Synergistic Enhancement of Oxygen Permeability in Silane-Modified Hydrogel Networks for Advanced Ophthalmic Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Polymerization Stability
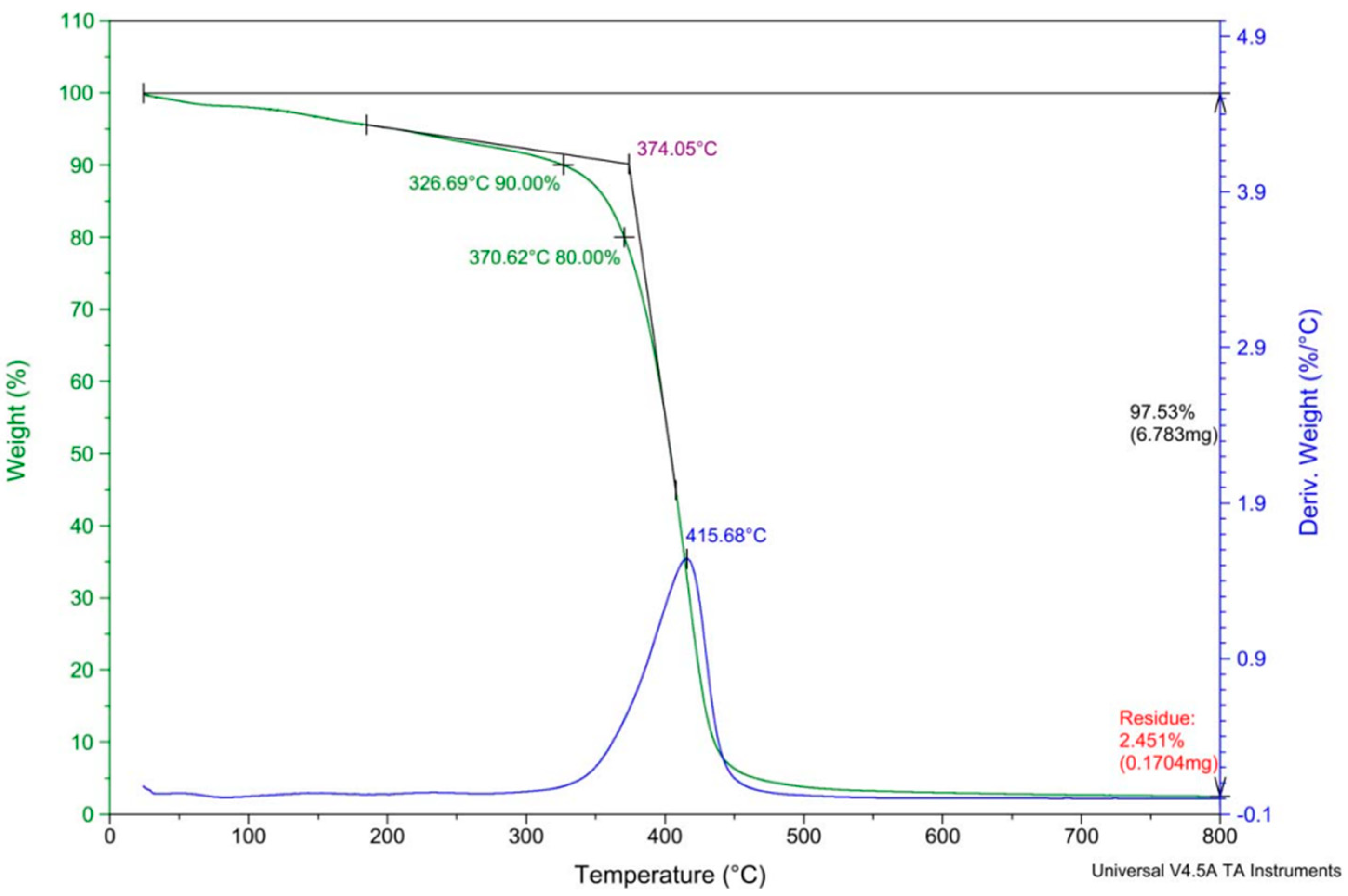
2.1.1. Thermal Analysis
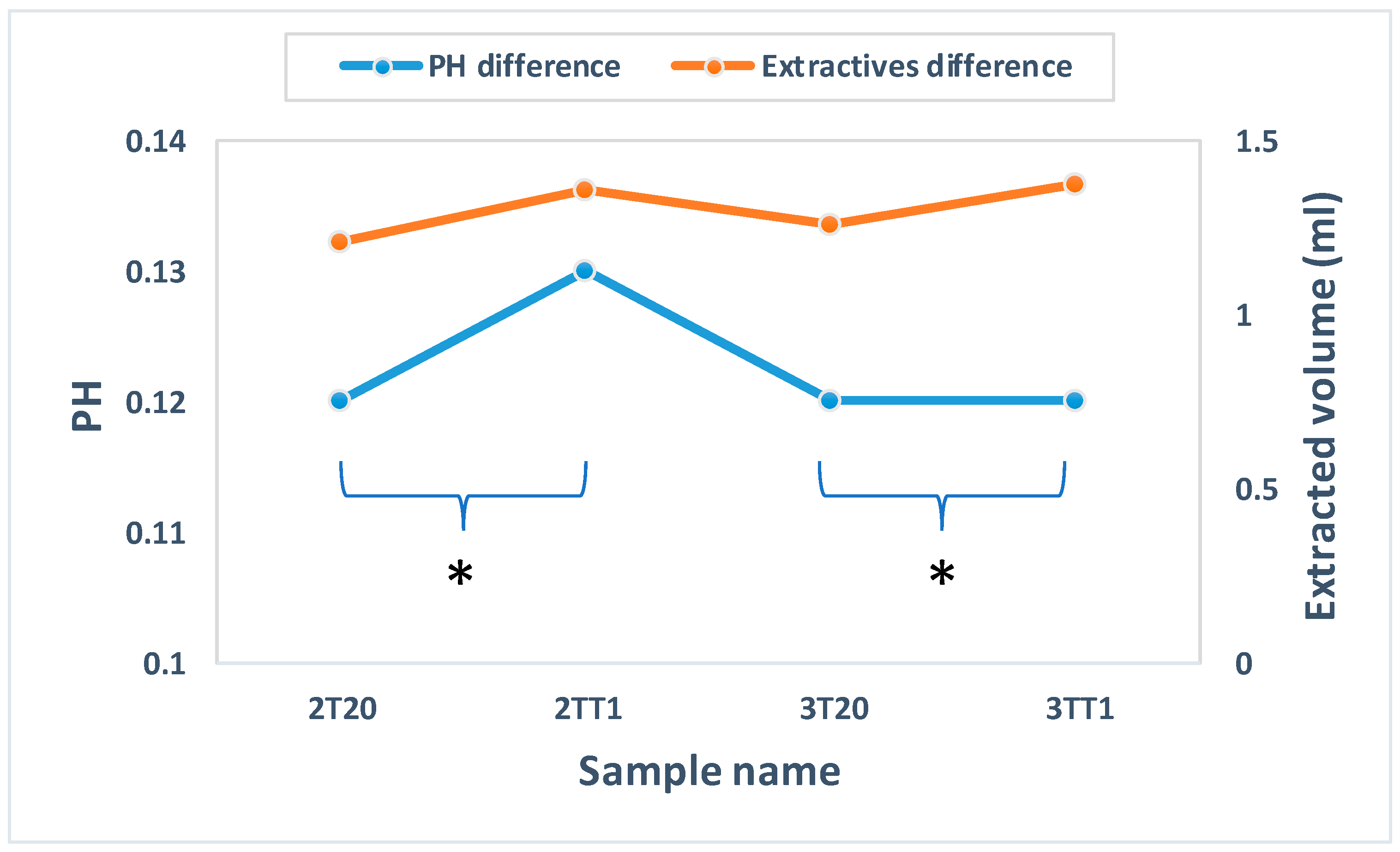
2.1.2. pH and Potassium Permanganate Reducing Analysis
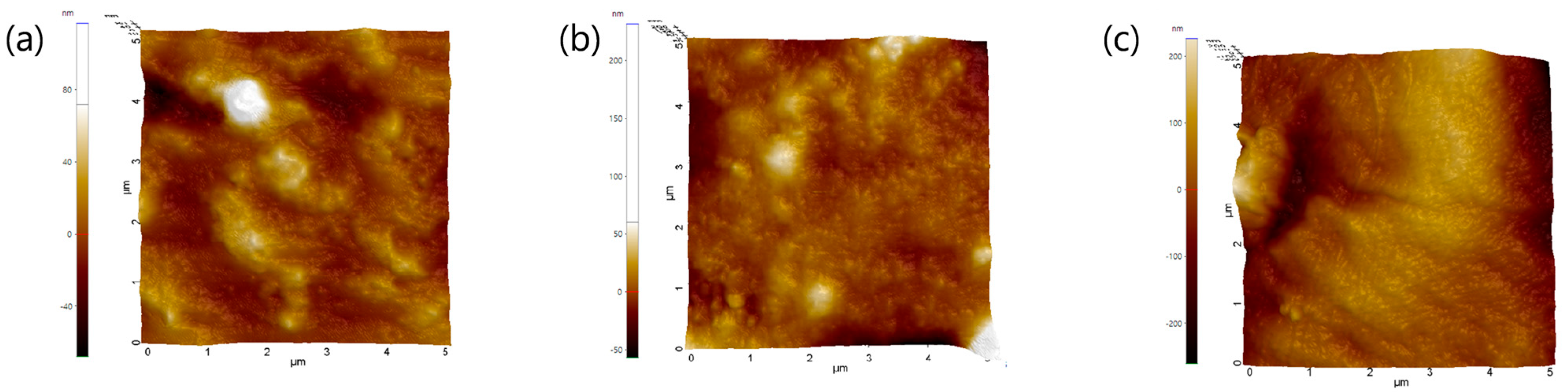
2.2. Physical Properties
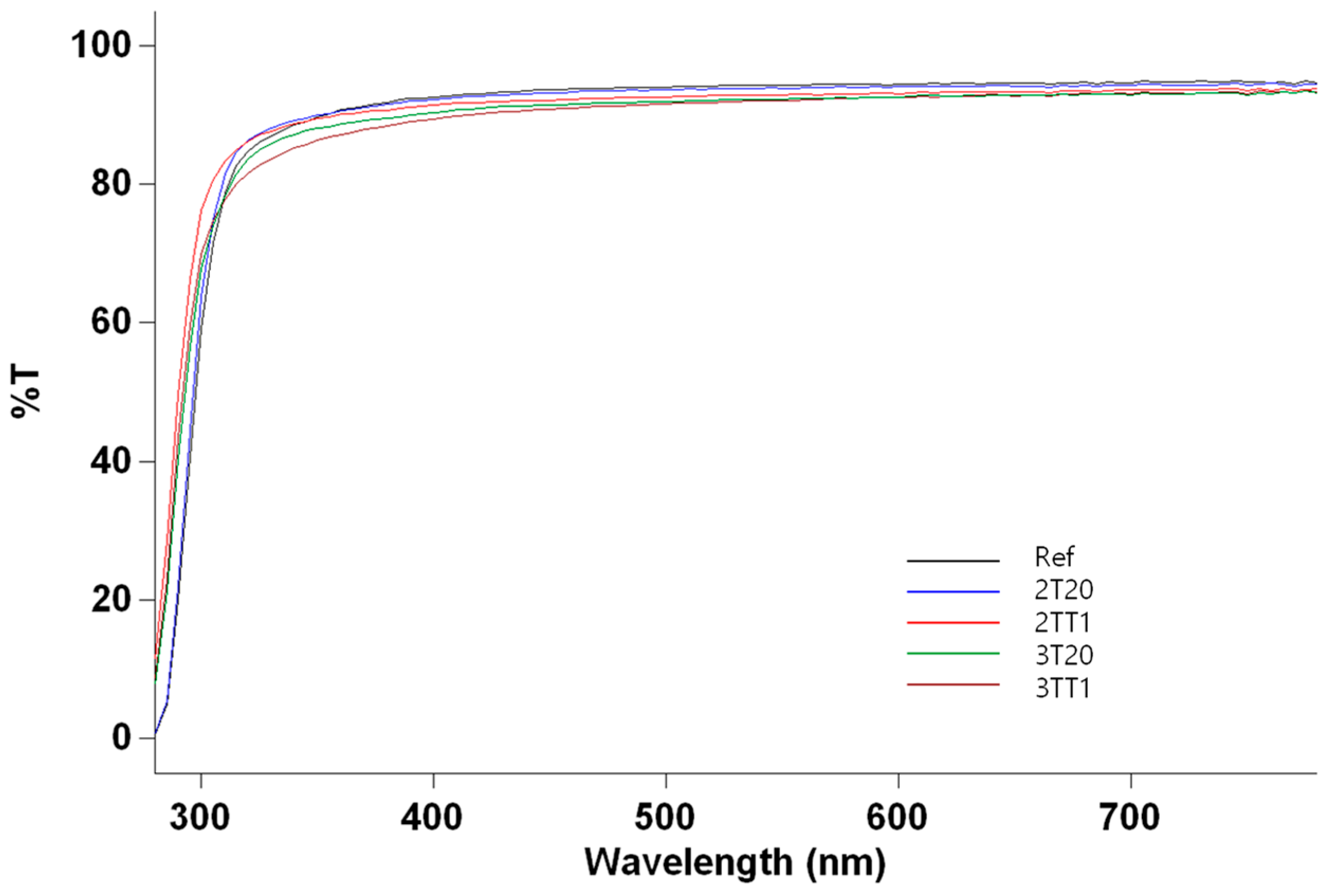
2.2.1. Optical Transmittance
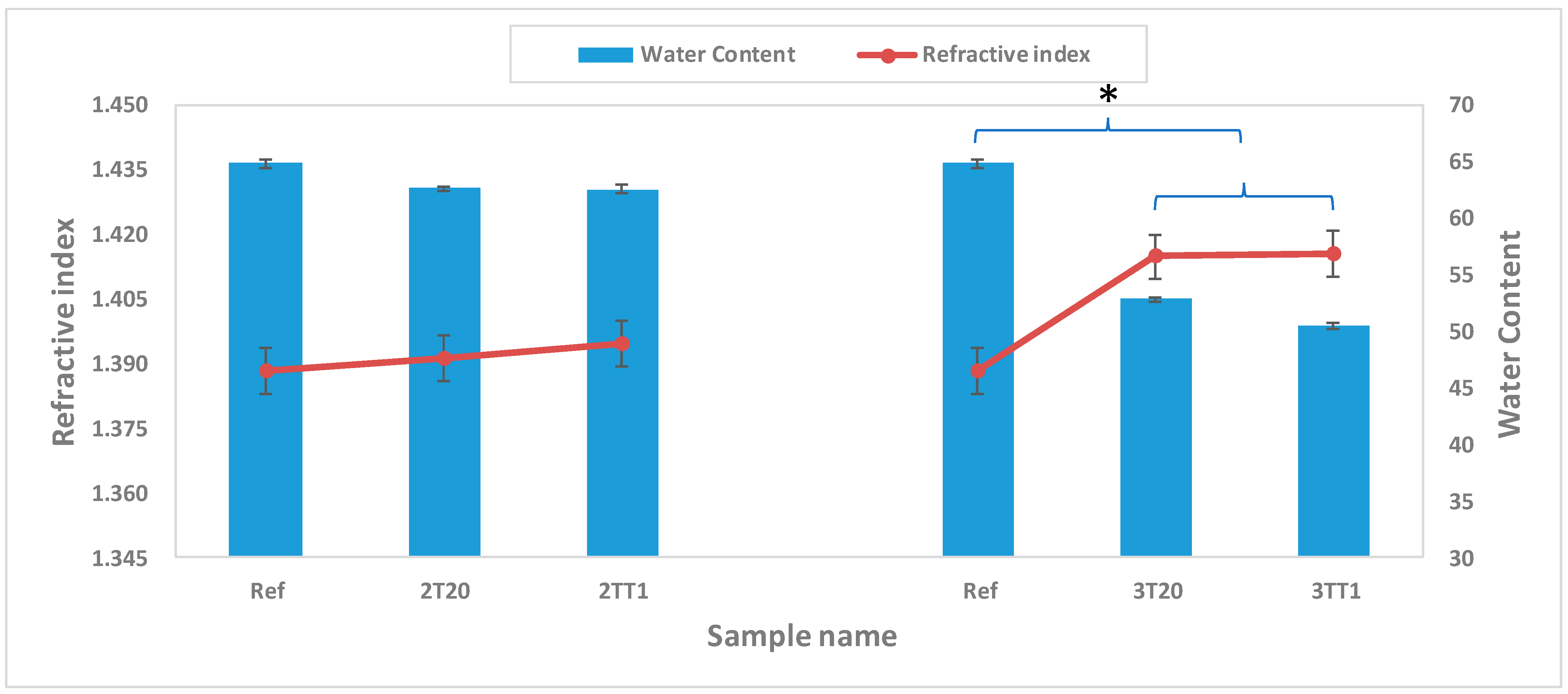
2.2.2. Refractive Index and Water Content
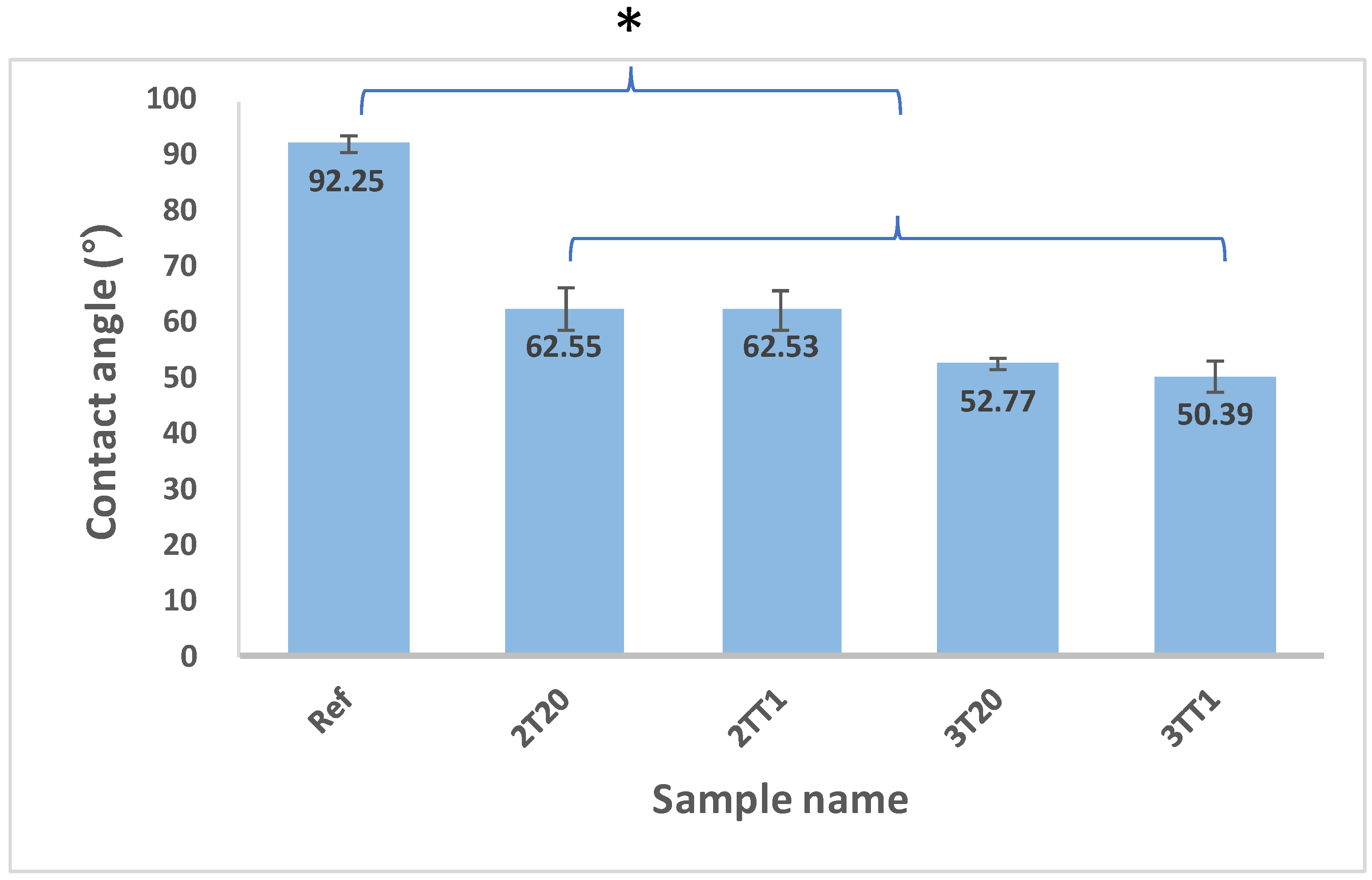
2.2.3. Contact Angle and Water Content
2.2.4. Oxygen Permeability and Water Content
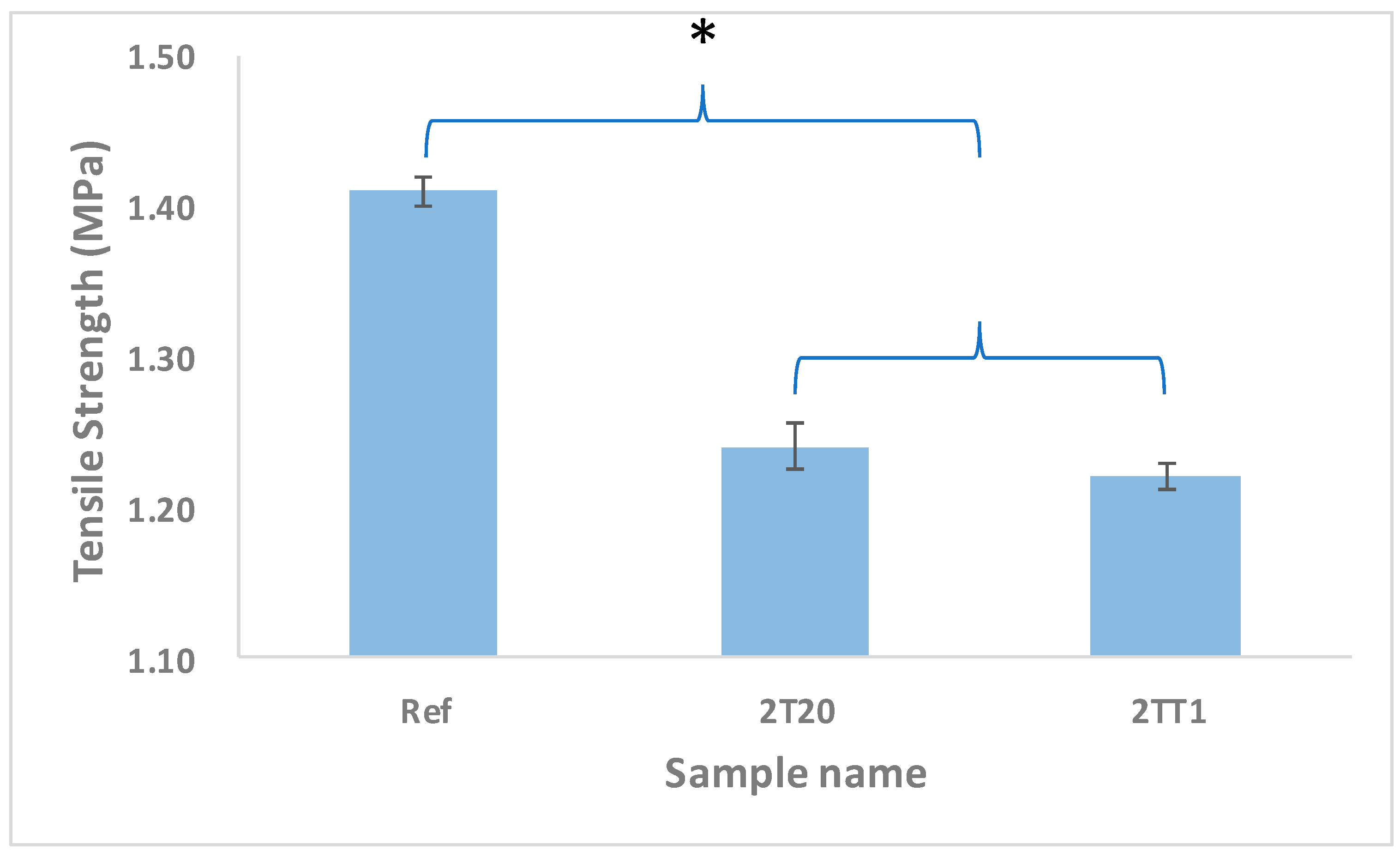
2.2.5. Tensile Strength
2.3. Limitations
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Polymerization
4.3. Experimental Method
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Correa, S.; Grosskopf, A.K.; Hernandez, H.L.; Chan, D.; Yu, A.C.; Stapleton, L.M.; Appel, E.A. Translational Applications of Hydrogels. Chem. Rev. 2021, 121, 11385–11457. [Google Scholar] [CrossRef]
- Kopeček, J. Hydrogels from Soft Contact Lenses and Implants to Self-Assembled Nanomaterials. J. Polym. Sci. A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in Pharmaceutical Formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Musgrave, C.S.A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Buch, J.; Dumbleton, K.; Woods, C.; Fonn, D. Hydration and Oxygen Permeability in Modern Hydrogel Contact Lenses. Eye Contact Lens 2018, 44 (Suppl. 2), S130–S138. [Google Scholar]
- Keir, N.; Jones, L. Wettability and Silicone Hydrogel Lenses: A Review. Eye Contact Lens 2013, 39, 100–108. [Google Scholar] [CrossRef]
- Newham, G.; Evans, S.D.; Ong, Z.Y. Mechanically Tuneable Physical Nanocomposite Hydrogels from Polyelectrolyte Complex Templated Silica Nanoparticles for Anionic Therapeutic Delivery. J. Colloid Interface Sci. 2022, 617, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Salih, A.E.; Elsherif, M.; Alam, F.; Alqattan, B.; Yetisen, A.K.; Butt, H. Syntheses of Gold and Silver Nanocomposite Contact Lenses via Chemical Volumetric Modulation of Hydrogels. ACS Biomater. Sci. Eng. 2022, 8, 2111–2120. [Google Scholar] [CrossRef] [PubMed]
- Sah, R.; Sharma, N.; Priyadarshini, K.; Titiyal, J.S. Contact Lenses for the Treatment of Ocular Surface Diseases. Indian J. Ophthalmol. 2023, 71, 1135–1141. [Google Scholar] [CrossRef]
- Sweeney, D. Clinical signs of hypoxia with high-Dk soft lens extended wear: Is the cornea convinced? Eye Contact Lens. 2003, 29, S22–S25. [Google Scholar] [CrossRef]
- Haworth, K.; Travis, D.; Abariga, S.A.; Fuller, D.; Pucker, A.D. Silicone Hydrogel versus Hydrogel Soft Contact Lenses for Extended Wear. Cochrane Database Syst. Rev. 2021, 10, CD014791. [Google Scholar]
- Tran, N.-P.-D.; Yang, M.-C.; Tran-Nguyen, P.L. Evaluation of silicone hydrogel contact lenses based on poly(dimethylsiloxane) dialkanol and hydrophilic polymers. Colloids Surf. B 2021, 206, 111957. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Z.; Zeng, W.; Zhang, Y.; Zhang, F.; Wu, D.; Wang, Y. Biocompatible Hydrogel Coating on Silicone Rubber with Improved Antifouling and Durable Lubricious Properties. Gels 2024, 10, 647. [Google Scholar] [CrossRef]
- McGrady, G.S.; Sirsch, P.; Chatterton, N.P.; Ostermann, A.; Gatti, C.; Altmannshofer, S.; Herz, V.; Eickerling, G.; Scherer, W. Nature of the bonding in metal-silane σ-complexes. Inorg Chem. 2009, 48, 1588–1598. [Google Scholar] [CrossRef] [PubMed]
- Indumathy, B.; Sathiyanathan, P.; Prasad, G.; Reza, M.S.; Prabu, A.A.; Kim, H. A Comprehensive Review on Processing, Development and Applications of Organofunctional Silanes and Silane-Based Hyperbranched Polymers. Polymer 2023, 15, 2517. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Ahmad, M.; Zhang, B.; Naik, M.-U.; Xie, H.; Zhang, Q. Desirable bonding interactions between organo-functional triazinedithiol groups and heavy metal ions for significantly improved adsorption or dispersion property. Chem. Eng. J. 2022, 442, 136220. [Google Scholar] [CrossRef]
- Réthoré, G.; Boyer, C.; Kouadio, K.; Toure, A.; Lesoeur, J.; Halgand, B.; Jordana, F.; Guicheux, J.; Weiss, P. Silanization of Chitosan and Hydrogel Preparation for Skeletal Tissue Engineering. Polymers 2020, 12, 2823. [Google Scholar] [CrossRef]
- Tran, N.-P.-D.; Yang, M.-C. The Ophthalmic Performance of Hydrogel Contact Lenses Loaded with Silicone Nanoparticles. Polymers. 2020, 12, 1128. [Google Scholar] [CrossRef] [PubMed]
- ISO 18369-4:2017; Ophthalmic Optics—Contact Lenses—Part 4: Physicochemical Properties of Contact Lens Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Walsh, K.; Jones, L.W.; Morgan, P.; Papas, E.B.; Sulley, A. Topical review: Twenty-five years of silicone hydrogel soft contact lenses. Optom. Vis. Sci. 2025, 102, 361–374. [Google Scholar] [CrossRef]
- Towler, J.; Lin, W.-P.; Wu, L.-Y.; Abass, R.; Wu, R.; Fathy, A.; Alanazi, R.; Davies, J.; Abass, A. Are Soft Silicone Hydrogel Contact Lenses More Compliant in a Warm, Hydrated Environment? Processes 2025, 13, 3290. [Google Scholar] [CrossRef]
- Bhamra, T.S.; Burns, J.; Callender, M.; Golding, T.; Allen, P.; Phillips, M. Mechanical properties of contact lens materials: The contribution of material structure and wear time. J. Optom. 2017, 10, 299–308. [Google Scholar]
- Mazhar, H.; Shehzad, F.; Hong, S.-G.; Al-Harthi, M.A. Thermal Degradation Kinetics Analysis of Ethylene-Propylene Copolymer and EP-1-Hexene Terpolymer. Polymer 2022, 14, 634. [Google Scholar] [CrossRef]
- Gao, J.; Mei, J.; Xiong, H.; Han, X. Effect of Silane Coupling Agents on Structure and Properties of Carbon Fiber/Silicon Rubber Composites Investigated by Positron Annihilation Spectroscopy. Molecules 2025, 30, 1658. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Yao, J.; Mu, Q.; Peng, D.; Zhao, H.; Yang, Z. Study on the Synthesis and Thermal Stability of Silicone Resin Containing Trifluorovinyl Ether Groups. Polymers 2020, 12, 2284. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Park, S.-Y.; Sung, A.-Y. Ophthalmic Hydrogel Contact Lens Material Containing Magnesium Oxide Nanoparticles and 3-(Trifluoromethyl)styrene for Biomedical Application. Micromachines 2022, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Park, S.-Y.; Sung, A.-Y. Characterization of Biocompatible Hydrogel Lenses Using Methacrylic Acid with Neodymium Oxide Nanoparticles. Polymers 2021, 13, 1575. [Google Scholar] [CrossRef]
- Tran, N.-P.-D.; Yang, M.-C.; Lin, C.-H. Synthesis and Characterization of Silicone Contact Lenses Based on TRIS-DMA-NVP-HEMA Hydrogels. Polymers 2019, 11, 944. [Google Scholar] [CrossRef]
- Wuchte, L.; DiPasquale, S.; Masterson, A.; Vance, A.; Goff, J.; Arkles, B.; Sulaiman, S.; Byrne, M. Characterization and analysis of extended-wear silicone hydrogel contact lenses utilizing novel silicone macromers. J. Biomed. Mater. Res. A 2022, 110, 1512–1523. [Google Scholar] [CrossRef]
- González-Méijome, J.M.; López-Alemany, A.; Rodríguez-Gascón, A.; Alió, J.L. Refractive Index and Equilibrium Water Content of Conventional and Silicone Hydrogel Contact Lenses. Ophthalmic Physiol. Opt. 2006, 26, 57–64. [Google Scholar] [CrossRef]
- González-Méijome, J.M.; López-Alemany, A. Equivalences between Refractive Index and Equilibrium Water Content of Conventional and Silicone Hydrogel Soft Contact Lenses from Automated and Manual Refractometry. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 82B, 49–61. [Google Scholar] [CrossRef]
- Qu, S.; Tang, Y.; Ning, Z.; Zhou, Y.; Wu, H. Desired properties of polymeric hydrogel vitreous substitute. Biomed. Pharmacother. 2024, 172, 116154. [Google Scholar] [CrossRef]
- Melčová, V.; Krobot, Š.; Šindelář, J.; Šebová, E.; Rampichová, M.K.; Přikryl, R. The effect of surface roughness and wettability on the adhesion and proliferation of Saos-2 cells seeded on 3D printed poly(3-hydroxybutyrate)/polylactide (PHB/PLA) surfaces. Results Surf. Interfaces 2024, 16, 100271. [Google Scholar] [CrossRef]
- Eftimov, P.; Sivakumar, P.; Bhat, S.; Mandal, B.; Zhou, M. Relationships between the Material Properties of Silicone Hydrogel Contact Lenses: Wettability, Modulus, Hydration, Contact Angle Hysteresis, and Friction. J. Biomater. Appl. 2011, 26, 85–99. [Google Scholar]
- Lin, C.-H.; Tsai, W.-Y.; Wang, J.-W. Improvement of the Surface Wettability of Silicone Hydrogel Materials by PEM (Polyelectrolyte Multilayer) Grafting. J. Colloid Interface Sci. 2015, 445, 96–104. [Google Scholar]
- Efron, N.; Morgan, P.B.; Cameron, I.D.; Brennan, N.A.; Goodwin, M. Oxygen Permeability and Water Content of Silicone Hydrogel Contact Lens Materials. Optom. Vis. Sci. 2007, 84, E328–E337. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wiseman, M.E.; Seitz, M.; Tomić, K.; Heise, A.; Brougham, D.F.; Litvinov, V.M. Impact of morphology on O2 permeability in silicone hydrogel membranes: New insights into domain percolation from experiments and simulations. J. Membr. Sci. 2021, 621, 118970. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, H.; An, S.; Jiang, Y. The relationship between oxygen permeability and phase separation morphology of the multicomponent silicone hydrogels. J. Phys. Chem. B 2014, 118, 14640–14647. [Google Scholar] [CrossRef]
- Chang, F.; Vogot, J. Method for Making Silicone Hydrogel Contact Lenses. U.S. Patent No. US8506856B2, 13 August 2013. [Google Scholar]
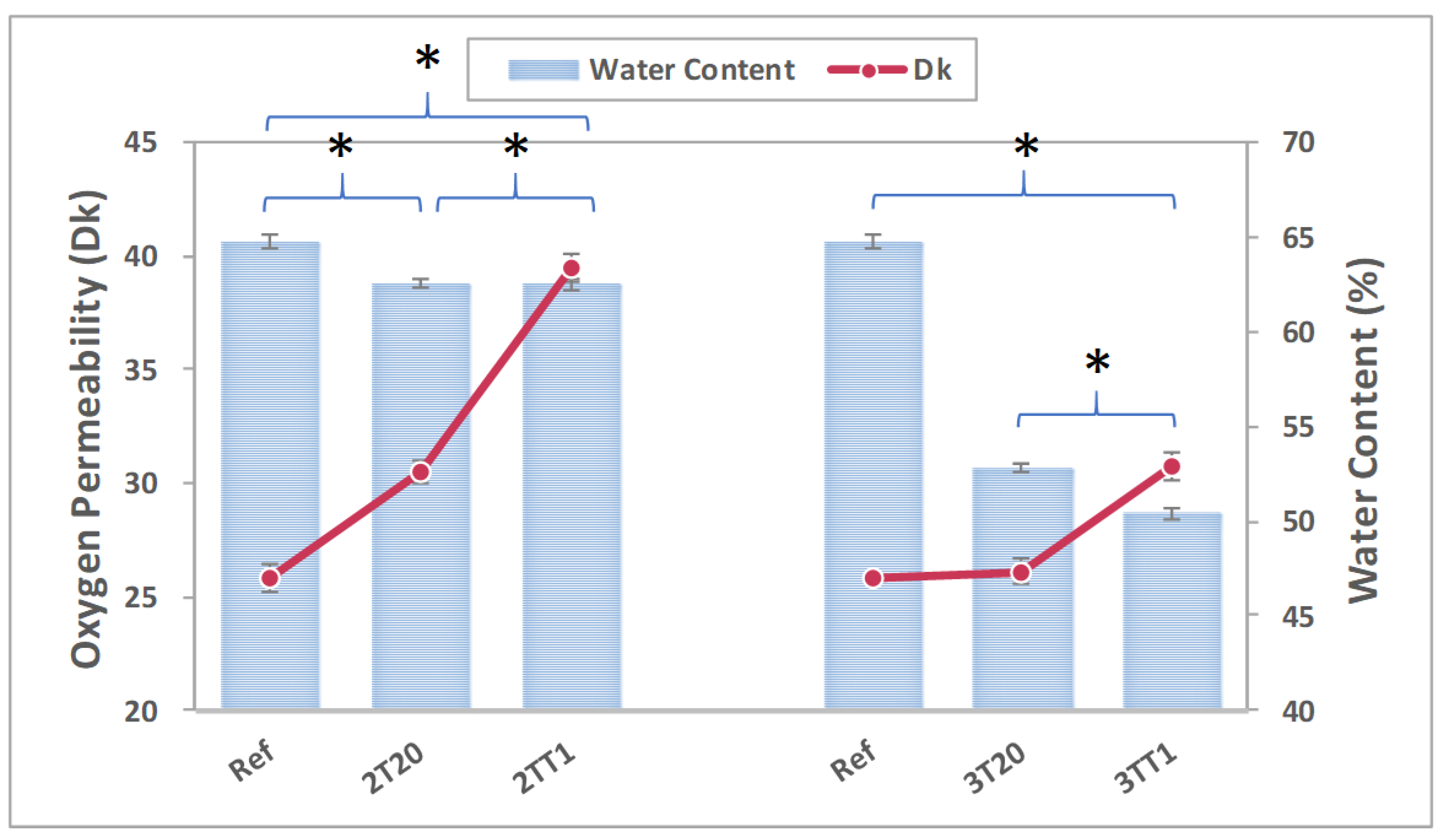
| Sample Name | Dk (Barrer) | ΔDk (Barrer) | Additive Expectation (Barrer) | Excess Over Additive Expectation (Barrer) | Synergy (%) |
|---|---|---|---|---|---|
| Ref | 25.79 ± 1.29 | ||||
| 2T20 | 30.53 ± 2.47 | +4.74 | |||
| 2TT1 | 39.52 ± 3.40 | +13.73 | 30.53 | 8.99 | 34.86 |
| Sil-OH | DMA | MMA | MA | EGDMA | AIBN | 2TSEMA * | 3TRIS ** | TRIS *** | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ref | 32.29 | 64.58 | 0.97 | 0.97 | 0.99 | 0.20 | - | - | - | 100 |
| 2T10 | 29.36 | 58.71 | 0.88 | 0.88 | 0.90 | 0.18 | 9.09 | - | - | 100 |
| 2T20 | 26.91 | 53.82 | 0.81 | 0.81 | 0.82 | 0.16 | 16.67 | - | - | 100 |
| 2T30 | 24.84 | 49.68 | 0.75 | 0.75 | 0.76 | 0.15 | 23.08 | - | - | 100 |
| 3T10 | 29.36 | 58.71 | 0.88 | 0.88 | 0.90 | 0.18 | - | 9.09 | - | 100 |
| 3T20 | 26.91 | 53.82 | 0.81 | 0.81 | 0.82 | 0.16 | - | 16.67 | - | 100 |
| 3T30 | 24.84 | 49.68 | 0.75 | 0.75 | 0.76 | 0.15 | - | 23.08 | - | 100 |
| 2TT1 | 26.64 | 53.29 | 0.80 | 0.80 | 0.82 | 0.16 | 16.50 | - | 0.99 | 100 |
| 3TT1 | 26.64 | 53.29 | 0.80 | 0.80 | 0.82 | 0.16 | 16.50 | 0.99 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-J.; Sung, A.-Y. Synergistic Enhancement of Oxygen Permeability in Silane-Modified Hydrogel Networks for Advanced Ophthalmic Applications. Gels 2025, 11, 987. https://doi.org/10.3390/gels11120987
Lee M-J, Sung A-Y. Synergistic Enhancement of Oxygen Permeability in Silane-Modified Hydrogel Networks for Advanced Ophthalmic Applications. Gels. 2025; 11(12):987. https://doi.org/10.3390/gels11120987
Chicago/Turabian StyleLee, Min-Jae, and A-Young Sung. 2025. "Synergistic Enhancement of Oxygen Permeability in Silane-Modified Hydrogel Networks for Advanced Ophthalmic Applications" Gels 11, no. 12: 987. https://doi.org/10.3390/gels11120987
APA StyleLee, M.-J., & Sung, A.-Y. (2025). Synergistic Enhancement of Oxygen Permeability in Silane-Modified Hydrogel Networks for Advanced Ophthalmic Applications. Gels, 11(12), 987. https://doi.org/10.3390/gels11120987






