of 2,4-DCBA Reduction
3.4. Stability of the Pd/MnO2 Particle Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBAs | Chlorobenzoic acids |
| ECH | Electrocatalytic hydrodechlorination |
| 2,4-DCBA | 2,4-dichlorobenzoic acid |
| H* | Atomic hydrogen |
References
- Ko, M.-S.; Roh, T.-H.; Desale, P.P.; Choi, S.-W.; Cho, D.-G. Effects of Electron-Withdrawing and Electron-Donating Groups on Aromaticity in Cyclic Conjugated Polyenes. J. Am. Chem. Soc. 2024, 146, 6266–6273. [Google Scholar] [CrossRef]
- Jote, C. A Review of 2,4-D Environmental Fate, Persistence and Toxicity Effects on Living Organisms. Org. Med. Chem. Int. J. 2019, 9, 555755. [Google Scholar] [CrossRef]
- Vrana, B.; Dercova, K.; Baláž, Š.; Ševčíková, A. Effect of chlorobenzoates on the degradation of polychlorinated biphenyls (PCB) by Pseudomonas stutzeri. World J. Microbiol. Biotechnol. 1996, 12, 323–326. [Google Scholar] [CrossRef]
- Kim, S.; Picardal, F.W. A novel bacterium that utilizes monochlorobiphenyls and 4-chlorobenzoate as growth substrates. FEMS Microbiol. Lett. 2000, 185, 225–229. [Google Scholar] [CrossRef]
- Adebusoye, S.A.; Picardal, F.W.; Ilori, M.O.; Amund, O.O.; Fuqua, C. Characterization of multiple novel aerobic polychlorinated biphenyl (PCB)-utilizing bacterial strains indigenous to contaminated tropical African soils. Biodegradation 2008, 19, 145–159. [Google Scholar]
- Magnoli, K.; Carranza, C.S.; Aluffi, M.E.; Magnoli, C.E.; Barberis, C.L. Herbicides based on 2,4-D: Its behavior in agricultural environments and microbial biodegradation aspects. A review. Environ. Sci. Pollut. Res. 2020, 27, 38501–38512. [Google Scholar] [CrossRef]
- Eggen, T.; Moeder, M.; Arukwe, A. Municipal landfill leachates: A significant source for new and emerging pollutants. Sci. Total Environ. 2010, 408, 5147–5157. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Quero, S.; Cárdenas-Lizana, F.; Keane, M.A. Unique selectivity in the hydrodechlorination of 2, 4-dichlorophenol over hematite-supported Au. J. Catal. 2013, 303, 41–49. [Google Scholar] [CrossRef]
- Zhou, J.; Lou, Z.; Xu, J.; Zhou, X.; Yang, K.; Gao, X.; Zhang, Y.; Xu, X. Enhanced electrocatalytic dechlorination by dispersed and moveable activated carbon supported palladium catalyst. Chem. Eng. J. 2019, 358, 1176–1185. [Google Scholar] [CrossRef]
- Adebusoye, S.A.; Miletto, M. Characterization of multiple chlorobenzoic acid-degrading organisms from pristine and contaminated systems: Mineralization of 2, 4-dichlorobenzoic acid. Bioresour. Technol. 2011, 102, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, Y.; Wang, S.; Chen, L.; Wen, X.; Huang, M.; Cheng, X.; Cheng, Z.; Tao, L. Bacterial networks mediate pentachlorophenol dechlorination across land-use types with citrate addition. J. Hazard. Mater. 2020, 384, 121295. [Google Scholar] [CrossRef] [PubMed]
- De Coster, J.; Vanherck, W.; Appels, L.; Dewil, R. Selective electrochemical degradation of 4-chlorophenol at a Ti/RuO2-IrO2 anode in chloride rich wastewater. J. Environ. Manag. 2017, 190, 61–71. [Google Scholar] [CrossRef]
- Zhang, M.; Shi, Q.; Song, X.; Wang, H.; Bian, Z. Recent electrochemical methods in electrochemical degradation of halogenated organics: A review. Environ. Sci. Pollut. Res. 2019, 26, 10457–10486. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, C.; Chen, W.; Xie, Q.; Guo, Q.; Huang, B. Electrochemical-driven nanoparticulate catalysis for highly efficient dechlorination of chlorinated environmental pollutant. J. Catal. 2021, 395, 362–374. [Google Scholar] [CrossRef]
- He, R.; Liang, H.; Zheng, Q.; Huang, H.; Yang, Y.; Li, X. Insight into the efficient degradation of 2,4,6-trichlorophenol by a three-electrode simultaneous oxidation-reduction system: Kinetics and mechanism. Environ. Technol. 2025, 46, 4583–4596. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Li, Y.; Pan, Y.; Liu, Y. Rational Design and Structural Regulation of Robust Catalysts for Electrocatalytic Hydrodechlorination: From Nanostructures to Single Atoms. ACS Catal. 2023, 13, 9633–9655. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of supported metal nanoparticles: Synthesis methodologies, advantages and application as catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Zhai, W.; Ma, Y.; Chen, D.; Ho, J.C.; Dai, Z.; Qu, Y. Recent progress on the long-term stability of hydrogen evolution reaction electrocatalysts. InfoMat 2022, 4, e12357. [Google Scholar] [CrossRef]
- Swain, N.; Mitra, A.; Saravanakumar, B.; Balasingam, S.K.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Construction of three-dimensional MnO2/Ni network as an efficient electrode material for high performance supercapacitors. Electrochim. Acta 2020, 342, 136041. [Google Scholar] [CrossRef]
- Huang, C.; Chen, C.-Y.; Whang, T.-J. An efficient foam Ni/MnO2/Pd composite cathode for the electrocatalytic degeneration of phenol. Mater. Chem. Phys. 2021, 263, 124401. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, B.; Liu, Z. Tailoring eletronic structure of Pd nanoparticles via MnO2 as electron transfer intermediate for enhanced hydrogen evolution reaction. Chem. Phys. Lett. 2020, 748, 137405. [Google Scholar] [CrossRef]
- Mohammadi, M. Development of Pb-MnO2 Composite Anodes for Electrowinning Application: Electrochemical and Corrosion Evaluations; Text; University of British Columbia: Vancouver, BC, Canada, 2016. [Google Scholar]
- Ahmed, S.; Khan, M.K.; Kim, J. Revolutionary advancements in carbon dioxide valorization via metal-organic framework-based strategies. Carbon Capture Sci. Technol. 2025, 15, 100405. [Google Scholar] [CrossRef]
- Ahmed, S.; Hussain, M.S.; Khan, M.K.; Kim, J. Innovations in catalysis towards efficient electrochemical reduction of CO2 to C1 chemicals. J. Energy Chem. 2025, 107, 622–649. [Google Scholar] [CrossRef]
- Yang, L.; Huang, C.; Yin, Z.; Meng, J.; Guo, M.; Feng, L.; Liu, Y.; Zhang, L.; Du, Z. Rapid electrochemical reduction of a typical chlorinated organophosphorus flame retardant on copper foam: Degradation kinetics and mechanisms. Chemosphere 2021, 264, 128515. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B Condens. Matter 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Liu, S.; Cheng, Y.; Zhang, C.; Jiao, J.; Lu, Y.; Wang, W.; Sun, K.; Bi, X. In-Situ doping-induced crystal form transition of amorphous Pd–P catalyst for robust electrocatalytic hydrodechlorination. Appl. Catal. B Environ. 2021, 284, 119713. [Google Scholar] [CrossRef]
- Pi, C.; Zhao, Z.; Zhang, X.; Gao, B.; Zheng, Y.; Chu, P.K.; Yang, L.; Huo, K. In situ construction of γ-MoC/VN heterostructured electrocatalysts with strong electron coupling for highly efficient hydrogen evolution reaction. Chem. Eng. J. 2021, 416, 129130. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, D.; Jiao, X. Highly selective adsorption on monolayer MoS2 doped with Pt, Ag, Au and Pd and effect of strain engineering: A DFT study. Sens. Actuators A Phys. 2021, 322, 112637, Erratum in Sens. Actuators A Phys. 2021, 324, 112683. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Yang, L. Ultra-highly sensitive and stable acetylcholinesterase biosensor based on TiO2-NRs and rGO. Microchem. J. 2021, 168, 106435. [Google Scholar] [CrossRef]
- Zhou, J.; Lou, Z.; Yang, K.; Xu, J.; Li, Y.; Liu, Y.; Baig, S.A.; Xu, X. Electrocatalytic dechlorination of 2,4-dichlorobenzoic acid using different carbon-supported palladium moveable catalysts: Adsorption and dechlorination activity. Appl. Catal. B Environ. 2019, 244, 215–224. [Google Scholar]
- Gopinath, R.; Lingaiah, N.; Sreedhar, B.; Suryanarayana, I.; Sai Prasad, P.S.; Obuchi, A. Highly stable Pd/CeO2 catalyst for hydrodechlorination of chlorobenzene. Appl. Catal. B Environ. 2003, 46, 587–594. [Google Scholar] [CrossRef]
- Fu, H.Q.; Zhou, M.; Liu, P.F.; Liu, P.; Yin, H.; Sun, K.Z.; Yang, H.G.; Al-Mamun, M.; Hu, P.; Wang, H.-F.; et al. Hydrogen Spillover-Bridged Volmer/Tafel Processes Enabling Ampere-Level Current Density Alkaline Hydrogen Evolution Reaction under Low Overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039. [Google Scholar] [CrossRef]
- Alhooshani, K.R. Adsorption of chlorinated organic compounds from water with cerium oxide-activated carbon composite. Arab. J. Chem. 2019, 12, 2585–2596. [Google Scholar] [CrossRef]
- Gupta, K.; Joshi, P.; Gusain, R.; Khatri, O.P. Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterials. Coord. Chem. Rev. 2021, 445, 214100. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Zou, Y.; Zhang, N.; Zhang, Y.; Wu, Y.; Wang, Y.; Chen, R.; Wang, S. Charge Transfer Modulated Activity of Carbon-Based Electrocatalysts. Adv. Energy Mater. 2020, 10, 1901227. [Google Scholar] [CrossRef]
- Shi, J. On the Synergetic Catalytic Effect in Heterogeneous Nanocomposite Catalysts. Chem. Rev. 2013, 113, 2139–2181. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, X.; Zhao, Z.; Chen, D.; Liu, R.; Ye, Z.; Lu, B.; Hou, Y.; Lu, J. Design of pH-universal electrocatalysts for hydrogen evolution reaction. Carbon Energy 2024, 6, e555. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Li, W.-H.; Zhang, X.-Y.; Xu, H.-Z.; Li, K.; Kai, Y.; Sun, L.-S.; Li, C.-Q.; Liu, F.-Q. Synthesis of Ag nanoparticles decorated MnO2/sulfonated graphene composites with 3D macroporous structure for high performance capacitors electrode materials. RSC Adv. 2016, 6, 94682–94686. [Google Scholar] [CrossRef]
- Pan, Y.; Lin, Y.; Liu, Y.; Liu, C. A novel CoP/MoS2-CNTs hybrid catalyst with Pt-like activity for hydrogen evolution. Catal. Sci. Technol. 2016, 6, 1611–1615. [Google Scholar] [CrossRef]
- Begum, H.; Ahmed, M.S.; Jeon, S. δ-MnO2 nanoflowers on sulfonated graphene sheets for stable oxygen reduction and hydrogen evolution reaction. Electrochim. Acta 2019, 296, 235–242. [Google Scholar]
- Xu, H.; Zhang, D.; Liu, M.; Ye, D.; Huo, S.; Chen, W.; Zhang, J. Self-supporting hierarchical Co3O4-nanowires@NiO-nanosheets core-shell nanostructure on carbon foam to form efficient bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2024, 654, 1293–1302. [Google Scholar] [CrossRef]
- Dabo, P.; Cyr, A.; Laplante, F.; Jean, F.; Ménard, H.; Lessard, J. Electrocatalytic dehydrochlorination of pentachlorophenol to phenol or cyclohexanol. Environ. Sci. Technol. 2000, 34, 1265–1268. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. Proc. Natl. Acad. Sci. USA 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, H.; Zhao, Y.; Liang, X.; Chen, Z.; Ji, M.; Wang, M. Creating hydrophobic nanopockets in metal–organic frameworks to promote hydrodeoxygenation of lignin derivatives under ambient conditions. Chem. Sci. 2025, 16, 15223–15230. [Google Scholar] [CrossRef]
- Lingaiah, N.; Seshu Babu, N.; Gopinath, R.; Siva Sankara Reddy, P.; Sai Prasad, P.S. Hydrodechlorination of chlorobenzene over supported metal catalysts. Catal. Surv. Asia 2006, 10, 29–39. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Huang, J. Electrocatalytic hydrodechlorination of 2, 4, 5-trichlorobiphenyl on a palladium-modified nickel foam cathode. Environ. Sci. Technol. 2007, 41, 7503–7508. [Google Scholar] [CrossRef]
- Li, J.; Peng, Y.; Zhang, W.; Shi, X.; Chen, M.; Wang, P.; Zhang, X.; Fu, H.; Lv, X.; Dong, F. Hierarchical Pd/MnO2 nanosheet array supported on Ni foam: An advanced electrode for electrocatalytic hydrodechlorination reaction. Appl. Surf. Sci. 2020, 509, 145369. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, C.; Zhang, G.; Xin, Y.; Wang, S. Electrocatalytic hydrodechlorination of pentachlorophenol on Pd-supported magnetic biochar particle electrodes. Sep. Purif. Technol. 2021, 258, 118017. [Google Scholar] [CrossRef]
- He, Z.; Zhan, L.; Wang, Q.; Song, S.; Chen, J.; Zhu, K.; Xu, X.; Liu, W. Increasing the activity and stability of chemi-deposited palladium catalysts on nickel foam substrate by electrochemical deposition of a middle coating of silver. Sep. Purif. Technol. 2011, 80, 526–532. [Google Scholar] [CrossRef]
- Nasako, K.; Ito, Y.; Hiro, N.; Osumi, M. Stress on a reaction vessel by the swelling of a hydrogen absorbing alloy. J. Alloys Compd. 1998, 264, 271–276. [Google Scholar] [CrossRef]
- Lou, Z.; Li, Y.; Zhou, J.; Yang, K.; Liu, Y.; Baig, S.A.; Xu, X. TiC doped palladium/nickel foam cathode for electrocatalytic hydrodechlorination of 2,4-DCBA: Enhanced electrical conductivity and reactive activity. J. Hazard. Mater. 2019, 362, 148–159. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Wang, M. MnO2-Supported Pd Nanocatalyst for Efficient Electrochemical Reduction of 2,4-Dichlorobenzoic Acid. Clean Technol. 2025, 7, 102. https://doi.org/10.3390/cleantechnol7040102
Peng Y, Wang M. MnO2-Supported Pd Nanocatalyst for Efficient Electrochemical Reduction of 2,4-Dichlorobenzoic Acid. Clean Technologies. 2025; 7(4):102. https://doi.org/10.3390/cleantechnol7040102
Chicago/Turabian StylePeng, Yaxuan, and Meiyan Wang. 2025. "MnO2-Supported Pd Nanocatalyst for Efficient Electrochemical Reduction of 2,4-Dichlorobenzoic Acid" Clean Technologies 7, no. 4: 102. https://doi.org/10.3390/cleantechnol7040102
APA StylePeng, Y., & Wang, M. (2025). MnO2-Supported Pd Nanocatalyst for Efficient Electrochemical Reduction of 2,4-Dichlorobenzoic Acid. Clean Technologies, 7(4), 102. https://doi.org/10.3390/cleantechnol7040102




