Abstract
Bioactive compounds are widely recognized for their ability to enhance health and prevent diseases due to their various biological activities. However, these compounds are very sensitive to environmental factors, which can reduce their solubility, bioavailability, permeability, and stability, necessitating carriers to protect and ensure targeted delivery. To develop an effective delivery system, it is essential to assess the key factors that influence the release behaviour of bioactive compounds. Therefore, the primary aim of this study is to evaluate how the conditions of the release media, the attributes of hydrogels, and the characteristics of the entrapped bioactive compounds regulate the release kinetics of these compounds. Prior to create suitable carriers, it is essential to comprehend the mechanisms of digestion and absorption of these compounds. Consequently, absorption and the factors influencing stability and bioavailability of bioactives were reviewed first. The conditions of release media, especially the pH, ionic characteristics, temperature, and the nature of solvent served as a critical determinant in the release of bioactive substances by affecting the functional groups, electrostatic interactions between carrier and entrapped bioactive compound, dissociation and conformational changes in polymers. The properties of delivery systems can be controlled using polymers, crosslinkers, plasticizers, and specific environmental factors. The application of dual crosslinkers or a combination of physical and chemical crosslinkers enhanced the efficiency of the crosslinking process, subsequently improving the overall release profile of bioactive compounds from the matrices. Therefore, this review explored several options for enhancing the delivery system.
1. Introduction
Currently, researchers are increasingly focused on bioactive compounds that originate from seeds, food sources, and products resulting from fermentation processes [1]. These compounds play a vital role in metabolic processes and can exert positive effects on human health, including antioxidant properties, modulation of enzyme activities, regulation of gene expression, and interaction with receptors, among various other functions [2]. The most notable bioactive compounds pertinent to the human diet include carotenoids, phenolic compounds, glucosinolates, vitamins, tocopherols, phytosterols, organosulfur compounds, flavonoids, fatty acids, dietary fibre, and folates, among others [3]. An effective strategy for creating novel functional foods involves the integration of peptides, probiotics, vitamins, antioxidants, and various other natural bioactives into food formulations [4,5]. However, the poor solubility, instability, and low bioavailability of these bioactive compounds have considerably restricted their application within the food industry [6].
Utilizing suitable delivery systems to entrap the compounds can alleviate these challenges to a degree [7]. Encapsulation is a technique for safeguarding and delivering bioactive compounds to their intended site by embedding these active ingredients within the structures of wall materials [8]. The food industry anticipates a growing demand for intricate characteristics in food ingredients, including delayed release, stability, thermal protection, and an appropriate sensory profile. Achieving these properties frequently necessitates the use of a delivery system [9]. Food encapsulation possesses numerous intriguing and distinctive characteristics; however, its application remains difficult, mainly because of various side effects, including toxicity, lack of specificity, low bioavailability, short duration of drug delivery, and swift degradation [10]. It is essential to use materials that are generally recognised as safe (GRAS) [11].
Recently, high concentration of biopolymers has been considered more advantageous for encapsulation, as it results in a more compact and coherent gel matrix that enhances the barrier effect [12]. With their unique eco-friendly and adaptable properties, biopolymers play a vital role in developing sustainable and innovative formulations [13]. The presence of hydroxyl, amino, or carboxyl groups in natural biopolymers increases their chemical reactivity and versatility, making them comparable to synthetic polymers [14,15]. Furthermore, a significant advantage of utilizing biopolymers is their capacity to be produced from the non-edible components of plants or animals. The primary categories of biopolymers that can be derived from biomass waste, along with examples of their respective sources are presented in Figure 1, adapted from [16].
Figure 1.
Strategies employed to regulate the release rate from biopolymeric hydrogels.
Controlled release systems provide sophisticated platforms for creating novel formulations and dietary approaches, allowing precise regulation of the release and activity of bioactive food components [17]. Controlled-release delivery systems can safeguard bioactives from hostile conditions, including light, pH levels, temperature fluctuations, and processing environments, thereby enhancing their bioavailability [18]. Additionally, controlled-release delivery systems have garnered interest in active food packaging, leading to an enhanced shelf life of food items [19]. As a controlled delivery system, hydrogels have attracted significant attention and have applications in various areas, including synthetic tendons [20], contact lenses [21], scaffolds for tissue engineering [22], environmental remediation [23], and drug delivery [24], as well as in agriculture and biosensors [25]. Hydrogels are 3D networks of polymers that absorb water or biological liquid while maintaining their structural integrity [26]. The properties of hydrogels, including their physicochemical, mechanical, and biocompatible characteristics, are influenced by the type of polymer used, the ratio of its constituents, the overall composition, and the methods employed in their fabrication [27].
As interest in controlled release delivery systems continues to rise within the food and pharmaceutical sectors, numerous efforts are being undertaken to assess the factors influencing the release characteristics of natural bioactive compounds from these delivery systems. Bioactives can be released from hydrogels through diffusion-controlled, swelling-controlled, or chemically controlled mechanisms [28]. The primary mechanism for the transfer of these ingredients is typically the process of diffusion [29]. It is common to study release kinetics using various mathematical models, the most widely applied being zero-order, first-order, Higuchi, Korsmeyer–Peppas, and Hixson–Crowell models [30]. When designing a delivery system for the micronutrients, it is essential to consider their physicochemical properties, the types and concentrations of additives (such as plasticizers, and crosslinkers) incorporated in the carriers, and environmental factors like pH, temperature, and ionic profile [31]. The effect of the release medium on drug release from biopolymeric matrices was investigated, revealing that varying drug release profiles were influenced by the medium’s pH, osmolarity, and temperature [32]. Another study investigated the impact of various crosslinkers on the release of bioactive compounds. Swelling and in vitro tests indicated that the release was significantly influenced by time, crosslinker type and concentration, and medium pH [33]. Crosslinkers, in fact, are regarded as effective tools for achieving sustained release by enhancing the structural characteristics of biopolymers [34]. A review showed that bioactive compounds, depending on their characteristics (concentration, structure, and reactive groups), modified the film structure and permeability by acting as either plasticizers, anti-plasticizers or crosslinkers [35].
Considering these key regulatory factors, this communication provides a comprehensive overview of the strategies utilized to regulate the release behaviour of bioactive molecules from biopolymeric matrices. This includes the selection of release media conditions, such as pH and temperature, various physical and chemical crosslinking treatments, the incorporation of additives, such as plasticizers, and the inherent characteristics of the bioactive compounds. Finally, we discuss the potential for predicting and regulating the release rate of bioactive components from biopolymeric hydrogels.
2. Absorption of Bioactive Compounds
Understanding the digestion and absorption mechanisms of different bioactive ingredients is essential prior to developing appropriate carriers for these substances. The gastrointestinal (GI) tract is the primary site for digesting and absorbing food components. In the stomach, food is mechanically broken down into a thick, semifluid mixture called chyme, which then moves into the duodenum for further processing. Some chemical digestion also occurs in the stomach, breaking down macromolecules such as proteins, fats, and polysaccharides. Once in the small intestine, these nutrients are absorbed into the body [6]. The physiological functions and absorption mechanisms of different bioactive compounds are outlined in Table 1.

Table 1.
A summary of the mechanisms of absorption and the environmental factors that affect bioactive compounds.
Vitamin D is mainly absorbed via passive diffusion as well as through a mechanism that includes membrane transporters, particularly cholesterol carriers [42]. Bioactive polypeptides, bioactive polysaccharides and unsaturated fatty acids are taken up through carrier-mediated transport, with the carriers being uniformly distributed throughout various regions of the small intestine [45,50,55]. Furthermore, the receptor-mediated transport mechanism plays a crucial role in the absorption of bioactive polypeptides [56]. Carrier-mediated diffusion primarily facilitates the absorption of polyphenols, including tea catechins [57]. A significant portion of polyphenols and certain larger molecules are not absorbed in the small intestine; consequently, these compounds arrive in the large intestine, where they undergo metabolism by the microbiota into smaller molecules [58]. Hydrophilic substances, including polyphenols and most of the drugs, exhibit a more straightforward absorption mechanism compared to lipids. Hydrophobic components are primarily absorbed through passive diffusion and receptor-mediated transport. For example, resveratrol may be taken up by passive diffusion or by interacting with membrane transporters such as integrins [52]. Additionally, the low aqueous solubility of hydrophobic compounds further leads to low absorption rate; thus, enhancing the aqueous solubility of these hydrophobic ingredients through the use of carriers is crucial [6].
3. Factors Influencing Stability and Bioavailability of Natural Bioactive Absorption of Bioactive Compounds
The low absorption efficiency of food bioactives leads to decreased bioavailability, as these compounds need to pass through gastrointestinal barriers before being taken up by the body. Moreover, their stability can be affected by multiple environmental factors. In this regard, the main factors influencing the absorption of bioactive ingredients are summarized in Table 1.
The stability of bioactive compounds is strongly influenced by environmental factors such as extreme pH, light exposure, heat, and oxygen. For example, the antioxidant activity of carotenoids largely depends on their conjugated double bonds, which are highly susceptible to oxidation [59]. Additionally, interactions with other food components, such as proteins and fats, can lessen their effectiveness [60]. The limited solubility of curcumin, a naturally occurring polyphenolic substance derived from turmeric, in water and its swift degradation at physiological pH levels lead to reduced bioavailability and suboptimal pharmacokinetics. Consequently, only a minor fraction of curcumin consumed orally is effectively digested and absorbed by the human body [61].
The harsh conditions of the gastrointestinal (GI) tract represent the second factor affecting bioavailability. Accurate knowledge of how food components are broken down in the stomach is essential for evaluating the bioaccessibility of phytochemicals. This stage of digestion involves both mechanical processes and the action of gastric secretions. Gastric juice is composed of hydrochloric acid, pepsinogens, lipase, mucus, electrolytes, and water. Under fasting conditions, the secretion rate of gastric juice ranges from about 1 to 4 mL/min, increasing to 1–10 mL/min following food intake. Hydrochloric acid contributes to protein denaturation and activates pepsin, while peristaltic waves help reduce the size of solid food particles to approximately 1–2 mm. In healthy individuals, fasting gastric pH typically ranges from 1.3 to 2.5, but it generally rises above 4.5 after a meal, depending on the food’s buffering capacity [62]. For instance, studies indicate that flavonoids and hydroxycinnamoyl acid experienced degradation rates of 84% and 80%, respectively, when subjected to in vitro GI environments, while vitamin C is reported to be degraded by 91% during intestinal digestion state [63]. Furthermore, digestive enzymes such as pepsin in the stomach and trypsin in the intestine can break down bioactive proteins and peptides, leading to a reduction in their activity. For example, quercetin is a well-known plant flavonoid that exhibits a variety of pharmacological effects. Nevertheless, its use in the pharmaceutical industry is constrained by several factors, including inadequate solubility, low bioavailability, limited permeability, and instability within the gastrointestinal tract [64]. Most importantly, prior to the bioactive components executing their intended functions, they might have already been degraded or digested. For this reason, the digestion occurring within the gastrointestinal tract is a great challenge that must be addressed to enhance their absorption.
Another barrier to absorption is the mucus layer that envelops the entire surface of the gastrointestinal tract. This mucus layer is mainly made up of glycoproteins, lipids, and discarded cellular materials [65]. Mucus is a semipermeable barrier that facilitates the exchange of various nutrients while simultaneously obstructing the entry of most bacteria and pathogens to the surfaces of epithelial cells [66]. The slow diffusion of food components through the mucus can restrict their access to enterocytes for absorption. Hydrophobic bioactive compounds, in particular, may interact with mucin proteins, which decreases their permeability through the mucus layer. The molecular structure of micronutrients significantly influences their absorption [67]. The results indicated that the bioaccessibility of phenolic compounds differed depending on their structures, with a clear relationship between molecular conformation and stability. For instance, it is well recognized that compounds with higher molecular weight, such as oligomeric proanthocyanidins and complex lipids, cannot permeate intestinal cells unless they undergo prior degradation [68]. Furthermore, the sugar component of flavonoids has been identified as an essential factor in their absorption in humans. When flavonoids are joined to an extra rhamnose unit, as seen with quercetin derived from tea, they must reach the large intestine to allow the intestinal microbiota to cleave the sugar moieties before absorption happens [69]. Still, it is not solely the chemical structure of bioactive food compounds that impacts their absorption; their isomeric configuration also plays a considerable role [6]. Like certain pharmaceuticals, flavonoids with varying stereochemistry show differing levels of bioavailability. This is exemplified by the bioavailability of (−)-epicatechin compared to (+)-catechin [70], and the superiority of Z-isomerization over all other isomers of carotenoids [71].
The various transport mechanisms, such as active transport, passive and facilitated diffusion, occurring within the intestinal lumen are also critical determinants of the bioavailability of consumed foods and drugs [58]. In summary, it is essential to develop strategies that overcome these challenges and improve the bioavailability of bioactive compounds. Each class of bioactives possesses unique chemical characteristics (hydrophilic or lipophilic), biological functions, and varying degrees of sensitivity. The limited solubility and susceptibility to oxidation of several vitamins, including vitamins A, D, E, and K, present significant obstacles to their application. Techniques such as nano-encapsulation and micro or nano-emulsion, utilizing nanoscale dimensions, can greatly enhance their availability and stability [72]. Polyunsaturated fatty acids, such as DHA and EPA, are especially prone to oxidation. Encapsulation techniques can effectively protect them from degradation [73]. Many bioactive proteins and peptides are prone to denaturation and exhibit low absorption in the gastrointestinal tract; however, encapsulation within nanoparticles or microparticles can protect these compounds and enable their targeted delivery to the intestine [74].
Numerous criteria must be met in the preparation of carriers as well. It is crucial that delivery carriers remain stable and prevent premature release of encapsulated compounds in the stomach’s acidic environment. Furthermore, carriers should be able to diffuse proficiently through the intestinal mucus layer to reach the surface of enterocytes rapidly, rather than being removed by the renewal of mucus. Particles that are hydrophilic and charge-neutral are more likely to pass the mucus layer with ease [75].
4. Entrapment of Bioactive Compounds
4.1. Polymers Used for Entrapment of Bioactive Compounds
A biopolymer is a type of polymer that originates from natural sources and is synthesized by living organisms. These polymers generally consist of repeating structural units, called monomers, which are linked together through covalent bonds. Biopolymers are defined as polymers obtained from renewable resources, including both biological and fossil-based biodegradable polymers [76]. Numerous polymers are employed as carrier materials for bioactive compounds. These include polylactic acid, polyhydroxyalkanoates, gelatin, β-glucans, alginate, dextran, starch, cellulose, chitosan, chitin, pectin, gums, collagen, zein, and hyaluronic acid, among various others [77].
4.2. Fabrication of Biopolymer Matrices
Hydrogels are widely utilized in biotechnology, including drug delivery, tissue engineering, food applications, cosmetics, and environmental remediation, requiring fabrication processes that ensure appropriate physico-mechanical properties. The properties of hydrogels are affected by factors such as the source of the biopolymer, its concentration or feed ratio, and the specific method used for hydrogel preparation. One common approach involves dissolving a single biopolymer in a solvent to form a three-dimensional hydrogel network. Another prevalent strategy is the creation of composite hydrogels, consisting of two or more polymers crosslinked through either single-step or multi-step processes. The presence of polar functional groups within the polymer chains, such as –NH2, –COOH, –OH, –CONH2, –CONH, and –SO3H, enhances the hydrophilic nature of the network and promotes water uptake [78]. The three-dimensional architecture of hydrogels is essential for maintaining their structural integrity and water absorption capacity [79].
4.2.1. Single-Polymer Hydrogel
A single-polymer hydrogel consists of a network formed from only one type of monomer, serving as the basic structural unit of any polymer network. This type of polymer can be formed with [80,81,82,83,84] or without [85,86,87,88,89,90,91] addition of any crosslinking agent. Owing to their unique features—including high molecular weight, porous 3D architecture, hydrophilicity, and biocompatibility—many natural polysaccharides, such as starch, cellulose, chitosan, agarose, and alginate, as well as proteins like collagen and gelatin, have been employed in hydrogel fabrication. However, in most instances, the effectiveness of this category of biopolymer hydrogels becomes limited when no crosslinker, such as genipin, is incorporated. Uncrosslinked biopolymer solutions often fail to form stable gels, leading to rapid dissolution and burst release, thereby necessitating crosslinking to achieve controlled release.
4.2.2. Multi-Polymer Hydrogel
Multi-polymer hydrogels are composed of two or more polymers, with at least one exhibiting hydrophilic properties. These hydrogels are typically fabricated using crosslinkers and/or fillers to improve their physico-mechanical characteristics. Composite hydrogels can be prepared via physical interactions, such as hydrogen bonding and hydrophobic forces, or through chemical methods like covalent crosslinking. A study indicates that hydrogels made from 1.5% genipin-crosslinked gelatin and chitosan demonstrate significant potential for on-demand drug delivery applications, particularly in the context of osteoarthritis treatment [92]. Xanthan gum–starch composite polymer has also been identified as a promising polysaccharide-based hydrogel, suitable for use as a drug delivery system [93]. Multi-polymer hydrogels also encompass interpenetrating polymer networks (IPNs), formed when a secondary hydrogel network is polymerized within an already polymerized hydrogel. This is typically achieved by immersing the pre-formed hydrogel in a solution containing monomers and a polymerization initiator. IPNs can be classified into two types: a full IPN, produced in the presence of a crosslinker, and a semi-IPN, formed without a crosslinking agent, where linear polymers are embedded within the original hydrogel network [94]. An example of a semi-IPN is the incorporation of linear cationic polyallylammonium chloride into acrylamide/acrylic acid multi-polymer hydrogels. This configuration not only enhanced the mechanical strength but also enabled a completely reversible pH-responsive release of theophylline [95]. The primary benefits of interpenetrating networks (IPNs) are the ability to make relatively dense hydrogel matrices that exhibit enhanced stiffness and toughness in their mechanical properties, along with the capacity to control their physical properties. Drug loading is usually done simultaneously with the polymerization of the interpenetrating hydrogel phase [96].
Polymer network systems (IPNs) consisting of carrageenan and guar gum, loaded with metronidazole as the model drug, were synthesized utilizing the microwave irradiation technique. The results indicated that the samples exhibited thermal stability and possessed a lower critical solution temperature ranging from 30 to 60 °C. Based on this investigation, it can be concluded that these IPNs are suitable for use as controlled release drug delivery systems, demonstrating potential for targeted drug delivery due to their responsiveness to external stimuli [97]. In another study, a pH-responsive interpenetrating polymer network was developed by combining two polysaccharides, Moringa bark gum (MOG) and carrageenan (CRG), using microwave irradiation, with metronidazole (MTZ) included as a model drug. The matrices possessed all the essential attributes necessary for an effective controlled delivery system, in addition to functioning as a tissue scaffold [98]. Successful in vitro trials have been conducted; however, extensive exploration is required, particularly in various animal models and through clinical trials, prior to application.
Hybrid Crystalline–Hydrogel Composites
While the multipolymer hydrogels discussed above combine two or more polymers to tune release properties, another emerging strategy involves hybrid crystalline–hydrogel composites. In these systems, crystalline drug domains are embedded within a hydrogel—often a single polymer—to achieve sustained and highly controlled release. Long-term stability studies at 4 °C demonstrated that crystalline amylase exhibits greater stability and prolonged release compared to its amorphous counterpart [99]. Amorphous systems, in contrast, frequently compromise protein stability due to exposure to stressors such as aqueous–organic interfaces, hydrophobic surfaces, detergents, elevated temperatures, and vigorous agitation. For instance, mechanical agitation of insulin can induce aggregation into amyloid-type fibrils, reducing its efficacy and bioavailability, while the gene therapy Zolgensma has a short 14-day shelf life, cannot be agitated, and must be stored at 2–8 °C [100]. These limitations restrict wider application. To address this, hybrid crystalline–hydrogel composites have been developed that stabilize proteins against thermal denaturation even at 50 °C and allow excipient-free protein delivery via mechanical release from a syringe [100]. Crystallization of insulin within agarose and fluorenylmethoxycarbonyl-dialanine (Fmoc-AA) hydrogels has also been successfully achieved, with insulin crystals remaining stable at 50 °C for seven days. Furthermore, insulin crystals grown in Fmoc-AA hydrogels exhibited enhanced thermal stability, remaining intact up to 60 °C over 24 h [101]. These findings highlight that the choice of hydrogel matrix significantly influences the physicochemical properties of the crystals, suggesting that crystallization within different hydrogel systems offers a promising approach to expanding the range of novel biopharmaceutical formulations.
5. Key Factors Regulating the Release Rate of Bioactive Compounds
The primary factors influencing controlled release include the characteristics of bioactive components, the properties of the matrix, and the conditions of the surrounding release media, as illustrated in Figure 1. Accordingly, the upcoming sections will explore these three major strategies, supported by a review of recent studies.
5.1. Conditions of Release Media
The environmental conditions play a significant role in determining the appropriate structure necessary for regulating the release of the bioactive compound. This is because the conditions of the release media are crucial in influencing the structure of the hydrogels, which in turn impacts the release rate of the entrapped nutrients. Some examples are listed in Table 2.

Table 2.
Effect of release media conditions on the release rate of bioactive compounds.
5.1.1. Boundary Conditions
Boundary conditions, either stationary or moving, play a vital role in controlling the mass flux of diffusants from the hydrogels. Purely molecular diffusion due to a concentration gradient in stationary conditions can be easily accelerated providing moving boundary conditions in the system where the release of the bioactives is the combined effect of the concentration gradient and the motion of the release medium. In stationary conditions, the concentration of the released substance remains higher near the boundary, meaning that the difference in concentration of bioactives between the outside and inside the hydrogels becomes less, resulting in a reduced release rate. External boundary conditions, such as agitation of the release medium, generally enhance the diffusive release of drugs from hydrogel delivery systems by promoting mass transport between the hydrogel and the surrounding medium. Drug release has been reported to vary with agitation speed (e.g., 50, 150, and 250 rpm), with higher speeds accelerating release [128]. Typically, agitation is applied in the range of 50–150 rpm, and a continuous speed of around 100 rpm is recommended, as it approximates the flow conditions encountered in physiological environments such as the bloodstream [30].
Under internal moving boundary conditions, polymeric matrices can experience a swelling-driven phase transition, shifting from a glassy (dry) state to a rubbery (hydrated) state [129,130]. During swelling, a rapidly advancing boundary forms within the shrinking glassy phase, where immobilized microconstituents remain fixed, allowing dissolved bioactive compounds to move through the rubbery phase and be released into the surrounding aqueous medium [131,132]. Therefore, the rate and position of the boundary between the glassy and rubbery regions of the polymer matrix are key determinants of the diffusion kinetics of solutes [81,133].
5.1.2. pH
The release of bioactive agents is significantly affected by pH through several mechanisms. In particular, pH can modify functional groups, such as carboxyl and amine groups, in polymers like polymethacrylic acid, polyacrylamide, and poly (dimethylaminoethyl methacrylate). Acting as a proton donor or acceptor, pH facilitates the release of bioactive compounds in Figure 2. This process occurs via changes in polymer hydrophobicity, structural deformation, conformational shifts, and polymer dissolution [134].

Figure 2.
A schematic illustrating the typical behavior of pH-responsive polymeric hydrogels.
Cationic hydrogels expand in acidic conditions and contract under basic conditions, releasing their payload, whereas anionic hydrogels behave in the opposite manner, adapted from reference [27].
Changing the pH from 5.5 to 8.5 led to an increase in the cumulative release of naringenin from carboxymethyl cellulose/2-hydroxyethyl acrylate, rising from 42% to 73% [135]. he release of β-carotene from genipin-crosslinked K-carrageenan/carboxymethyl cellulose beads was approximately 2.5 times lower in acidic buffer (pH 1.2) compared to near-neutral conditions (pH 7.4) [136]. At a pH 7.4, the beads exhibited swelling as a result of the electrostatic repulsion among the ionized carboxyl groups of carboxymethyl cellulose, which engage in hydrogen bonding under acidic conditions. Conversely, a different trend was noted regarding the release of lysozyme from gelatin/genipin films [137]. Lysozyme was released rapidly under acidic conditions (pH 3.8) compared to neutral pH (7.0). This burst release from the composite system was linked to film degradation, triggered by acid hydrolysis of gelatin networks and the breakdown of genipin–gelatin crosslinks at low pH. Similarly, lowering the pH from 7.4 to 3.0 increased the release of curcumin from 1,3-bis(N,N-dimethyl-N-cetylammonium)-2-propyl acrylate dibromide, which was attributed to reduced electrostatic interactions due to protonation of functional groups [138], whereas curcumin release from alginate hydrogel nanoemulsions was significantly higher under alkaline conditions (pH 9.0), due to accelerated degradation of sodium alginate gel beads [139]. The structure of genipin-crosslinked BSA networks was examined using scanning electron microscopy (SEM), as illustrated in Figure 3. The swelling at alkaline pH levels resulted in changes to the structural characteristics of the BSA matrix. A qualitative correlation was observed between the progressively more open structures of the protein matrix, as indicated by SEM, and the decline in mechanical properties noted through rheological measurements [140].
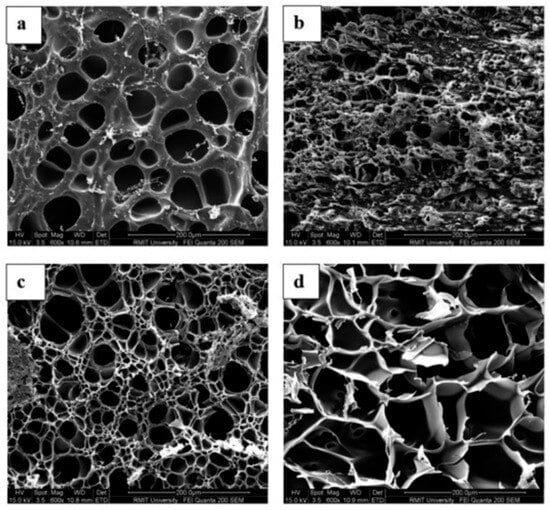
Figure 3.
The morphology of BSA networks crosslinked with 0.5 g/100 g genipin was visualized by SEM at pH (a) 2.0, (b) 4.8, (c) 7.0, and (d) 11, using 200× magnification at 24 °C, adapted from reference [136].
This pH sensitivity was utilized in entrapping and releasing of probiotics from ethylenediaminetetraacetic-calcium-alginate (EDTA-Ca-Alg) system. In an acidic environment, the hydrogel maintained a compact structure with minimal pore formation, ensuring the protection of probiotics, L. rhamnosus ATCC 53103. Conversely, in a neutral intestinal environment, the hydrogel structure progressively disintegrated due to the release of Ca2+ from the hydrogel, which led to the release of cells [109]. Similarly, isoquercitin (IQ) loaded okra polysaccharides/gelatin complex coacervates (OGIQ) demonstrated pH responsiveness and targeted delivery to the intestine. The release rate of IQ from OGIQ was measured at 89.4% in intestinal fluid, while it remained below 2% during acidic and simulated gastric digestion [102].
Indeed, among the various chemical factors, the influence of pH stands out as a critical determinant in the release of bioactive substances [141]. It acts as an independent regulator of release behavior by altering the ionization state of functional groups, polymer conformation, and network integrity. For instance, in gelatin-based hydrogels, low pH conditions can trigger burst release due to acid-induced hydrolysis of the gelatin. Conversely, in BSA based hydrogels, alkaline conditions lead to swelling and structural degradation, which progressively opens the network and facilitates diffusion. These observations collectively demonstrate that pH-driven structural transitions directly govern the release mechanism. Additionally, pH acts synergistically with other factors, including ionic strength. The electrostatic interactions between carrier and entrapped bioactive compound, which ultimately govern its release, are significantly influenced by pH levels.
5.1.3. Ionic Profile
The ionic properties of the release medium can facilitate the diffusion of bioactive compounds by promoting the loosening of crosslinking points within the biopolymer network or by accelerating matrix erosion [142,143,144]. Ions in the release medium can significantly affect the release rate of bioactive compounds. In cases where bioactive molecules interact with polymers through coordinative bonds, the presence of ions or competing ligands may alter these interactions. To evaluate the bioaccessibility and release of vitamin E encapsulated in oil-in-water emulsions, a simulated small intestinal fluid was employed. The results showed that the addition of calcium ions enhanced the release rate of vitamin E in this system [145]. The ionic strength influences the expansion of polymers containing ionizable groups by modifying their solubility and swelling characteristics. In contrast, nonionizable polymers remain unaffected by changes in ionic strength. When the ionic strength exceeds a certain level, complexes can dissociate as a result of reduced electrostatic interactions, a process referred to as the electrostatic screening effect. Additionally, the swelling behavior of hydrogels made from sodium carboxymethyl cellulose is influenced by the ionic strength of the surrounding medium [146].
In vitro drug release from biopolymeric matrices is influenced not only by the pH and ionic strength of the dissolution medium but also by the presence of electrolytes [147]. The effect of the dissolution medium on the in vitro release of drugs from ionically gelled pectinate beads was investigated. It was observed that rutin was released more rapidly from calcium pectinate beads when phosphate buffers were used, likely due to the formation of CaHPO4 precipitate, which can create a “pumping” effect on calcium ions, potentially disrupting the gel network and enhancing rutin release. In the case of zinc pectinate beads, two distinct precipitates may form depending on the electrolyte composition of the medium. The formation of Zn3(PO4)2, which can coat the beads, may slow rutin release in Sorensen’s buffer, whereas the generation of ZnHPO4 could promote zinc ion release and thereby increase rutin release in a citrate-phosphate buffer [148]. Another study was conducted to evaluate the effect of the conditions of release medium changing bilayer numbers of the matrix, temperature, pH, and ionic strength. It was observed that bioactive release (tannic acid) from antioxidative cellulose nanofiber films (polyethylene glycol/tannic acid films) was found to increase with a higher number of bilayers, as well as with elevated pH and temperature; however, it decreased with an increase in ionic strength [122].
5.1.4. Nature of Solvent
The capacity of the solvent to dissolve the bioactive compound is essential for its liberation from the hydrogel. It is important that the polarity of the solvent and bioactive compound is compatible. Polar bioactives tend to dissolve better in polar solvents (like water), while non-polar bioactives prefer non-polar solvents. The solvent’s interaction with the hydrogel polymer matrix is also important. The release kinetics of a bioactive compound are influenced by the ability of the solvent to penetrate the hydrogel matrix and by the partitioning behavior of the bioactive between the solvent and the hydrogel [149]. Several studies have shown that the polarity of the surrounding medium can affect the release of bioactive compounds from different carrier systems. For example, the release behavior of gallic acid encapsulated in poly(lactic acid) nanofibers was assessed in aqueous ethanol solutions at concentrations of 10% and 95% [150]. Due to the hydrophilic nature of gallic acid, its highest release rates were observed in both pure aqueous and 10% ethanol solutions. Similarly, the release behavior of oregano essential oil and green tea extract encapsulated in ethylene-PVA films was studied in 3% acetic acid, 10% ethanol, and 50% ethanol solutions. The films demonstrated the greatest antioxidant release in the 50% ethanol medium [151]. The release behavior of polyphenols from starch–chitosan films is strongly influenced by both the pH and polarity of the solvent [151], such that fewer polyphenols are released under acidic conditions, and the release occurs at a slower rate compared to both hydrophilic and lipophilic environments.
A high diffusion rate of bioactive compounds in low-polarity media has also been reported for carvacrol released from poly(ε-caprolactone) [152], and catechin (tea polyphenol) from chitosan-PVA film [153]. In contrast, The rate of release of rosemary polyphenols into ethanol, which serves as a fatty food simulant, was observed to be slower in comparison to the release into an aqueous medium [154]. Comparable results were reported in a study investigating the release of Mentha longifolia L. essential oil from Balangu seed gum–polyvinyl alcohol (PVA) nanocapsules in different media, including distilled water, 10% ethanol, 50% ethanol, and 3% acetic acid. The essential oil exhibited the highest release in distilled water, followed sequentially by 10% ethanol, 50% ethanol, and 3% acetic acid. The study also highlighted the considerable solubility and swelling ability of Balangu seed gum and PVA in water, whereas both components were poorly soluble in ethanol and acetic acid, with the gum being only slightly soluble in acetic acid [155]. An opposite trend was observed in another investigation, where the release kinetics of polyphenols from thyme extract (TE) incorporated in chitosan (CH) films were assessed using three different solvents: acetic acid solution, distilled water, and ethanol. The highest quantity of polyphenols was observed in the acetic acid solution, attributed to the enhanced solubility of chitosan. Consequently, the polarity of the solvents significantly influenced the release of polyphenols from the films [116].
5.1.5. Temperature
Temperature serves as a crucial physical environmental factor. It is widely recognized that variations in temperature significantly influence molecular motion. The diffusion process accelerates with an increase in temperature, as structural relaxation generates voids within the matrix, facilitating the swift movement of penetrants through these channels [156]. Initial research on breakfast cereals has shown a significant correlation between water absorption and temperature increase, consistent with the principles outlined in the Arrhenius law [157,158]. The glass transition temperature serves as a useful indicator for observing diffusion processes. As temperature rises and approaches the glass transition zone, there is an increase in the available voids, which facilitates the movement of diffusing component [156,159]. Rising temperatures supply thermal energy that enables the overcoming of attractive forces between polymer chains and bioactive molecules, thereby improving transport phenomena. In an investigation, novel stimuli-responsive hybrid hydrogels were developed using succinylated cellulose nanocrystals (Su-CNC). The innovation involved incorporating Su-CNC with varying degrees of substitution (DS) into the hydrogel matrix, enabling it to exhibit pH and thermal responsiveness through a free radical polymerization reaction with poly(N-isopropylacrylamide) (PNIPAm). It demonstrated a significant response to temperature variations, as its swelling behaviour and hydrophilicity decreased at temperatures of 35 °C and above. This change resulted in increased hydrophobicity and subsequent hydrogel contraction [123]. To observe the effect of temperature on release profile, an investigation was conducted on an Amazonian fruit named camu-camu. The fruit extract, including both pulp and peel, was microencapsulated via spray drying using maltodextrin, inulin, and oligofructose as carriers. The resulting microcapsules were evaluated for their physicochemical and thermal properties, as well as their controlled release behavior at different temperatures (25 °C and 35 °C). Increasing the temperature from 25 °C to 35 °C led to an enhanced release of bioactive compounds in all formulations [121]. Thermo-responsive hydrogels alter their hydrophilic–hydrophobic balance at the lower critical solution temperature (LCST); for example, PNIPAm has an LCST of ~32 °C. Bioactive release increases below the LCST due to swelling and thermal activation, but decreases above it as the hydrogel’s hydrophilic character diminishes with the loss of intermolecular hydrogen bonds.
5.2. Hydrogel Properties
In a delivery system, natural bioactives are entrapped within different biopolymers, which significantly influence the release rate of these entrapped compounds according to requirement.
5.2.1. Polymer Types
Various biopolymers, such as carbohydrates, proteins, and their blends, can be employed to develop delivery systems for different bioactive compounds. The structural characteristics of biomaterials, built by molecular organization and concentration, play a vital role in the diffusion of bioactives from either individual or composite formulations. Material composites are typically favoured for their ability to provide the necessary techno-functionality that supports bio-functionality through the regulation of drug release. The combination of natural polymers leads to the formation of binary systems characterized by either a phase-separated or an associated network structure [160]. Polymers of lower molecular weight enhance the available free volume within the matrix, reduce the activation energy required for conformational changes between states, and promote faster diffusion kinetics. In contrast, higher molecular weight polymers form entanglements that resemble a cage-like structure, thereby limiting the mobility of diffusing substances [161,162]. In vitro studies showed that β-carotene encapsulated in zein protein was rapidly released in the gastric environment, due to the positive charge of zein and protonation of β-carotene, which weakened their interaction.
In contrast, the presence of pepsin leads to the degradation of zein, weakening its structure and facilitating the release of β-carotene in the gastric environment. On the other hand, encapsulation of β-carotene using whey protein isolates and gum acacia produced a more sustained release during the gastric phase, due to the protective effect of these wall materials [163,164,165]. Curcumin encapsulated in Lactoferrin/pectin polyelectrolyte complex nanoparticles (PEC NPs) showed rapid release under acidic conditions. This phenomenon can be attributed to the electrostatic interactions among the carrier components, which lead to a disruption in the structure of the PEC NPs, resulting in a swift release. Conversely, curcumin encapsulated with chitosan and gum Arabic demonstrated a slower release in the gastric environment. This behavior suggests that the polymer matrix has a more stable structure and greater swelling capacity, attributed to the amino groups present in chitosan [166,167].
5.2.2. Polymer Crosslinking
The strategy of employing proteins in carrier-mediated transport presents promising opportunities for advancements in biology and chemistry, particularly in the realm of drug delivery. However, the unavoidable infiltration of water molecules results in network degradation and an unintended rapid release of the drug, which should be prevented, as it diminishes the treatment’s effectiveness [168,169]. Crosslinking is considered a valuable strategy for designing delivery systems that can achieve tailored release kinetics for specific therapeutic needs [170]. Cross-linking is a polymer chemistry technique that improves the stability of polymer chains by creating a network structure through multidimensional connections. A cross-link is a bond that links one polymer chain to another, which may be either covalent or ionic. This process converts a liquid polymer into a gel or solid state by restricting chain mobility. Moreover, cross-linking leads to an increase in the overall molecular weight of the polymer [171]. Crosslinking agents are generally classified into two categories: chemical and physical crosslinkers.
Chemical Crosslinking
A summary of chemical crosslinkers used in polymeric matrices has been listed in Table 3. Crosslinking agents that are suitable for oral delivery include genipin, transglutaminase, anhydrous tripolyphosphate, glutaraldehyde, and citric acid. These agents have been used to alter the structural characteristics of delivery systems by either forming interactions with oppositely charged polymer chains or through enzyme-mediated crosslinking mechanisms [29]. However, the production of hydrogels for biomaterial applications necessitates meticulous planning and the appropriate choice of the crosslinking method. This process significantly influences the chemical and mechanical characteristics of the hydrogel, which in turn impacts the cellular responses elicited by the hydrogel. The selection process must consider factors such as solubility, reaction conditions (including pH and temperature), potential side reactions, and the overall reactivity. Depending on the intended use, it is essential to evaluate the crosslinking rate, density, hydrogel stability, and the biocompatibility of both the crosslinker and the crosslinked biopolymers [172]. Chemical crosslinkers that exhibit cytotoxicity, such as glutaraldehyde [173], can raise cytocompatibility concerns, whereas genipin exhibits much lower toxicity [174]. A low level of crosslinking may lead to a hydrogel exhibiting poor mechanical strength and stability characteristics, while an excessive degree of crosslinking can cause reduced porosity, adversely affecting cell viability and biodegradation [172].

Table 3.
Chemical crosslinkers used in biopolymeric matrices for controlled delivery systems.
Genipin is a non-toxic crosslinking agent increasingly used in functional foods and pharmaceutical formulations. In the presence of oxygen, it reacts with lysine residues from neighboring protein chains, forming stable crosslinked structures [230]. In contrast to traditional chemical cross-linkers such as formaldehyde and glutaraldehyde, genipin offers several safety benefits, including anti-inflammatory, anticancer, and antibacterial properties. Consequently, genipin has been the subject of extensive research over recent decades, establishing itself as a safe and efficient chemical cross-linker across a wide range of delivery systems [29]. Genipin has been demonstrated to effectively limit the burst release of entrapped agent while prolonging the release duration [207]. It enhanced the stability of hydrogels under acidic conditions by reducing the availability of free amino groups in the polymer chain necessary for ionization, which led to a decreased swelling ratio and extent of release [193,196,202], especially a lower release rate at higher pH (pH 8), and a higher release rate in lower pH (pH 1.5) [209]. Moreover, genipin enhanced resistance to proteolytic degradation, thereby decreasing curcumin release in the gastric environment [203]. Compared with glutaraldehyde-crosslinked gels, genipin-crosslinked chitosan hydrogels exhibited lower swelling and more controlled release due to their greater rigidity and stability [205]. Furthermore, genipin was found to be effective in the entrapment and sustained release of vitamin B6 [80,81,140], n-3 fatty acids [210], lidocaine [192], and vitamins C and E [82,199], among others.
The enzymatic approach to crosslinking offers advantages including efficient catalysis, gentle reaction conditions, and non-toxicity. Hydrogels prepared enzymatically are highly biocompatible and degradable, positioning them as promising materials for use in food processing, tissue engineering, sustained-release formulations, bioplastics, and biosensing devices [231,232]. Derived from Streptoverticillium mobaraense, microbial transglutaminase (mTGase) is a transferase that promotes inter- and intramolecular crosslinking through amide bond formation between the carboxyamide moieties of acyl donors and lysine amino groups of acyl acceptors [233,234]. Microbial transglutaminase was employed to crosslink bitter apricot kernel protein, enabling a controlled release of riboflavin. The results indicated that the enzyme successfully generated crosslinks that increased the gel’s hardness, elasticity, and cohesiveness, leading to a more sustained release profile. This formulation was identified as a promising carrier for protecting sensitive bioactive molecules, with potential utility in both food and pharmaceutical applications [176]. In other studies, transglutaminase crosslinking was found to be a promising approach for biopolymeric gels, offering improved stability, regulated release, and an enhanced system for delivering bioactive compounds [175,177,179,182]. However, it is essential to screen and assess cross-linking enzymes capable of crosslinking various substrates, given the extensive range and diversity of these substrates. Comprehensive research into the molecular level crosslinking mechanisms of enzymes and their substrates is also essential to establish a theoretical foundation for enhancing the properties of hydrogels.
Glutaraldehyde is widely used as a crosslinking agent in the preparation of polymer-based particles, films, and fibers designed for controlled delivery applications. Numerous investigations have shown that glutaraldehyde-crosslinked biopolymer matrices can modulate the release of therapeutic and bioactive compounds. In one study, post-treatment with glutaraldehyde (GTA) vapors was applied to electrospun zein nanofibers loaded with eugenol to improve their physicochemical properties and release behavior. Relative to the uncrosslinked fibers, the crosslinked systems preserved their fibrous morphology but exhibited increased fiber diameter and a denser structure. Achieving an appropriate crosslinking level was essential for enhancing the stability and mechanical integrity of the zein network. The GTA vapor-treated nanofibers provided a controlled release of eugenol in phosphate-buffered saline (PBS), demonstrating strong potential for enriched food products as well as smart or active food packaging applications [183].
In a similar vein, other researchers have documented comparable findings regarding the development of chitosan microspheres [184,185,187,190], gelatin nanoparticles [186,188], and silk fibroin film [189] intended for the delivery and controlled release of functional agents, utilizing glutaraldehyde as a crosslinking agent. Whey protein-based microcapsules were synthesized utilizing glutaraldehyde. Casein microspheres crosslinked with glutaraldehyde were shown to function effectively as carriers capable of regulating the diffusion of bioactive compounds. At pH 7.4, the microspheres exhibited enhanced pore development, resulting in greater swelling and consequently facilitating drug diffusion [235]. Similarly, the release observed under simulated intestinal conditions exceeded that occurred under simulated gastric conditions [236]. Although numerous studies have examined in vitro release profiles, far fewer have evaluated in vitro functional activities—such as antioxidant and antimicrobial effects, microbial penetration, and cell cytotoxicity—or conducted in vivo assessments of crosslinked delivery systems.
Dual Crosslinking
In certain instances, the use of dual crosslinkers improves the effectiveness of the crosslinking process, thereby enhancing the overall release profile of bioactive compounds from the matrices. A research investigation focused on the development of gelatin-chitosan microcapsules utilizing a dual cross-linking method involving transglutaminase (TGase) and tannic acid (TA). The application of dual cross-linking resulted in improved structural integrity of the microcapsules. The presence of TA allowed the dual cross-linked microcapsules to demonstrate superior controlled-release characteristics compared to those that were single cross-linked, as evidenced by a reduced cumulative release rate [227]. Likewise, studies on bovine serum albumin gels treated with both microbial transglutaminase and ribose to modulate caffeine release showed that the dual-crosslinking approach was more effective in simulated saliva and gastric fluids [237]. The comparable results were observed in other research studies [198,215,225,226].
Hydrogel crosslinking can occur via covalent or non-covalent interactions between polymer chains. Covalent bonds provide energetic stability, producing mechanically robust networks, while non-covalent interactions, such as hydrogen bonding, are sensitive to environmental factors like pH and temperature, influencing hydrogel formation, stability, and functional properties. By combining covalent and non-covalent mechanisms—i.e., using dual crosslinkers—it is possible to fine-tune network structure, mechanical strength, and release behavior, offering a versatile design strategy to optimize hydrogel performance. Overall, reported successful dual-crosslinking systems include transglutaminase–tannic acid, transglutaminase–ribose, genipin–disulfide, citric acid–calcium chloride, tripolyphosphate–dextran sulfate, and epichlorohydrin–calcium chloride–tannic acid.
Physical Crosslinking
A significant transformation has taken place in the food processing industry with the introduction of advanced non-thermal technologies, which have enhanced the quality, safety, and functionality of processed foods. Non-thermal methods, such as gamma radiation, ultrasound, and near-infrared techniques, utilize mechanical waves and the phenomenon of acoustic cavitation to rapidly alter physical structures, thereby promoting chemical reactions in proteins that affect their physicochemical and functional characteristics [238]. Physical crosslinking approaches help control the release of bioactive compounds by generating additional crosslinks—formed through interactions between atoms—and also improve the technological and functional properties of biopolymers by reinforcing their physical and mechanical structure. The research conducted in this area is compiled in Table 4.

Table 4.
Physical crosslinkers employed in controlled delivery systems.
Protein matrices can be formed through physical crosslinking methods, such as gamma irradiation and dehydrothermal treatment, which rely on various non-covalent interactions including ionic bonds, hydrogen bonding, van der Waals forces, and hydrophobic interactions. The effect of electron beam irradiation on fish gelatin films containing bamboo leaf antioxidants was investigated, showing that irradiation enhanced tensile strength, opacity, denaturation temperature, and microstructural properties. Infrared spectroscopy and microstructural analyses suggested that appropriate irradiation doses promoted the formation of crosslinking interactions between gelatin and the antioxidants, resulting in compact structures that slowed antioxidant release [243]. In a fibrous chitosan–curcumin polymer, exposure to 1 Gy of gamma irradiation resulted in the release of 5 ± 1% of the conjugated curcumin, whereas exposure to 6 Gy led to the release of 98 ± 1% of the conjugated curcumin [108,126,153,241]. Similarly, various physical crosslinking treatments, such as ultrasound [228], near-infrared [127], ultraviolet radiation [111], and microwave [248] demonstrated improved mechanical and release properties of crosslinked polymeric structures.
A combination of gelatin and phenylazide-conjugated poly(acrylic acid) was subjected to electrospinning under ultraviolet light. In contrast to the conventional crosslinking method utilizing glutaraldehyde vapor, the gelatin electrospun fibres (GESFs) that underwent UV crosslinking exhibited enhanced characteristics, including improved preservation of GESF morphology, consistent crosslinking throughout the fibres, reduced cytotoxicity, and maintained bio-functionality. L929 cells demonstrated superior growth on the UV-crosslinked GESF scaffolds in comparison to those crosslinked with glutaraldehyde [246]. In contrast, The films made from chemically (glutaraldehyde) crosslinked methylcellulose exhibited a homogeneous gel structure, while those produced from radiation crosslinked methylcellulose displayed a less uniform crosslinked composition [252].
Physical crosslinking treatments can accelerate the chemical crosslinking process. Vanillin cross-linked chitosan was synthesized using both the refluxing and microwave irradiation methods, which were subsequently compared. The refluxing method necessitated a reaction time of 6 h, while the microwave irradiation method achieved completion in just 1 to 5 min [224]. The sustained release of drug from a microwave-assisted pH-responsive hybrid polymer network, which consists of chitosan and gelatin cross-linked with glutaraldehyde, was documented by [103]. However, without microwave assistance, the same behaviour was not observed. That is why, in depth comparative analysis is needed to fill up the gaps.
In summary, chemical crosslinkers offer the advantage of easily designing and precisely controlling hydrogel release profiles over time. Naturally derived crosslinkers, such as microbial transglutaminase and genipin, serve as safer alternatives to conventional chemical crosslinkers. Moreover, the use of dual crosslinkers, combining complementary mechanisms, can further enhance network stability, mechanical strength, and tunable release behavior.
5.2.3. Effect of Plasticisers
Plasticizers are frequently incorporated into biopolymeric films to overcome certain limitations. These substances (outlined in Table 5) enhance macromolecules’ mobility by decreasing the interconnections between polymer chains.

Table 5.
Plasticizers used in biopolymeric matrices.
These additives increase the intermolecular spacing, or free volume, similar to the effect of temperature, and act as “mobility enhancers” that ultimately lower Tg [265,266,267]. Plasticisers also reduce the melting temperatures and crystallization [267]. The distinct characteristics of plasticisers contribute to an increased presence of the amorphous phase in the intermolecular structure of polymers. The integration of plasticisers with polymers enhances the ionic conductivity of polymeric films, all the while preserving their flexibility and thermal stability [268]. Findings from reference [269] indicate that an increase in plasticiser concentration resulted in a reduction in melting temperature and an enhancement of crystallinity in poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) when various types and concentrations of plasticisers were employed to enhance flexibility and elongation. The plasticizers evaluated in the study included propylene glycol, glycerol, triethyl citrate, castor oil, epoxidized soybean oil, and polyethylene glycol. It was found that medium molecular weight compounds possessing ether or ketone functionalities, which can interact effectively with PHBV, acted as the most efficient plasticizers. In contrast, long side chains appeared to hinder the interaction of ketone or hydroxyl groups with the PHBV matrix, while low molecular weight compounds containing hydroxyl groups showed negligible plasticizing effects. Among the tested agents, triethyl citrate and polyethylene glycol produced the most favorable thermal, mechanical, and barrier properties. These findings suggest that further research focusing on plasticizer selection based on their physicochemical characteristics may be beneficial.
In a separate investigation, it was observed that glycerol and sorbitol diminished the intermolecular forces between neighbouring chain segments, thereby increasing the spacing between them. This modification allowed water molecules to penetrate the more hydrated, solid-like matrices and supported the diffusion of small bioactive compounds into the surrounding medium [270]. Thus, the transport of potassium sorbate from chitosan films increased four times when palmitic acid was present. The improved diffusion was ascribed to the formation of voids within the films, arising from the multilayered architecture of the lipid–chitosan emulsion. Substantial swelling of the matrix, reaching roughly 40% of a single chitosan film’s thickness, allowed potassium sorbate to migrate freely through the chitosan network [271]. Another study explored the influence of plasticizers, specifically sorbitol and Tween 80 (T80), on the drug release behavior of rice starch wafers. Sorbitol produced a more compact structure, exhibiting higher puncture strength (PS) but lower water absorption capacity (WAC), whereas T80 led to a looser matrix, decreasing PS and increasing WAC. The smaller molecular size of sorbitol likely enabled stronger interactions with starch molecules, resulting in a denser wafer structure. This rigid network also contributed to reduced WAC and a slower, more sustained drug release [272].
5.2.4. Swelling and Microstructural Properties
Understanding the swelling behavior in food-based systems allows precise control over mechanical properties and diffusion rates, facilitating targeted delivery for a variety of biomedical and therapeutic applications [82]. he movement of entrapped molecules is subsequently affected by both the volume and rate of water penetrating the matrix. The swelling behavior of hydrogels is determined by factors such as crosslinking density, network mesh size, wall thickness, and the presence of hydrophilic functional groups within the gel [273]. Hydrogels with lower chemical crosslinking exhibited greater volumetric changes, which took place over shorter time intervals [84]. These distinct swelling properties likely result from the strong chemical crosslinks formed by genipin between gelatin chains, unlike the temporary physical interactions present in other matrices [171]. Interestingly, the results also indicated that incorporating bioactive compounds had little effect on the swelling behavior of the matrices. Instead, the extent of swelling was primarily governed by the concentration of genipin [92,274].
Although scanning electron microscopy imaging offers a topographical perspective of the superficial pores of the gel (Figure 3), the diameter of the mesh size can be estimated by applying the Flory-Rehner theory in conjunction with swelling measurements. Key parameters obtained from the swelling analysis include the polymer volume fraction, the molecular weight between crosslinks, the crosslink density, and the network mesh size of the polymer [81]. With increasing swelling, conventional triple-helix structures form through hydrogen bonding with water molecules, accompanied by the creation of chemical crosslinks. This leads to an apparent increase in the molecular weight between crosslinks. This phenomenon persists until the state of equilibrium swelling is achieved. The size of the mesh also increases in a comparable way, as the infusion of water molecules causes the voids between neighbouring chain segments to expand [82]. Because Flory–Rehner parameters directly determine network porosity, they also govern drug release kinetics: larger mesh sizes allow faster diffusion-driven release, whereas smaller meshes restrict molecular mobility and shift release toward relaxation- or erosion-controlled mechanisms. Thus, the structural parameters predicted by Flory–Rehner theory provide a quantitative basis for interpreting and predicting hydrogel release behavior. In a system crosslinked with 0.1% genipin, the initial measurements for polymer volume fraction, molecular weight between crosslinks, crosslink density, and mesh size were 0.901, 8.6 g/mol, 8985, and 3.22 nm, respectively, which changed to 0.756, 25.9 g/mol, 2904, and 5.97 nm after 24 h of immersion [84]. Swelling also decreases the total solids content of the system as water molecules are absorbed from the initial dry state of 93% (w/w) total solids to 42% (w/w), reaching equilibrium swelling after approximately 48 h (2900 min) [82]. It is noteworthy that the absorption of water during the swelling process does not interfere with the covalent bonds between genipin and gelatin molecules. This swelling causes the overall volume of the sample to expand, thereby decreasing the crosslink density, which represents the number of crosslinks per unit volume.
Within the gel system, the polymer volume fraction influences properties such as mechanical stability, opacity, and permeability. Increasing the amount of genipin crosslinker raises the polymer volume fraction by creating a more compact structure that restricts water uptake [275]. With higher polymer concentrations in the hydrogel, the release of bioactive compounds slows down, as their diffusion paths become more convoluted, requiring adjustments to the diffusion coefficients to account for increased tortuosity [276]. The swollen polymer volume fraction is determined by factors such as initial polymer content, molecular weight, branching, charge density, flexibility, crosslink type and density, configuration, and solvent–polymer interactions [277]. Raising the genipin concentration in BSA networks from 0.5% to 4% (w/w) resulted in a marked decrease in the swelling ratio, from 14.7 to 0.2, while the polymer volume fraction increased from 0.1 to 0.85. This denser network structure consequently slowed the release of the encapsulated vitamin B6 [80]. For the hydrogel crosslinked with 0.1% genipin, the polymer volume fraction declined from 0.901 to 0.756 over 1440 min, whereas a higher genipin concentration of 2% resulted in a decrease from 0.971 to 0.803 [84].
The mesh size, or correlation length (ξ), represents the linear distance between neighboring crosslinks and has been identified as a key structural factor influencing the controlled release of bioactive compounds from swellable carriers [278]. Most frequently, hydrogel mesh size is determined utilizing equilibrium swelling theory (i.e., Flory–Rehner) [279] or rubber elasticity theory [280], although the Mackintosh theory [281], the blob model [282], NMR [283], small-angle X-ray scattering [284], small angle neutron scattering [285], and correlations based on dextran diffusion [286] have also been used with success. The mesh size can be adjusted to regulate the controlled release of bioactive compounds, depending on factors such as the polymer type and concentration, the nature or extent of crosslinking, and external stimuli including pH, ionic strength, and temperature [277,287]. Increasing the genipin crosslinking concentration resulted in smaller mesh sizes, with values decreasing from 5.97 nm at 0.1% genipin to 3.97 nm at 2%, as stronger crosslinking drew gelatin polymer chains closer together [84]. Gelatin networks crosslinked at 25 °C exhibited pore sizes below 50 μm, while crosslinking at lower temperatures produced less compact structures with larger pores [288]. At neutral pH, the release of vitamin B6 was markedly slowed due to a reduction in mesh size from 59.1 to 1.1 nm, whereas at alkaline pH (11.0), the protein network’s molecular pore size expanded, facilitating the release of vitamin B6 into the surrounding medium [80].
5.3. Characteristics of Bioactive Compounds
In addition to the matrix density, crosslinking, and molecular interactions between the polymer network and the bioactive agent discussed earlier, the morphological characteristics of penetrant molecules—such as size, shape, conformational chemistry, and ionic nature—also significantly influence the behavior of delivery vehicles. The ability of a penetrant to diffuse into the polymeric matrix depends on both these interactions and the available free space, and this step often becomes the rate-limiting factor in the release of the bioactive compound [289]. The outcome can be either beneficial or detrimental, depending on the properties of the active compound and the specific biopolymers used as the matrix [35].
Penetrant compounds can enhance the physical and functional characteristics of edible films by facilitating the formation of cross-links among proteins and/or polysaccharides [290,291]. Furthermore, modifying the film structure via these cross-linking reactions can affect the retention of active compounds within the polymer network, thereby enabling controlled adjustments in their release [241,292]. Ascorbic acid was identified as a plasticizer for films made from potato starch and polyvinyl alcohol (PVA). The addition of ascorbic acid at a concentration of 20% resulted in a 35% reduction in the tensile strength (TS) of the PVA-based films, while simultaneously increasing the elongation at break by a factor of five [293]. The presence of functional groups in ascorbic acid facilitates a more effective interaction between starch and PVA molecules, potentially improving their mechanical properties. In contrast, the incorporation of tartaric acid at 1% w/w of dry matter into carboxymethylcellulose films did not exhibit any anti-plasticizing or crosslinking effects, nor did it enhance the mechanical properties of the films. This lack of effect may be attributed to the insufficient formation or absence of chemical bonds at this concentration of organic acid. In a separate study, the addition of lysozyme was frequently found to adversely affect the mechanical properties of edible films and coatings, primarily due to its degradative impact on polymer chains, particularly in protein-based films. Specifically, the tensile strength (TS) and elongation at break (EAB) of chitosan films decreased with increasing lysozyme concentration [294].
When the particle size of the solute exceeds the mesh size of the polymer network, the solute cannot penetrate the matrix; this phenomenon is commonly referred to as the “screening effect” of the polymer [161]. Evidence of this phenomenon was observed in genipin-crosslinked casein systems during the preparation of “drug” formulations, where the infusion of a BSA solution into the casein xerogel was hindered due to the polymer network’s screening effect [295]. The movement of large penetrant molecules can be regulated through chain disentanglement, structural relaxation, or swelling of the matrix, which effectively increases the network’s “apparent pore size” and enhances its utility [296]. Recently, a theoretical approach has been presented that predicts the diffusion coefficients of solutes diffusing from genipin crosslinked gelatin matrices [297]. This model showed a linear correlation between diffusion coefficient and molecular size of hydrophilic diffusants. The diffusants used were small molecules, with molecular weights ranging from 58.44 to 342 g/mol. Therefore, investigating diffusants with higher molecular weights could offer valuable insights into the influence of solute size on diffusion and holds strong potential for further exploration.
The shape of the penetrant molecule also exerts a significant influence on transport phenomena [298]. The atomic arrangement of molecules, which determines their well-defined morphology, influences their binding capacity to neighboring penetrant molecules and/or polymeric network chains [299].
6. Conclusions and Prospects
For the sustained release and targeted delivery of bioactive compounds, it is essential to comprehend the mechanisms of digestion and absorption of these compounds before creating suitable carriers for their transport. It is crucial for carriers to uphold stability in the face of adverse environmental conditions and to prevent the premature release of entrapped compounds. Additionally, solute must possess the capability to diffuse effectively into the targeted area and release the bioactive compounds as required. Various strategies were proposed to create resilient structures that can release bioactives in a controlled and preferred manner. To effectively manage the release of bioactive compounds from various structures, it is essential to consider several critical factors, including the properties of the release medium, the characteristics of the hydrogel, and the inherent properties of the bioactive compounds themselves. The release conditions of media, particularly pH, ionic properties, temperature, and solvent characteristics, play a vital role in the liberation of bioactive substances. These factors influence the functional groups, the electrostatic interactions between the carrier and the encapsulated bioactive compound, as well as the dissociation and conformational alterations of polymers.
The properties of delivery systems can be modulated through the selection of polymers, cross-linkers, plasticizers, and specific environmental conditions. Despite the considerable volume of research on release profiles, there remains a significant shortage of studies examining the functional activities of delivery systems crosslinked by chemical crosslinkers, including antioxidant and antimicrobial properties, microbial penetration assays, and cell cytotoxicity evaluations. Also, in-depth investigation into the crosslinking mechanism in molecular level between enzymic crosslinkers and their substrates is crucial for developing a theoretical framework aimed at improving the characteristics of hydrogels. Comparative investigations of crosslinkers, both single and multiple, remain inadequately documented. Therefore, a thorough comparative analysis would be beneficial in addressing these gaps. Furthermore, a critical gap exists in understanding the in vivo degradation rate of crosslinked hydrogels and its direct impact on the release mechanism—diffusion-controlled versus erosion-controlled. Future research could explore the development of smart, stimuli-responsive hydrogels to achieve more precise and controlled delivery of bioactive compounds.
Author Contributions
Conceptualization, M.R., S.T. and S.K.; Methodology, M.R., S.T., A.R. and S.K.; Software, M.R.; Validation, M.R., S.T., P.R.R., H.A.A., A.R. and S.K.; Formal Analysis, M.R.; Investigation, M.R., S.T., P.R.R., H.A.A., A.R. and S.K.; Resources, M.R., S.T., P.R.R., H.A.A., A.R. and S.K.; Data Curation, M.R., S.T., P.R.R., H.A.A., A.R. and S.K.; Writing—Original Draft Preparation, M.R.; Writing—Review & Editing, M.R., S.T., P.R.R., H.A.A., A.R. and S.K.; Supervision, S.T. and S.K.; Project Administration, S.T., A.R. and S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
This research is supported by RMIT Research Stipend Scholarship.
Conflicts of Interest
Author Shahla Teimouri was employed by the company Lactalis Australia. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Banwo, K.; Olojede, A.O.; Adesulu-Dahunsi, A.T.; Verma, D.K.; Thakur, M.; Tripathy, S.; Singh, S.; Patel, A.R.; Gupta, A.K.; Aguilar, C.N. Functional importance of bioactive compounds of foods with Potential Health Benefits: A review on recent trends. Food Biosci. 2021, 43, 101320. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Bento, C.; Gonçalves, A.; Jesus, F.; Simões, M.; Silva, L. Bioactive Compounds: Sources, Properties and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2017. [Google Scholar]
- Joye, I.J.; Nelis, V.A.; McClements, D.J. Gliadin-based nanoparticles: Fabrication and stability of food-grade colloidal delivery systems. Food Hydrocoll. 2015, 44, 86–93. [Google Scholar] [CrossRef]
- Vozza, G.; Khalid, M.; Byrne, H.J.; Ryan, S.M.; Frias, J.M. Nutraceutical formulation, characterisation, and in-vitro evaluation of methylselenocysteine and selenocystine using food derived chitosan: Zein nanoparticles. Food Res. Int. 2019, 120, 295–304. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Costa, A.M.; Nunes, J.; Lima, B.; Pedrosa, C.; Calado, V.; Torres, A.; Pierucci, A. Effective stabilization of CLA by microencapsulation in pea protein. Food Chem. 2015, 168, 157–166. [Google Scholar] [CrossRef]
- Katouzian, I.; Jafari, S.M. Nano-encapsulation as a promising approach for targeted delivery and controlled release of vitamins. Trends Food Sci. Technol. 2016, 53, 34–48. [Google Scholar] [CrossRef]
- Đorđević, V.; Balanč, B.; Belščak-Cvitanović, A.; Lević, S.; Trifković, K.; Kalušević, A.; Kostić, I.; Komes, D.; Bugarski, B.; Nedović, V. Trends in encapsulation technologies for delivery of food bioactive compounds. Food Eng. Rev. 2015, 7, 452–490. [Google Scholar] [CrossRef]
- Opriș, O.; Mormile, C.; Lung, I.; Stegarescu, A.; Soran, M.-L.; Soran, A. An overview of biopolymers for drug delivery applications. Appl. Sci. 2024, 14, 1383. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A. Encapsulation and controlled release of bioactive compounds in lactoferrin-glycomacropeptide nanohydrogels: Curcumin and caffeine as model compounds. J. Food Eng. 2016, 180, 110–119. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Llavata-Cabrero, B.; Martínez-Sanz, M.; Fabra, M.J.; López-Rubio, A. Self-assembled gelatin-ι-carrageenan encapsulation structures for intestinal-targeted release applications. J. Colloid Interface Sci. 2018, 517, 113–123. [Google Scholar] [CrossRef]
- Fazal, T.; Murtaza, B.N.; Shah, M.; Iqbal, S.; Rehman, M.-u.; Jaber, F.; Dera, A.A.; Awwad, N.S.; Ibrahium, H.A. Recent developments in natural biopolymer based drug delivery systems. RSC Adv. 2023, 13, 23087–23121. [Google Scholar] [CrossRef]
- Dumontel, B.; Conejo-Rodríguez, V.; Vallet-Regí, M.; Manzano, M. Natural biopolymers as smart coating materials of mesoporous silica nanoparticles for drug delivery. Pharmaceutics 2023, 15, 447. [Google Scholar] [CrossRef]
- Che, E.; Gao, Y.; Wan, L.; Zhang, Y.; Han, N.; Bai, J.; Li, J.; Sha, Z.; Wang, S. Paclitaxel/gelatin coated magnetic mesoporous silica nanoparticles: Preparation and antitumor efficacy in vivo. Microporous Mesoporous Mater. 2015, 204, 226–234. [Google Scholar] [CrossRef]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Esfanjani, A.F.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Kuai, L.; Liu, F.; Chiou, B.-S.; Avena-Bustillos, R.J.; McHugh, T.H.; Zhong, F. Controlled release of antioxidants from active food packaging: A review. Food Hydrocoll. 2021, 120, 106992. [Google Scholar] [CrossRef]
- Far, B.F.; Naimi-Jamal, M.R.; Jahanbakhshi, M.; Rostamani, H.; Karimi, M.; Keihankhadiv, S. Synthesis and characterization of chitosan/collagen/polycaprolactone hydrogel films with enhanced biocompatibility and hydrophilicity for artificial tendon applications. Int. J. Biol. Macromol. 2023, 253, 127448. [Google Scholar]
- Russo, E.; Villa, C. Poloxamer hydrogels for biomedical applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Alammar, A.; Park, S.-H.; Ibrahim, I.; Arun, D.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Wang, L.; Luo, Y.; Song, Y.; He, X.; Xu, T.; Zhang, X. Hydrogel-functionalized bandages with Janus wettability for efficient unidirectional drug delivery and wound care. ACS Nano 2024, 18, 3468–3479. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, C.; Wang, X.; Wang, Y.; Sun, K.; Li, L.; Fan, Y.; Hu, L. A hydrogel-based biosensor for stable detection of glucose. Biosens. Bioelectron. 2023, 221, 114908. [Google Scholar] [CrossRef]
- Dragan, E.S.; Perju, M.M.; Dinu, M.V. Preparation and characterization of IPN composite hydrogels based on polyacrylamide and chitosan and their interaction with ionic dyes. Carbohydr. Polym. 2012, 88, 270–281. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Raina, N.; Pahwa, R.; Bhattacharya, J.; Paul, A.K.; Nissapatorn, V.; de Lourdes Pereira, M.; Oliveira, S.M.; Dolma, K.G.; Rahmatullah, M.; Wilairatana, P. Drug delivery strategies and biomedical significance of hydrogels: Translational considerations. Pharmaceutics 2022, 14, 574. [Google Scholar] [CrossRef] [PubMed]
- Sabaghi, M.; Tavasoli, S.; Hoseyni, S.Z.; Mozafari, M.; Degraeve, P.; Katouzian, I. A critical review on approaches to regulate the release rate of bioactive compounds from biopolymeric matrices. Food Chem. 2022, 382, 132411. [Google Scholar] [CrossRef]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, D.; Regenstein, J.M.; Xia, W.; Dong, J. A comprehensive review on natural bioactive films with controlled release characteristics and their applications in foods and pharmaceuticals. Trends Food Sci. Technol. 2021, 112, 690–707. [Google Scholar] [CrossRef]
- Faisant, N.; Akiki, J.; Siepmann, F.; Benoit, J.; Siepmann, J. Effects of the type of release medium on drug release from PLGA-based microparticles: Experiment and theory. Int. J. Pharm. 2006, 314, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Saikia, C.; Das, M.K.; Ramteke, A.; Maji, T.K. Effect of crosslinker on drug delivery properties of curcumin loaded starch coated iron oxide nanoparticles. Int. J. Biol. Macromol. 2016, 93, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- GhavamiNejad, A.; Ashammakhi, N.; Wu, X.Y.; Khademhosseini, A. Crosslinking strategies for 3D bioprinting of polymeric hydrogels. Small 2020, 16, 2002931. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef]
- Harrison, E.H. Mechanisms involved in the intestinal absorption of dietary vitamin A and provitamin A carotenoids. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2012, 1821, 70–77. [Google Scholar] [CrossRef]
- Carazo, A.; Macáková, K.; Matoušová, K.; Krčmová, L.K.; Protti, M.; Mladěnka, P. Vitamin A update: Forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients 2021, 13, 1703. [Google Scholar] [CrossRef]
- Guéant, J.-L.; Guéant-Rodriguez, R.-M.; Alpers, D.H. Vitamin B12 absorption and malabsorption. Vitam. Horm. 2022, 119, 241–274. [Google Scholar]
- Temova Rakuša, Ž.; Roškar, R.; Hickey, N.; Geremia, S. Vitamin B12 in foods, food supplements, and medicines—A review of its role and properties with a focus on its stability. Molecules 2022, 28, 240. [Google Scholar] [CrossRef]
- Bianchi, J.; Wilson, F.A.; Rose, R.C. Dehydroascorbic acid and ascorbic acid transport systems in the guinea pig ileum. Am. J. Physiol.-Gastrointest. Liver Physiol. 1986, 250, G461–G468. [Google Scholar] [CrossRef]
- Coelho, S.C.; Estevinho, B.N.; Rocha, F. Recent advances in water-soluble vitamins delivery systems prepared by mechanical processes (electrospinning and spray-drying techniques) for food and nutraceuticals applications—A review. Foods 2022, 11, 1271. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; Furlanetto, T.W. Intestinal absorption of vitamin D: A systematic review. Nutr. Rev. 2018, 76, 60–76. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Pišlar, M.; Kristl, A.; Roškar, R. Comprehensive stability study of vitamin D3 in aqueous solutions and liquid commercial products. Pharmaceutics 2021, 13, 617. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E bioavailability: Mechanisms of intestinal absorption in the spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, S.; Ji, Y.; Chen, H.; Zhang, H.; Chen, W.; Gu, Z.; Chen, Y.Q. Dietary intake of n-3 PUFAs modifies the absorption, distribution and bioavailability of fatty acids in the mouse gastrointestinal tract. Lipids Health Dis. 2017, 16, 10. [Google Scholar] [CrossRef]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In vivo formed metabolites of polyphenols and their biological efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Piscopo, R.; Coppola, F.; Almeida, Â.; De Marchi, L.; Russo, T.; Esteves, V.I.; Soares, A.M.; Pretti, C.; Chiellini, F.; Polese, G. Effects of temperature on caffeine and carbon nanotubes co-exposure in Ruditapes philippinarum. Chemosphere 2021, 271, 129775. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tang, K.; Wu, S.; Liu, L.; Qiang, C.; Lin, X.; Liu, B. Astragalus polysaccharides lowers plasma cholesterol through mechanisms distinct from statins. PLoS ONE 2011, 6, e27437. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.; Gomez-Cabrera, M.; Vina, J. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxidative Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, W.; Wang, J.; Wang, Y.; Zhang, Y.; Cao, J. The intestinal delivery systems of ferulic acid: Absorption, metabolism, influencing factors, and potential applications. Food Front. 2024, 5, 1126–1144. [Google Scholar] [CrossRef]
- Zhao, Z.; Egashira, Y.; Sanada, H. Digestion and absorption of ferulic acid sugar esters in rat gastrointestinal tract. J. Agric. Food Chem. 2003, 51, 5534–5539. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef]
- Gammeltoft, S. Receptor-mediated endocytosis and degradation of polypeptide hormones, growth factors, and neuropeptides. In Degradation of Bioactive Substances; CRC Press: Boca Raton, FL, USA, 2024; pp. 81–111. [Google Scholar]
- Li, Z.; Jiang, H.; Xu, C.; Gu, L. A review: Using nanoparticles to enhance absorption and bioavailability of phenolic phytochemicals. Food Hydrocoll. 2015, 43, 153–164. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Aslam, S.; Ahmad, M.; Riaz, M. Stability of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 251–315. [Google Scholar]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids diet: Digestion, gut microbiota modulation, and inflammatory diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yin, R.; Tian, Y.; Xu, S.; Meng, X. Curcumin nanopreparations: Recent advance in preparation and application. Biomed. Mater. 2024, 19, 052009. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In vitro gastrointestinal digestion study of broccoli inflorescence phenolic compounds, glucosinolates, and vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of quercetin: Problems and promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Abdulkarim, M.; Agulló, N.; Cattoz, B.; Griffiths, P.; Bernkop-Schnürch, A.; Borros, S.G.; Gumbleton, M. Nanoparticle diffusion within intestinal mucus: Three-dimensional response analysis dissecting the impact of particle surface charge, size and heterogeneity across polyelectrolyte, pegylated and viral particles. Eur. J. Pharm. Biopharm. 2015, 97, 230–238. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Aura, A.-M.; Hollman, P.C.; Gruppen, H. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl) acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J. Agric. Food Chem. 2009, 57, 1084–1092. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, Y.; Liang, Y.; Ma, X.; Qi, X.; Xiao, J.; Xue, W.; Luo, Y.; Yue, T. Advance on the absorption, metabolism, and efficacy exertion of quercetin and its important derivatives: Absorption, metabolism and function of quercetin. Food Front. 2020, 1, 420–434. [Google Scholar] [CrossRef]
- Ottaviani, J.I.; Momma, T.Y.; Heiss, C.; Kwik-Uribe, C.; Schroeter, H.; Keen, C.L. The stereochemical configuration of flavanols influences the level and metabolism of flavanols in humans and their biological activity in vivo. Free. Radic. Biol. Med. 2011, 50, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Renard, C.M. A Perspective on Carotenoids: Z/E-Isomerization, Extraction by Deep Eutectic Solvents and Applications. In Dietary Carotenoids-Sources, Properties, and Role in Human Health; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Yuan, D.; Guo, Y.; Pu, F.; Yang, C.; Xiao, X.; Du, H.; He, J.; Lu, S. Opportunities and challenges in enhancing the bioavailability and bioactivity of dietary flavonoids: A novel delivery system perspective. Food Chem. 2024, 430, 137115. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.; Jaiswal, S.G.; Dave, J.; Wei, S.; Hailu, G.G. Recent trends in the encapsulation of functional lipids: Comprehensive review. Sustain. Food Technol. 2024, 2, 1610–1630. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, J.; Chandrapala, J.; Majzoobi, M.; Brennan, C.; Zeng, X.A.; Sun, B. Recent progress of food-derived bioactive peptides: Extraction, purification, function, and encapsulation. Food Front. 2024, 5, 1240–1264. [Google Scholar] [CrossRef]
- Liu, B.; Jiao, L.; Chai, J.; Bao, C.; Jiang, P.; Li, Y. Encapsulation and targeted release. In Food Hydrocolloids: Functionalities and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 369–407. [Google Scholar]
- Niaounakis, M. Biopolymers: Applications and Trends; William Andrew: Norwich, NY, USA, 2015. [Google Scholar]
- Zabot, G.L.; Schaefer Rodrigues, F.; Polano Ody, L.; Vinícius Tres, M.; Herrera, E.; Palacin, H.; Córdova-Ramos, J.S.; Best, I.; Olivera-Montenegro, L. Encapsulation of bioactive compounds for food and agricultural applications. Polymers 2022, 14, 4194. [Google Scholar] [CrossRef]
- Dergunov, S.A.; Mun, G.A. γ-irradiated chitosan-polyvinyl pyrrolidone hydrogels as pH-sensitive protein delivery system. Radiat. Phys. Chem. 2009, 78, 65–68. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Teimouri, S.; Kasapis, S. Morphology of genipin-crosslinked BSA networks yields a measurable effect on the controlled release of vitamin B6. Food Chem. 2020, 314, 126204. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, S.; Morrish, C.; Panyoyai, N.; Small, D.M.; Kasapis, S. Diffusion and relaxation contributions in the release of vitamin B6 from a moving boundary of genipin crosslinked gelatin matrices. Food Hydrocoll. 2019, 87, 839–846. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Structural relaxation and glass transition in high-solid gelatin systems crosslinked with genipin. Int. J. Biol. Macromol. 2019, 141, 867–875. [Google Scholar] [CrossRef]
- Dimida, S.; Barca, A.; Cancelli, N.; De Benedictis, V.; Raucci, M.G.; Demitri, C. Effects of genipin concentration on cross-linked chitosan scaffolds for bone tissue engineering: Structural characterization and evidence of biocompatibility features. Int. J. Polym. Sci. 2017, 2017, 8410750. [Google Scholar] [CrossRef]
- Morrish, C.; Teimouri, S.; Kasapis, S. Structural manipulation of the gelatin/genipin network to inform the molecular transport of caffeine. Food Hydrocoll. 2023, 140, 108616. [Google Scholar] [CrossRef]
- Qu, X.; Wirsén, A.; Albertsson, A.C. Structural change and swelling mechanism of pH-sensitive hydrogels based on chitosan and D, L-lactic acid. J. Appl. Polym. Sci. 1999, 74, 3186–3192. [Google Scholar] [CrossRef]
- Willfahrt, A.; Steiner, E.; Hötzel, J.; Crispin, X. Printable acid-modified corn starch as non-toxic, disposable hydrogel-polymer electrolyte in supercapacitors. Appl. Phys. A 2019, 125, 474. [Google Scholar] [CrossRef]
- Ku, J.; Seonwoo, H.; Park, S.; Jang, K.-J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-laden thermosensitive chitosan hydrogel bioinks for 3D bioprinting applications. Appl. Sci. 2020, 10, 2455. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Dehabadi, L.; Wilson, L.D. Renewable starch carriers with switchable adsorption properties. ACS Sustain. Chem. Eng. 2018, 6, 4603–4613. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Lima, D.C.; Junior, M.D.M.; Le-Bail, P.; Le-Bail, A.; Augusto, P.E. Hydrogels based on ozonated cassava starch: Effect of ozone processing and gelatinization conditions on enhancing 3D-printing applications. Int. J. Biol. Macromol. 2019, 138, 1087–1097. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Tunable collagen I hydrogels for engineered physiological tissue micro-environments. PLoS ONE 2015, 10, e0122500. [Google Scholar] [CrossRef]
- Antoine, E.E.; Vlachos, P.P.; Rylander, M.N. Review of collagen I hydrogels for bioengineered tissue microenvironments: Characterization of mechanics, structure, and transport. Tissue Eng. Part B Rev. 2014, 20, 683–696. [Google Scholar] [CrossRef]
- Moshayedi, S.; Sarpoolaky, H.; Khavandi, A. Fabrication, swelling behavior, and water absorption kinetics of genipin-crosslinked gelatin–chitosan hydrogels. Polym. Eng. Sci. 2021, 61, 3094–3103. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Kaith, B.S.; Kaur, M.; Sharma, N.; Kumar, V. Cross-linked xanthan gum–starch hydrogels as promising materials for controlled drug delivery. Cellulose 2020, 27, 4565–4589. [Google Scholar] [CrossRef]
- Bayat, M.; Nasri, S. Injectable microgel–hydrogel composites “plum pudding gels”: New system for prolonged drug delivery. In Nanomaterials for Drug Delivery and Therapy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 343–372. [Google Scholar]
- Zhang, Y.; Wu, F.; Li, M.; Wang, E. pH switching on-off semi-IPN hydrogel based on cross-linked poly (acrylamide-co-acrylic acid) and linear polyallyamine. Polymer 2005, 46, 7695–7700. [Google Scholar] [CrossRef]
- Liou, G.-S.; Lin, P.-H.; Yen, H.-J.; Yu, Y.-Y.; Tsai, T.-W.; Chen, W.-C. Highly flexible and optical transparent 6F-PI/TiO2 optical hybrid films with tunable refractive index and excellent thermal stability. J. Mater. Chem. 2010, 20, 531–536. [Google Scholar] [CrossRef]
- Swain, S.; Bal, T. Carrageenan-guar gum microwave irradiated micro-porous interpenetrating polymer network: A system for drug delivery. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 256–265. [Google Scholar] [CrossRef]
- Roy, D.; Bal, T.; Swain, S. Fabrication and evaluation of pH-sensitive biocompatible microwave irradiated moringa barkgum-carrageenan (MOG-CRG-IPN) interpenetrating isotropic polymeric network for controlled delivery of pharmaceuticals. Sustain. Chem. Pharm. 2020, 18, 100325. [Google Scholar] [CrossRef]
- Pechenov, S.; Shenoy, B.; Yang, M.X.; Basu, S.K.; Margolin, A.L. Injectable controlled release formulations incorporating protein crystals. J. Control. Release 2004, 96, 149–158. [Google Scholar] [CrossRef]
- Bianco, S.; Hasan, M.; Ahmad, A.; Richards, S.-J.; Dietrich, B.; Wallace, M.; Tang, Q.; Smith, A.J.; Gibson, M.I.; Adams, D.J. Mechanical release of homogenous proteins from supramolecular gels. Nature 2024, 631, 544–548. [Google Scholar] [CrossRef]
- Contreras-Montoya, R.; Arredondo-Amador, M.; Escolano-Casado, G.; Manas-Torres, M.C.; González, M.; Conejero-Muriel, M.; Bhatia, V.; Díaz-Mochón, J.J.; Martínez-Augustin, O.; de Medina, F.S. Insulin crystals grown in short-peptide supramolecular hydrogels show enhanced thermal stability and slower release profile. ACS Appl. Mater. Interfaces 2021, 13, 11672–11682. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Li, X.; Zhang, Z.; Wei, Z.; Xing, Z.; Deng, S.; Duan, F. Okra polysaccharides/gelatin complex coacervate as pH-responsive and intestine-targeting delivery protects isoquercitin bioactivity. Int. J. Biol. Macromol. 2020, 159, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Jindal, R.; Jindal, D. RSM-CCD optimized microwave-assisted synthesis of chitosan and gelatin-based pH sensitive, inclusion complexes incorporated hydrogels and their use as controlled drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 48, 161–173. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, W.; Wang, X.; Zhao, Z.; Wang, Z.; Zhang, L. Multifunctional self-healing and pH-responsive hydrogel dressing based on cationic guar gum and hyaluronic acid for on-demand drug release. Int. J. Biol. Macromol. 2025, 301, 140326. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Network formation of Moringa oleifera gum by radiation induced crosslinking: Evaluation of drug delivery, network parameters and biomedical properties. Int. J. Biol. Macromol. 2018, 108, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Singh, S.K.; Mishra, S.; Pandey, J.P.; Sen, G. Gum ghatti based hydrogel: Microwave synthesis, characterization, 5-Fluorouracil encapsulation and ‘in vitro’drug release evaluation. Carbohydr. Polym. 2019, 222, 114979. [Google Scholar] [CrossRef]
- Shi, M.; Bai, J.; Zhao, L.; Yu, X.; Liang, J.; Liu, Y.; Nord, W.; Li, Y. Co-loading and intestine-specific delivery of multiple antioxidants in pH-responsive microspheres based on TEMPO-oxidized polysaccharides. Carbohydr. Polym. 2017, 157, 858–865. [Google Scholar] [CrossRef]
- Nisar, S.; Pandit, A.H.; Nadeem, M.; Pandit, A.H.; Rizvi, M.M.A.; Rattan, S. γ-Radiation induced L-glutamic acid grafted highly porous, pH-responsive chitosan hydrogel beads: A smart and biocompatible vehicle for controlled anti-cancer drug delivery. Int. J. Biol. Macromol. 2021, 182, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Gao, M.; Ren, Y.; Lou, R.; Xie, H.; Yu, W.; Liu, X.; Ma, X. An improved pH-responsive carrier based on EDTA-Ca-alginate for oral delivery of Lactobacillus rhamnosus ATCC 53103. Carbohydr. Polym. 2017, 155, 329–335. [Google Scholar] [CrossRef]
- Li, F.; Zhang, Z.; Wang, X.; Yin, X.; Fu, M.; Qin, T.; Ji, X.; Yang, G.; Sun, S. A physical crosslinked pH-sensitive hydrogel based on hemicellulose/graphene oxide for controlled oral drug delivery. Int. J. Biol. Macromol. 2025, 289, 138875. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, B.; Jiang, Y.; Liu, G.; Pu, S.; Feng, Y.; Jia, D.; Zhou, Y. pH-responsive UV crosslinkable chitosan hydrogel via “thiol-ene” click chemistry for active modulating opposite drug release behaviors. Carbohydr. Polym. 2021, 251, 117101. [Google Scholar] [CrossRef]
- Chen, N.; Nicolai, T.; Chassenieux, C.; Wang, Y. pH and ionic strength responsive core-shell protein microgels fabricated via simple coacervation of soy globulins. Food Hydrocoll. 2020, 105, 105853. [Google Scholar] [CrossRef]
- Tahmasebi, S.; Farmanbordar, H.; Mohammadi, R. Synthesis of magnetic bio-nanocomposite hydrogel beads based on sodium alginate and β-cyclodextrin: Potential pH-responsive oral delivery anticancer systems for colorectal cancer. Int. J. Biol. Macromol. 2025, 305, 140748. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, Q. Developing organogel-based Pickering emulsions with improved freeze-thaw stability and hesperidin bioaccessibility. Food Hydrocoll. 2019, 93, 68–77. [Google Scholar] [CrossRef]
- de Farias, N.S.; Silva, B.; de Oliveira Costa, A.C.; Müller, C.M.O. Alginate based antioxidant films with yerba mate (Ilex paraguariensis St. Hil.): Characterization and kinetics of phenolic compounds release. Food Packag. Shelf Life 2021, 28, 100548. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Vargas, M.; Chiralt, A.; González-Martínez, C. Release of polyphenols from starch-chitosan based films containing thyme extract. Carbohydr. Polym. 2017, 175, 122–130. [Google Scholar] [CrossRef]
- Qiao, Z.; Liu, H.-Y.; Zha, J.-C.; Mao, X.-X.; Yin, J. Completely degradable backbone-type hydrogen peroxide responsive curcumin copolymer: Synthesis and synergistic anticancer investigation. Polym. Chem. 2019, 10, 4305–4313. [Google Scholar] [CrossRef]
- Bernardos, A.; Božik, M.; Montero, A.; Pérez-Esteve, É.; García-Casado, E.; Lhotka, M.; Fraňková, A.; Marcos, M.D.; Barat, J.M.; Martínez-Máñez, R. Secreted enzyme-responsive system for controlled antifungal agent release. Nanomaterials 2021, 11, 1280. [Google Scholar] [CrossRef]
- Coulter, S.M.; Pentlavalli, S.; An, Y.; Vora, L.K.; Cross, E.R.; Moore, J.V.; Sun, H.; Schweins, R.; McCarthy, H.O.; Laverty, G. In situ forming, enzyme-responsive peptoid-peptide hydrogels: An advanced long-acting injectable drug delivery system. J. Am. Chem. Soc. 2024, 146, 21401–21416. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.A.; Nayak, R.R. Supramolecular phenoxy-alkyl maleate-based hydrogels and their enzyme/pH-responsive curcumin release. New J. Chem. 2019, 43, 5559–5567. [Google Scholar] [CrossRef]
- de Abreu Figueiredo, J.; Teixeira, M.A.; Campelo, P.H.; Lago, A.M.T.; de Souza, T.P.; Yoshida, M.I.; de Oliveira, C.R.; Pereira, A.P.A.; Pastore, G.M.; Sanches, E.A. Encapsulation of camu-camu extracts using prebiotic biopolymers: Controlled release of bioactive compounds and effect on their physicochemical and thermal properties. Food Res. Int. 2020, 137, 109563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Hu, X.; Zhu, J.; Wang, Z.; Wang, X.; Wang, M. Release properties of tannic acid from hydrogen bond driven antioxidative cellulose nanofibrous films. Int. J. Biol. Macromol. 2016, 91, 68–74. [Google Scholar] [CrossRef]
- Emam, H.E.; Shaheen, T.I. Design of a dual pH and temperature responsive hydrogel based on esterified cellulose nanocrystals for potential drug release. Carbohydr. Polym. 2022, 278, 118925. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Li, M.; Chen, H.; Xu, Z.; Li, J.; Kong, Y.; Zuo, X. Synthesis of stimuli-responsive copolymeric hydrogels for temperature, reduction and pH-controlled drug delivery. J. Ind. Eng. Chem. 2025, 143, 252–261. [Google Scholar] [CrossRef]
- Chou, K.F.; Chiu, H.S.; Lin, J.H.; Huang, W.Y.; Chen, P.Y.; Xiao, W.L.; Chen, T.K.; Wang, L.W. The effect of microwave treatment on the drug release property of gelatin microspheres. In Proceedings of the 15th International Conference on Biomedical Engineering: ICBME 2013, Singapore, 4–7 December 2013; pp. 726–729. [Google Scholar]
- Chauhan, R.; Kinney, K.; Akalkotkar, A.; Nunn, B.M.; Keynton, R.S.; Soucy, P.A.; O’Toole, M.G. Radiation-induced curcumin release from curcumin–chitosan polymer films. RSC Adv. 2020, 10, 16110–16117. [Google Scholar] [CrossRef]
- Gulfam, M.; Jo, S.-H.; Vu, T.T.; Ali, I.; Rizwan, A.; Joo, S.-B.; Park, S.-H.; Lim, K.T. NIR-degradable and biocompatible hydrogels derived from hyaluronic acid and coumarin for drug delivery and bio-imaging. Carbohydr. Polym. 2023, 303, 120457. [Google Scholar] [CrossRef]
- Han, Y.; Shchukin, D.; Fernandes, P.; Mutihac, R.-C.; Möhwald, H. Mechanism and kinetics of controlled drug release by temperature stimuli responsive protein nanocontainers. Soft Matter 2010, 6, 4942–4947. [Google Scholar] [CrossRef]
- Khalf, A.; Madihally, S.V. Modeling the permeability of multiaxial electrospun poly (ε-caprolactone)-gelatin hybrid fibers for controlled doxycycline release. Mater. Sci. Eng. C 2017, 76, 161–170. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Swelling behaviour and glass transition in genipin-crosslinked chitosan systems. Int. J. Biol. Macromol. 2020, 164, 3075–3083. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef]
- Teimouri, S.; Kasapis, S.; Dokouhaki, M. Diffusional characteristics of food protein-based materials as nutraceutical delivery systems: A review. Trends Food Sci. Technol. 2022, 122, 201–210. [Google Scholar] [CrossRef]
- Kelly, S.M.; Upadhyay, A.K.; Mitra, A.; Narasimhan, B. Analyzing drug release kinetics from water-soluble polymers. Ind. Eng. Chem. Res. 2019, 58, 7428–7437. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Gowd, V.; Suo, H.; Wang, M.; Wang, Q.; Chen, F.; Cheng, K.W. Advances in smart delivery of food bioactive compounds using stimuli-responsive carriers: Responsive mechanism, contemporary challenges, and prospects. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5449–5488. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Shin, H.S.; Park, S.N. A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Muhamad, I.I.; Fen, L.S.; Hui, N.H.; Mustapha, N.A. Genipin-cross-linked kappa-carrageenan/carboxymethyl cellulose beads and effects on beta-carotene release. Carbohydr. Polym. 2011, 83, 1207–1212. [Google Scholar] [CrossRef]
- Ma, W.; Tang, C.-H.; Yin, S.-W.; Yang, X.-Q.; Qi, J.-R. Genipin-crosslinked gelatin films as controlled releasing carriers of lysozyme. Food Res. Int. 2013, 51, 321–324. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Zhang, J.; Zhu, Y.; Wang, J. Self-assembled pH-responsive supramolecular hydrogel for hydrophobic drug delivery. RSC Adv. 2018, 8, 31581–31587. [Google Scholar] [CrossRef]
- Xu, W.; Huang, L.; Jin, W.; Ge, P.; Shah, B.R.; Zhu, D.; Jing, J. Encapsulation and release behavior of curcumin based on nanoemulsions-filled alginate hydrogel beads. Int. J. Biol. Macromol. 2019, 134, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Teimouri, S.; Morrish, C.; Kasapis, S. Release profile of vitamin B6 from a pH-responsive BSA network crosslinked with genipin. LWT 2020, 128, 109458. [Google Scholar] [CrossRef]
- Sabaghi, M.; Tavasoli, S.; Taheri, A.; Jamali, S.N.; Faridi Esfanjani, A. Controlling release patterns of the bioactive compound by structural and environmental conditions: A review. J. Food Meas. Charact. 2023, 17, 2261–2284. [Google Scholar] [CrossRef]
- Seeli, D.S.; Dhivya, S.; Selvamurugan, N.; Prabaharan, M. Guar gum succinate-sodium alginate beads as a pH-sensitive carrier for colon-specific drug delivery. Int. J. Biol. Macromol. 2016, 91, 45–50. [Google Scholar] [CrossRef]
- Mondal, S.; Li, C.; Wang, K. Bovine serum albumin adsorption on gluteraldehyde cross-linked chitosan hydrogels. J. Chem. Eng. Data 2015, 60, 2356–2362. [Google Scholar] [CrossRef]
- Kono, H.; Otaka, F.; Ozaki, M. Preparation and characterization of guar gum hydrogels as carrier materials for controlled protein drug delivery. Carbohydr. Polym. 2014, 111, 830–840. [Google Scholar] [CrossRef]
- Yang, Y.; Decker, E.A.; Xiao, H.; McClements, D.J. Enhancing vitamin E bioaccessibility: Factors impacting solubilization and hydrolysis of α-tocopherol acetate encapsulated in emulsion-based delivery systems. Food Funct. 2015, 6, 83–96. [Google Scholar] [CrossRef]
- Akar, E.; Altınışık, A.; Seki, Y. Preparation of pH-and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- Ponnusami, V. Polyelectrolyte complex-based ionically gelled biopolymeric systems for sustained drug release. In Ionically Gelled Biopolysaccharide Based Systems in Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 105–120. [Google Scholar]
- Assifaoui, A.; Chambin, O.; Cayot, P. Drug release from calcium and zinc pectinate beads: Impact of dissolution medium composition. Carbohydr. Polym. 2011, 85, 388–393. [Google Scholar] [CrossRef]
- Sepe, F.; Valentino, A.; Marcolongo, L.; Petillo, O.; Conte, R.; Margarucci, S.; Peluso, G.; Calarco, A. Marine-Derived Polysaccharide Hydrogels as Delivery Platforms for Natural Bioactive Compounds. Int. J. Mol. Sci. 2025, 26, 764. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and antimicrobial properties of ethylene vinyl alcohol copolymer films based on the release of oregano essential oil and green tea extract components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Release kinetics and antimicrobial properties of carvacrol encapsulated in electrospun poly-(ε-caprolactone) nanofibres. Application in starch multilayer films. Food Hydrocoll. 2018, 79, 158–169. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Kashiri, M.; Shakeri, A. Evaluation of release mechanism of catechin from chitosan-polyvinyl alcohol film by exposure to gamma irradiation. Carbohydr. Polym. 2020, 230, 115589. [Google Scholar] [CrossRef]
- López-Córdoba, A.; Medina-Jaramillo, C.; Piñeros-Hernandez, D.; Goyanes, S. Cassava starch films containing rosemary nanoparticles produced by solvent displacement method. Food Hydrocoll. 2017, 71, 26–34. [Google Scholar] [CrossRef]
- Rezaeinia, H.; Ghorani, B.; Emadzadeh, B.; Tucker, N. Electrohydrodynamic atomization of Balangu (Lallemantia royleana) seed gum for the fast-release of Mentha longifolia L. essential oil: Characterization of nano-capsules and modeling the kinetics of release. Food Hydrocoll. 2019, 93, 374–385. [Google Scholar] [CrossRef]
- Panyoyai, N.; Kasapis, S. A free-volume interpretation of the decoupling parameter in bioactive-compound diffusion from a glassy polymer. Food Hydrocoll. 2016, 54, 338–341. [Google Scholar] [CrossRef]
- Bakshi, A.S.; Singh, R.P. Kinetics of water diffusion and starch gelatinization during rice parboiling. J. Food Sci. 1980, 45, 1387–1392. [Google Scholar] [CrossRef]
- Nicolin, D.J.; Neto, R.M.; Paraíso, P.R.; Jorge, R.M.M.; Jorge, L.M.M. Analytical solution and experimental validation of a model for hydration of soybeans with variable mass transfer coefficient. J. Food Eng. 2015, 149, 17–23. [Google Scholar] [CrossRef]
- Paramita, V.D.; Bannikova, A.; Kasapis, S. Release mechanism of essential fatty acids in polysaccharide matrices undergoing glass transition. In Gums and Stabilisers for the Food Industry 18: Hydrocolloid Functionality for Affordable and Sustainable Global Food Solutions; Royal Society of Chemistry: London, UK, 2016; Volume 18, p. 157. [Google Scholar]
- Shi, W.; Weitz, D.A. Polymer phase separation in a microcapsule shell. Macromolecules 2017, 50, 7681–7686. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Paramita, V.D.; Piccolo, J.D.L.; Kasapis, S. Effect of co-solute concentration on the diffusion of linoleic acid from whey protein matrices. Food Hydrocoll. 2017, 70, 277–285. [Google Scholar] [CrossRef]
- Bouman, J.; Belton, P.; Venema, P.; Van Der Linden, E.; De Vries, R.; Qi, S. Controlled release from zein matrices: Interplay of drug hydrophobicity and pH. Pharm. Res. 2016, 33, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.; Shivhare, U. Microencapsulation by complex coacervation using whey protein isolates and gum acacia: An approach to preserve the functionality and controlled release of β-carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.; Anandharamakrishnan, C. Micro-and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Yan, J.-K.; Qiu, W.-Y.; Wang, Y.-Y.; Wu, J.-Y. Biocompatible polyelectrolyte complex nanoparticles from lactoferrin and pectin as potential vehicles for antioxidative curcumin. J. Agric. Food Chem. 2017, 65, 5720–5730. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Teng, M.-J.; Wei, Y.-S.; Hu, T.-G.; Zhang, Y.; Feng, K.; Zong, M.-H.; Wu, H. Citric acid cross-linked zein microcapsule as an efficient intestine-specific oral delivery system for lipophilic bioactive compound. J. Food Eng. 2020, 281, 109993. [Google Scholar] [CrossRef]
- Zhu, L.; Yu, Z.-L.; Li, S.; Xu, C.-Z.; Hou, Y.-J.; Liao, L.-X.; Xu, Y.-L.; Zhang, J.-T.; Wei, B.-M.; Wen, W. Recent advances on collagen biomaterial: From extraction, cross-linking to tissue regeneration. Polym. Rev. 2024, 64, 1031–1059. [Google Scholar] [CrossRef]
- Maitra, J.; Shukla, V.K. Cross-linking in hydrogels-a review. Am. J. Polym. Sci 2014, 4, 25–31. [Google Scholar]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, Z.; Deng, D.; Li, J.; Qi, X.; Song, M.; Liu, Y.; Wu, Q.; Xie, X.; Chen, Z. Improved cytocompatibility and reduced calcification of glutaraldehyde-crosslinked bovine pericardium by modification with glutathione. Front. Bioeng. Biotechnol. 2022, 10, 844010. [Google Scholar] [CrossRef]
- Ahmed, R.; ul ain Hira, N.; Wang, M.; Iqbal, S.; Yi, J.; Hemar, Y. Genipin, a natural blue colorant precursor: Source, extraction, properties, and applications. Food Chem. 2024, 434, 137498. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.; Zhang, Y.; Nardin, C. Core-shell gelatin-chitosan nanoparticles with lysozyme responsiveness formed via pH-drive and transglutaminase cross-linking. Int. J. Biol. Macromol. 2025, 292, 138802. [Google Scholar] [CrossRef]
- Wen, X.; Jin, F.; Regenstein, J.M.; Wang, F. Transglutaminase induced gels using bitter apricot kernel protein: Chemical, textural and release properties. Food Biosci. 2018, 26, 15–22. [Google Scholar] [CrossRef]
- Mzoughi, J.; Tran, Q.H.; Schrodj, G.; Vandamme, T.; Luchnikov, V. Rolled-up gastroretentive oral dosages for controlled release of riboflavin and propranolol. J. Drug Deliv. Sci. Technol. 2024, 95, 105563. [Google Scholar] [CrossRef]
- Yin, W.; Su, R.; Qi, W.; He, Z. A casein-polysaccharide hybrid hydrogel cross-linked by transglutaminase for drug delivery. J. Mater. Sci. 2012, 47, 2045–2055. [Google Scholar] [CrossRef]
- Lin, Y.; Roos, Y.H.; Miao, S. Transglutaminase crosslinked fish gelatin emulsion gels: Structure, rheology behaviors, and delivery functionality. Food Hydrocoll. 2025, 162, 111001. [Google Scholar] [CrossRef]
- De Souza, P.M.; Fernández, A.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Modified sodium caseinate films as releasing carriers of lysozyme. Food Hydrocoll. 2010, 24, 300–306. [Google Scholar] [CrossRef]
- Fang, M.; Ma, F.; Yu, L.; Wang, D.; Wen, C.; Zhang, L.; Li, P. Fabrication of resveratrol-loaded soy peptide nanogels with transglutaminase and in vitro gastrointestinal delivery and cholesterol-lowering effect. Food Hydrocoll. 2024, 157, 110437. [Google Scholar] [CrossRef]
- Wu, X.; Wang, K.; Liu, Y.; Liu, A.; Ye, R. Microstructure of transglutaminase-induced gelatin-natamycin fungistatic composite films. Int. J. Food Prop. 2017, 20, 3191–3203. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Wang, P.; Zhang, M.; Liu, S.; Wang, R.; Li, Y.; Ren, F.; Fang, B. Improvement in the Sustained-Release Performance of Electrospun Zein Nanofibers via Crosslinking Using Glutaraldehyde Vapors. Foods 2024, 13, 1583. [Google Scholar] [CrossRef]
- Jayanudin; Fahrurrozi, M.; Wirawan, S.K.; Rochmadi. Preparation of chitosan microcapsules containing red ginger oleoresin using emulsion crosslinking method. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800018809917. [Google Scholar] [CrossRef]
- Ofokansi, K.; Kenechukwu, F.; Isah, A.; Okigbo, E. Formulation and evaluation of glutaraldehyde-crosslinked chitosan microparticles for the delivery of ibuprofen. Trop. J. Pharm. Res. 2013, 12, 19–25. [Google Scholar] [CrossRef]
- Liao, J.; Lin, Y.; Xu, M.; Luo, Z.; Jiang, G.; Chen, F.; Li, H.; Yang, L. Preparation of hydrophobic hard gelatin capsules for slow-release fertilizers. Polym. Test. 2024, 134, 108427. [Google Scholar] [CrossRef]
- Hiwale, P.; Lampis, S.; Conti, G.; Caddeo, C.; Murgia, S.; Fadda, A.M.; Monduzzi, M. In vitro release of lysozyme from gelatin microspheres: Effect of cross-linking agents and thermoreversible gel as suspending medium. Biomacromolecules 2011, 12, 3186–3193. [Google Scholar] [CrossRef]
- Fatima, W.; Batool, S.R.; Mushtaq, F.; Aslam, M.; Raza, Z.A.; Nazeer, M.A. Controlled release of doxorubicin from gelatin-based nanoparticles: Theoretical and experimental approach. Mater. Adv. 2024, 5, 2347–2358. [Google Scholar] [CrossRef]
- Wang, Y.X.; Qin, Y.P.; Kong, Z.J.; Wang, Y.J.; Ma, L. Glutaraldehyde cross-linked silk fibroin films for controlled release. Adv. Mater. Res. 2014, 887, 541–546. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Boonme, P. Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater. Sci. Eng. C 2013, 33, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.; Cavusoglu, K. Glutaraldehyde cross-linked agarose carriers: Design, characterization and insulin release behaviour. Turk. J. Biochem 2008, 33, 148–153. [Google Scholar]
- Tseng, T.-H.; Chang, J.-H.; Chang, L.-C.; Wang, M.-L.; Yang, S.-J.; Chang, C.-H. Indocyanine green-mediated photothermal release of lidocaine from genipin-crosslinked gelatin hydrogel in nerve block. Int. J. Biol. Macromol. 2025, 297, 139518. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Yuan, G. Chitosan/squid ring teeth protein hydrogels for the controlled release of curcumin. Int. J. Biol. Macromol. 2025, 291, 139163. [Google Scholar] [CrossRef]
- Li, F.; Jin, H.; Xiao, J.; Yin, X.; Liu, X.; Li, D.; Huang, Q. The simultaneous loading of catechin and quercetin on chitosan-based nanoparticles as effective antioxidant and antibacterial agent. Food Res. Int. 2018, 111, 351–360. [Google Scholar] [CrossRef]
- Li, M.; Wang, K.; Wang, Y.; Han, Q.; Ni, Y.; Wen, X. Effects of genipin concentration on cross-linked β-casein micelles as nanocarrier of naringenin: Colloidal properties, structural characterization and controlled release. Food Hydrocoll. 2020, 108, 105989. [Google Scholar] [CrossRef]
- Thakare, S.; Gorle, A. Design of Jackfruit Gum-Based Genipin Crosslinked Nanoparticles for Sustained Release of Curcumin: Optimization and in Vitro Characterization. Cellul. Chem. Technol. 2024, 58, 67–79. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Paramita, V.D.; Teimouri, S.; Young, S.; Kasapis, S. Controlled release of ascorbic acid from genipin-crosslinked gelatin matrices under moving boundary conditions. Food Hydrocoll. 2019, 89, 171–179. [Google Scholar] [CrossRef]
- Uddin, M.S.; Khand, S.; Dong, C. Effect of crosslinking agents on chitosan hydrogel carriers for drug loading and release for targeted drug delivery. Gels 2024, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Fraj, J.; Petrović, L.; Đekić, L.; Budinčić, J.M.; Bučko, S.; Katona, J. Encapsulation and release of vitamin C in double W/O/W emulsions followed by complex coacervation in gelatin-sodium caseinate system. J. Food Eng. 2021, 292, 110353. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Guaresti, O.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Gabilondo, N.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. β-Glycerol phosphate/genipin chitosan hydrogels: A comparative study of their properties and diclofenac delivery. Carbohydr. Polym. 2020, 248, 116811. [Google Scholar] [CrossRef]
- Zhu, N.; Bi, D.; Huang, J.; Yao, L.; Wu, Y.; Jiang, Z.; Hu, Z.; Zhu, B.; Li, S.; Xu, X. Genipin crosslinked sodium caseinate-chitosan oligosaccharide nanoparticles for optimizing β-carotene stability and bioavailability. Int. J. Biol. Macromol. 2025, 297, 139626. [Google Scholar] [CrossRef]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Sun, X.; Zhang, S.; Yuan, Y.; Wang, D.; Xu, Y. Fabrication and characterization of cold-gelation whey protein-chitosan complex hydrogels for the controlled release of curcumin. Food Hydrocoll. 2020, 103, 105619. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, R.; Yu, S.; Li, J.; Wang, Y.; Song, Y.; Yang, X.; Pan, W.; Li, S. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int. J. Biol. Macromol. 2018, 114, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.; Philip, J.; Rodrigues, F.C.; Thakur, G. Transport of curcumin from cross-linked chitosan matrices: A comparative study. IOP Conf. Ser. Mater. Sci. Eng. 2019, 561, 012029. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Shi, Y.; Cai, T.; Miao, Q.; Gao, Z.; Cui, Z. Lightweight pH-responsive chitosan hydrogel iron fertilizer: Efficient performance, controlled-release, and tomato application. J. Environ. Chem. Eng. 2024, 12, 113428. [Google Scholar] [CrossRef]
- Chen, B.Z.; Ashfaq, M.; Zhu, D.D.; Zhang, X.P.; Guo, X.D. Controlled Delivery of Insulin Using Rapidly Separating Microneedles Fabricated from Genipin-Crosslinked Gelatin. Macromol. Rapid Commun. 2018, 39, 1800075. [Google Scholar] [CrossRef] [PubMed]
- Samprasit, W.; Akkaramongkolporn, P.; Jaewjira, S.; Opanasopit, P. Design of alpha mangostin-loaded chitosan/alginate controlled-release nanoparticles using genipin as crosslinker. J. Drug Deliv. Sci. Technol. 2018, 46, 312–321. [Google Scholar] [CrossRef]
- Koc, F.E.; Altıncekic, T.G. Investigation of gelatin/chitosan as potential biodegradable polymer films on swelling behavior and methylene blue release kinetics. Polym. Bull. 2021, 78, 3383–3398. [Google Scholar] [CrossRef]
- Ghaleshahi, A.Z.; Rajabzadeh, G. The influence of sodium alginate and genipin on physico-chemical properties and stability of WPI coated liposomes. Food Res. Int. 2020, 130, 108966. [Google Scholar] [CrossRef]
- Liu, L.; Yang, S.; Jin, L.; Niu, C.; Liang, H.; Chen, C.; Ban, Z. Development of controlled-release soy β-conglycinin nanoparticles containing curcumin using genipin as crosslinker. Food Biosci. 2023, 54, 102826. [Google Scholar] [CrossRef]
- Tuan Mohamood, N.F.A.-Z.; Abdul Halim, A.H.; Zainuddin, N. Carboxymethyl cellulose hydrogel from biomass waste of oil palm empty fruit bunch using calcium chloride as crosslinking agent. Polymers 2021, 13, 4056. [Google Scholar] [CrossRef]
- O-chongpian, P.; Na Takuathung, M.; Chittasupho, C.; Ruksiriwanich, W.; Chaiwarit, T.; Baipaywad, P.; Jantrawut, P. Composite nanocellulose fibers-based hydrogels loading clindamycin HCl with Ca2+ and citric acid as crosslinking agents for pharmaceutical applications. Polymers 2021, 13, 4423. [Google Scholar] [CrossRef]
- Neto, L.A.A.; Silva, L.P. Influence of biopolymer composition and crosslinking agent concentration on the micro-and nanomechanical properties of hydrogel-based filaments. J. Mech. Behav. Biomed. Mater. 2024, 150, 106316. [Google Scholar]
- Alves-Silva, G.F.; Romani, V.P.; Martins, V.G. Different crosslinking as a strategy to improve films produced from external mesocarp of pequi (Caryocar brasiliense). Food Chem. 2024, 432, 137202. [Google Scholar] [CrossRef] [PubMed]
- Medina-Jaramillo, C.; Quintero-Pimiento, C.; Díaz-Díaz, D.; Goyanes, S.; López-Córdoba, A. Improvement of andean blueberries postharvest preservation using carvacrol/alginate-edible coatings. Polymers 2020, 12, 2352. [Google Scholar] [CrossRef] [PubMed]
- Kimi, M.; Chong, C.J. Facile Preparation of Chitosan-Alginate Crosslinked with Calcium Chloride Hydrogel as Sustained Release Fertilizers. Res. Sq. 2024. preprint. [Google Scholar] [CrossRef]
- Saad, S.; Alali, H. Developing and Evaluating a Novel Drug Delivery System, Calcium-Alginate Beads loaded with Valsartan. Iraqi J. Pharm. Sci. 2024, 33, 146–153. [Google Scholar] [CrossRef]
- Sumitha, N.S.; Prakash, P.; Nair, B.N.; Sailaja, G.S. Degradation-dependent controlled delivery of doxorubicin by glyoxal cross-linked magnetic and porous chitosan microspheres. ACS Omega 2021, 6, 21472–21484. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Szczepańska, B.; Mazur, O.; Michalska-Sionkowska, M.; Łukowicz, K.; Osyczka, A.M. The preparation and characterization of chitosan-based hydrogels cross-linked by glyoxal. Materials 2021, 14, 2449. [Google Scholar] [CrossRef]
- Krishna, D.V.; Sankar, M.R.; Reddy, T.N. Effect of glyoxal concentration and nanoparticles reinforcement on the functional properties of composite hydrogel for biomedical applications. Macromol. Res. 2025, 33, 569–592. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Durpekova, S.; Filatova, K.; Cisar, J.; Ronzova, A.; Kutalkova, E.; Sedlarik, V. A novel hydrogel based on renewable materials for agricultural application. Int. J. Polym. Sci. 2020, 2020, 8363418. [Google Scholar] [CrossRef]
- Abraham, S.; Rajamanickam, D.; Srinivasan, B. Research article preparation, characterization and cross-linking of chitosan by microwave assisted synthesis. Sci. Int 2018, 6, 18–30. [Google Scholar] [CrossRef]
- Motiei, M.; Sedlařík, V.; Lucia, L.A.; Fei, H.; Münster, L. Stabilization of chitosan-based polyelectrolyte nanoparticle cargo delivery biomaterials by a multiple ionic cross-linking strategy. Carbohydr. Polym. 2020, 231, 115709. [Google Scholar] [CrossRef]
- Shi, X.; Xu, S.; Xu, J.; He, J. Preparation and properties of a multi-crosslinked chitosan/sodium alginate composite hydrogel. Mater. Lett. 2024, 354, 135414. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Yang, W.; Gong, Y.; Zhang, X.; Li, J.; Wu, D. Dual cross-linking with tannic acid and transglutaminase improves microcapsule stability and encapsulates lemon essential oil for food preservation. Food Chem. 2025, 465, 142173. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. Integrated ultrasonic-transglutaminase modification of lesser mealworm protein isolate: A pioneering cobalamin delivery vehicle in gluten-free breads. Food Chem. 2024, 448, 139069. [Google Scholar] [CrossRef] [PubMed]
- Arjeh, E.; Rostami, H.; Pirsa, S.; Fathi, M. Synthesis and characterization of novel Spirulina protein isolate (SPI)-based hydrogels through dual-crosslinking with genipin/Zn2+. Food Res. Int. 2022, 162, 112107. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.-M. Gelation modification of soy protein isolate by a naturally occurring cross-linking agent and its potential biomedical application. Ind. Eng. Chem. Res. 2009, 48, 7077–7083. [Google Scholar] [CrossRef]
- Li, Z.; Lu, F.; Liu, Y. A review of the mechanism, properties, and applications of hydrogels prepared by enzymatic cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Mirabelli, V.; Majidi Salehi, S.; Angiolillo, L.; Belviso, B.D.; Conte, A.; Del Nobile, M.A.; Di Profio, G.; Caliandro, R. Enzyme crystals and hydrogel composite membranes as new active food packaging material. Glob. Chall. 2018, 2, 1700089. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, S.; Liu, K.; Liu, X.; Zhang, X.; Wang, H.; Lu, F. High-level expression of the Streptomyces mobaraense CICC 11018 transglutaminase in Corynebacterium glutamicum ATCC 13032. Appl. Biochem. Microbiol. 2014, 50, 456–462. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, W.; Li, Y.; Gao, G.; Zhang, K.; Zhou, J.; Wu, Z. Cross-linking and film-forming properties of transglutaminase-modified collagen fibers tailored by denaturation temperature. Food Chem. 2019, 271, 527–535. [Google Scholar] [CrossRef]
- Feng, S.; Yang, W.; Zhang, L.; Wang, X.; Wang, S.; Tao, S. Casein-hydroxyapatite composite microspheres for strontium-containing wastewater treatment. ACS EST Water 2021, 1, 900–909. [Google Scholar] [CrossRef]
- Lee, S.; Rosenberg, M. Preparation and properties of glutaraldehyde cross-linked whey protein-based microcapsules containing theophylline. J. Control. Release 1999, 61, 123–136. [Google Scholar] [CrossRef]
- Gan, C.-Y.; Cheng, L.-H.; Phuah, E.-T.; Chin, P.-N.; AlKarkhi, A.F.; Easa, A.M. Combined cross-linking treatments of bovine serum albumin gel beadlets for controlled-delivery of caffeine. Food Hydrocoll. 2009, 23, 1398–1405. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. Ultrasound-assisted alcoholic extraction of lesser mealworm larvae oil: Process optimization, physicochemical characteristics, and energy consumption. Antioxidants 2022, 11, 1943. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Karbowiak, T.; Debeaufort, F. Release behavior of quercetin from chitosan-fish gelatin edible films influenced by electron beam irradiation. Food Control 2016, 66, 315–319. [Google Scholar] [CrossRef]
- Abu Ghalia, M.; Dahman, Y. Radiation crosslinking polymerization of poly (vinyl alcohol) and poly (ethylene glycol) with controlled drug release. J. Polym. Res. 2015, 22, 218. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Assifaoui, A.; Karbowiak, T.; Debeaufort, F.; Chambin, O. Controlled release of tyrosol and ferulic acid encapsulated in chitosan–gelatin films after electron beam irradiation. Radiat. Phys. Chem. 2016, 118, 81–86. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma ray-induced polymerization and cross-linking for optimization of PPy/PVP hydrogel as biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef]
- Huang, T.; Fang, Z.; Zhao, H.; Xu, D.; Yang, W.; Yu, W.; Zhang, J. Physical properties and release kinetics of electron beam irradiated fish gelatin films with antioxidants of bamboo leaves. Food Biosci. 2020, 36, 100597. [Google Scholar] [CrossRef]
- Lin, J.; Yong, K.Y.A.; Zhou, Y.; Wang, Y.; Zhou, W. Improved in vitro bioaccessibility of quercetin by nanocomplexation with high-intensity ultrasound treated soy protein isolate. Food Chem. 2023, 406, 135004. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Olvera, D.; Sawkins, M.J.; Cryan, S.-A.; Kimmins, S.D.; Da Silva, T.E.; Kelly, D.J.; Duffy, G.P.; Kearney, C.; Heise, A. Direct UV-Triggered thiol–ene cross-linking of electrospun polyester fibers from unsaturated poly (macrolactone) s and their drug loading by solvent swelling. Biomacromolecules 2017, 18, 4292–4298. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-H.; Tsai, W.-B. In situ UV-crosslinking gelatin electrospun fibers for tissue engineering applications. Biofabrication 2013, 5, 035008. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Dong, P.; Gravesande, J.; Cheng, B.; Xing, J. UV-mediated solid-state cross-linking of electrospinning nanofibers of modified collagen. Int. J. Biol. Macromol. 2018, 120, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Microwave-assisted synthesis of nutgall tannic acid–based salecan polysaccharide hydrogel for tunable release of β-lactoglobulin. Int. J. Biol. Macromol. 2020, 161, 1431–1439. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Li, S.; Zeng, R.; Zhang, F.; Wang, Z.; Guan, S. Corrosion resistance and drug release profile of gentamicin-loaded polyelectrolyte multilayers on magnesium alloys: Effects of heat treatment. J. Colloid Interface Sci. 2019, 547, 309–317. [Google Scholar] [CrossRef]
- Mahmud, M.M.; Zaman, S.; Perveen, A.; Jahan, R.A.; Islam, M.F.; Arafat, M.T. Controlled release of curcumin from electrospun fiber mats with antibacterial activity. J. Drug Deliv. Sci. Technol. 2020, 55, 101386. [Google Scholar] [CrossRef]
- Sullivan, S.T.; Tang, C.; Kennedy, A.; Talwar, S.; Khan, S.A. Electrospinning and heat treatment of whey protein nanofibers. Food Hydrocoll. 2014, 35, 36–50. [Google Scholar] [CrossRef]
- Rimdusit, S.; Somsaeng, K.; Kewsuwan, P.; Jubsilp, C.; Tiptipakorn, S. Comparison of gamma radiation crosslinking and chemical crosslinking on properties of methylcellulose hydrogel. Eng. J. 2012, 16, 15–28. [Google Scholar] [CrossRef]
- Basu, T.; Bhutani, U.; Majumdar, S. Cross-linker-free sodium alginate and gelatin hydrogels: A multiscale biomaterial design framework. J. Mater. Chem. B 2022, 10, 3614–3623. [Google Scholar] [CrossRef] [PubMed]
- Basu, T.; Goswami, D.; Majumdar, S. Fabrication of crosslinker free hydrogels with diverse properties: An interplay of multiscale physical forces within polymer matrix. iScience 2024, 27, 111227. [Google Scholar] [CrossRef]
- Kayati, F.N.; Purnomo, C.W.; Kusumastuti, Y. Physical properties comparison of hydrogel from cassava starch using two different non toxic crosslinkers. Next Sustain. 2024, 4, 100043. [Google Scholar] [CrossRef]
- Sulastri, E.; Zubair, M.S.; Lesmana, R.; Mohammed, A.F.A.; Wathoni, N. Development and characterization of ulvan polysaccharides-based hydrogel films for potential wound dressing applications. Drug Des. Dev. Ther. 2021, 15, 4213–4226. [Google Scholar] [CrossRef]
- Kim, S.; Kim, B.S.; Bai, J.; Chang, Y. Antibacterial κ-carrageenan/konjac glucomannan-based edible hydrogel film containing Salmonella phage PBSE191 and its application in chicken meat. LWT 2023, 180, 114707. [Google Scholar] [CrossRef]
- Treenate, P.; Monvisade, P.; Yamaguchi, M. The effect of glycerol/water and sorbitol/water on the plasticization of hydroxyethylacryl chitosan/sodium alginate films. In Proceedings of the MATEC Web of Conferences, Singapore, 26–28 October 2015; p. 02006. [Google Scholar]
- Bialik-Wąs, K.; Pluta, K.; Malina, D.; Barczewski, M.; Malarz, K.; Mrozek-Wilczkiewicz, A. The effect of glycerin content in sodium alginate/poly (vinyl alcohol)-based hydrogels for wound dressing application. Int. J. Mol. Sci. 2021, 22, 12022. [Google Scholar] [CrossRef]
- Xie, J.; Jin, X.; Cheng, H.; Chen, W.; Yu, W.; Wang, L. Molecular origin of the plasticizing effect difference of glycerol with other polyols on plasticizing polyvinyl alcohol (PVA) as elucidated by solid-state NMR. Ind. Crops Prod. 2024, 220, 119246. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and characterization of PVA/starch hydrogel membranes incorporating essential oils aimed to be used in wound dressing applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of plasticiser on the barrier, mechanical and grease resistance properties of alginate cast films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Kadzińska, J.; Bryś, J.; Ostrowska-Ligęza, E.; Estéve, M.; Janowicz, M. Influence of vegetable oils addition on the selected physical properties of apple–sodium alginate edible films. Polym. Bull. 2020, 77, 883–900. [Google Scholar] [CrossRef]
- Sharmin, N.; Sone, I.; Walsh, J.L.; Sivertsvik, M.; Fernández, E.N. Effect of citric acid and plasma activated water on the functional properties of sodium alginate for potential food packaging applications. Food Packag. Shelf Life 2021, 29, 100733. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, S.; Liu, Y.; Chen, S.; Li, L. Modelling and assessment of plasticizer migration and structure changes in hydrophobic starch-based films. Int. J. Biol. Macromol. 2022, 195, 41–48. [Google Scholar] [CrossRef]
- Paramita, V.D.; Kasapis, S. Molecular dynamics of the diffusion of natural bioactive compounds from high-solid biopolymer matrices for the design of functional foods. Food Hydrocoll. 2019, 88, 301–319. [Google Scholar] [CrossRef]
- Wadkin-Snaith, D.; Mulheran, P.A.; Johnston, K. The impact of plasticisers on crystal nucleation, growth and melting in linear polymers. Polymer 2024, 304, 127095. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Mohamad, A.B. Study of the plasticising effect on polymer and its development in fuel cell application. Renew. Sustain. Energy Rev. 2017, 79, 794–805. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.-C. Effect of different plasticisers on the mechanical and barrier properties of extruded cast PHBV films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Wagner, J.R.; Salvay, A.G. Hydration and water vapour transport properties in yeast biomass based films: A study of plasticizer content and thickness effects. Eur. Polym. J. 2018, 99, 9–17. [Google Scholar] [CrossRef]
- Yoshida, C.M.; Bastos, C.E.N.; Franco, T.T. Modeling of potassium sorbate diffusion through chitosan films. LWT-Food Sci. Technol. 2010, 43, 584–589. [Google Scholar] [CrossRef]
- Lee, J.Y.; Tan, L.W.; Lee, K.V.; Beh, K.P.; Goh, C.F. Effects of polyol and surfactant plasticisers on lyophilised rice starch wafers for buccal drug delivery. Int. J. Biol. Macromol. 2024, 261, 129935. [Google Scholar] [CrossRef] [PubMed]
- Oustadi, F.; Imani, R.; Haghbin Nazarpak, M.; Sharifi, A.M. Genipin-crosslinked gelatin hydrogel incorporated with PLLA-nanocylinders as a bone scaffold: Synthesis, characterization, and mechanical properties evaluation. Polym. Adv. Technol. 2020, 31, 1783–1792. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Z. Effects of gelatin-polyphenol and gelatin–genipin cross-linking on the structure of gelatin hydrogels. Int. J. Food Prop. 2017, 20, S2822–S2832. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Han, Q. Modulation of spatial and topological inhomogeneities of linear polymer hydrogel. Mater. Today Commun. 2022, 31, 103700. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, B.; Whent, M.; Yu, L.L.; Wang, Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids Surf. B Biointerfaces 2011, 85, 145–152. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Chen, L.; Tong, Q.; McClements, D.J. Designing hydrogel particles for controlled or targeted release of lipophilic bioactive agents in the gastrointestinal tract. Eur. Polym. J. 2015, 72, 698–716. [Google Scholar] [CrossRef]
- Prusty, K.; Biswal, A.; Biswal, S.B.; Swain, S.K. Synthesis of soy protein/polyacrylamide nanocomposite hydrogels for delivery of ciprofloxacin drug. Mater. Chem. Phys. 2019, 234, 378–389. [Google Scholar] [CrossRef]
- Tong, X.; Lee, S.; Bararpour, L.; Yang, F. Long-Term controlled protein release from poly (ethylene glycol) hydrogels by modulating mesh size and degradation. Macromol. Biosci. 2015, 15, 1679–1686. [Google Scholar] [CrossRef]
- Schwartz, M.P.; Fairbanks, B.D.; Rogers, R.E.; Rangarajan, R.; Zaman, M.H.; Anseth, K.S. A synthetic strategy for mimicking the extracellular matrix provides new insight about tumor cell migration. Integr. Biol. 2010, 2, 32–40. [Google Scholar] [CrossRef]
- Nagy-Smith, K.; Yamada, Y.; Schneider, J.P. Protein release from highly charged peptide hydrogel networks. J. Mater. Chem. B 2016, 4, 1999–2007. [Google Scholar] [CrossRef]
- Yu, Y.; Chau, Y. Formulation of in situ chemically cross-linked hydrogel depots for protein release: From the blob model perspective. Biomacromolecules 2015, 16, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Adams, D.J.; Iggo, J.A. Analysis of the mesh size in a supramolecular hydrogel by PFG-NMR spectroscopy. Soft Matter 2013, 9, 5483–5491. [Google Scholar] [CrossRef]
- Waters, D.J.; Engberg, K.; Parke-Houben, R.; Hartmann, L.; Ta, C.N.; Toney, M.F.; Frank, C.W. Morphology of photopolymerized end-linked poly (ethylene glycol) hydrogels by small-angle X-ray scattering. Macromolecules 2010, 43, 6861–6870. [Google Scholar] [CrossRef]
- Saffer, E.M.; Lackey, M.A.; Griffin, D.M.; Kishore, S.; Tew, G.N.; Bhatia, S.R. SANS study of highly resilient poly (ethylene glycol) hydrogels. Soft Matter 2014, 10, 1905–1916. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Samavedi, S.; Grigoryan, B.; Hahn, M.S. Impact of secondary reactive species on the apparent decoupling of poly (ethylene glycol) diacrylate hydrogel average mesh size and modulus. Polymer 2015, 77, 227–238. [Google Scholar] [CrossRef][Green Version]
- McClements, D.J. Encapsulation, protection, and release of hydrophilic active components: Potential and limitations of colloidal delivery systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef]
- Thakur, G.; Mitra, A.; Rousseau, D.; Basak, A.; Sarkar, S.; Pal, K. Crosslinking of gelatin-based drug carriers by genipin induces changes in drug kinetic profiles in vitro. J. Mater. Sci. Mater. Med. 2011, 22, 115–123. [Google Scholar] [CrossRef]
- Pal, K.; Paulson, A.T.; Rousseau, D. Biopolymers in controlled-release delivery systems. In Modern Biopolymer Science; Elsevier: Amsterdam, The Netherlands, 2009; pp. 519–557. [Google Scholar]
- Nie, X.; Gong, Y.; Wang, N.; Meng, X. Preparation and characterization of edible myofibrillar protein-based film incorporated with grape seed procyanidins and green tea polyphenol. LWT-Food Sci. Technol. 2015, 64, 1042–1046. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef]
- Cheng, S.-Y.; Wang, B.-J.; Weng, Y.-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT-Food Sci. Technol. 2015, 63, 115–121. [Google Scholar] [CrossRef]
- Yoon, S.-D. Cross-linked potato starch-based blend films using ascorbic acid as a plasticizer. J. Agric. Food Chem. 2014, 62, 1755–1764. [Google Scholar] [CrossRef]
- Ulbin-Figlewicz, N.; Zimoch-Korzycka, A.; Jarmoluk, A. Antibacterial activity and physical properties of edible chitosan films exposed to low-pressure plasma. Food Bioprocess Technol. 2014, 7, 3646–3654. [Google Scholar] [CrossRef]
- Song, F.; Zhang, L.-M.; Yang, C.; Yan, L. Genipin-crosslinked casein hydrogels for controlled drug delivery. Int. J. Pharm. 2009, 373, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Schweizer, K.S. Theory of activated penetrant diffusion in viscous fluids and colloidal suspensions. J. Chem. Phys. 2015, 143, 144906. [Google Scholar] [CrossRef]
- Rahman, M.; Teimouri, S.; Morrish, C.; Kasapis, S. A theoretical approach is presented that follows the effect of solute size on their diffusion through genipin-crosslinked gelatin networks. Food Hydrocoll. 2025, 170, 111567. [Google Scholar] [CrossRef]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Leahy-Dios, A.; Firoozabadi, A. Molecular and Thermal Diffusion Coefficients of Alkane-Alkane and Alkane-Aromatic Binary Mixtures: Effect of Shape and Size of Molecules. J. Phys. Chem. B 2007, 111, 191–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).