- Article
Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics
- Lidija Ćurković,
- Sanja Štefančić and
- Irena Žmak
- + 3 authors
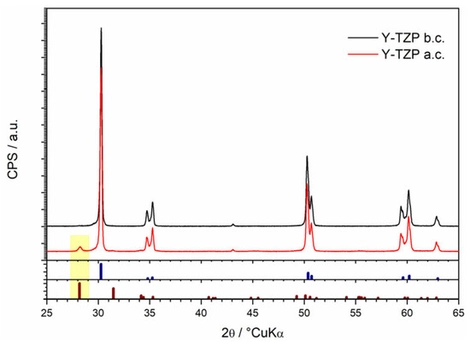
In this study, the chemical solubility (stability) of yttria-partially stabilized zirconia (2.3Y-TZP) dental ceramics, both glazed (Group 2) and non-glazed samples (Group 1), was evaluated using a modified testing protocol based on ISO 6872:2024. Chemical stability was assessed by measuring ion release with inductively coupled plasma mass spectrometry (ICP-MS) and by analyzing phase composition with X-ray diffraction (XRD). While ISO 6872 prescribes chemical stability testing in a 4 wt.% aqueous acetic acid solution at 80 °C for 16 h, the exposure duration in this study was extended to 768 h (32 days) to allow a more accurate determination of long-term solubility behavior. Additionally, the surface roughness parameters (Ra, Rmax, Rz, Sa, Sq) were analyzed and evaluated before and after solubility testing. Kinetic analysis revealed that degradation followed a near-parabolic rate law, with power-law exponents of n = 2.261 for Group 1 and n = 1.935 for Group 2. The corresponding dissolution rate constants were 3.85 × 10−5 µgn·cm−2n·h−1 for Group 1 and 132.3 µgn·cm−2n·h−1 for Group 2. XRD results indicated that the long exposure to acetic acid induced a partial phase transformation of zirconia from the tetragonal to the monoclinic phase. Under prolonged acetic exposure, the glaze layer on 2.3Y-TZP exhibited significantly higher dissolution, whereas the zirconia (polished, unglazed) showed low ion release. The temporal change in the total amount of dissolved ions was statistically analyzed for Group 1 and Group 2. The samples showed a strong correlation, but ANOVA confirmed significant differences between them.
8 October 2025