Recent Advances in 3D Bioprinting of Porous Scaffolds for Tissue Engineering: A Narrative and Critical Review
Abstract
1. Introduction
2. Porosity
2.1. Chemically Derived Porosity or Molecular Porosity
- Intraparticle porosity refers to the voids confined within a particle, microstructure, or polymer network. These pores may be closed, open, or interconnected, and their size can fall within the micro or nanometric range depending on the synthesis method and the base material [27]. This type of porosity is essential in adsorbent materials such as activated carbon, widely used in purification and filtering processes for gaseous or liquid contaminants, as in the adsorption of toxic compounds present in tobacco smoke [28]. At the biological level, although the term porosity is not strictly applied, internal functional cavities have been described in transmembrane proteins such as the Na+/K+-ATPase, whose conformational structure enables the opening and closing of ion channels, allowing for the active transport of ions against the electrochemical gradient [29].
- Interparticle porosity, in contrast, corresponds to the void spaces generated between particles, fibers, or polymer chains. This type of porosity depends mainly on the size, morphology, and packing density of the structural units, as well as the interaction forces between them [30]. In molecular or colloidal systems, these interactions determine the degree of compaction and therefore the volumetric fraction of free pore space [31]. A representative case is found in cementitious materials, where hydration leads to the formation of new solid products that generate an interconnected porous network, modifying the mechanical properties and permeability of the concrete [32]. Another example can be seen in hydrogels, which are materials composed of three-dimensional networks of hydrated polymers, where interparticle porosity is regulated by polymer concentration, degree of crosslinking, and the drying methods used. Techniques such as freeze-drying or solvent drying remove water and generate secondary pores, which are useful for tuning the structure and functionality of the material [33].
2.2. Processing-Induced Porosity
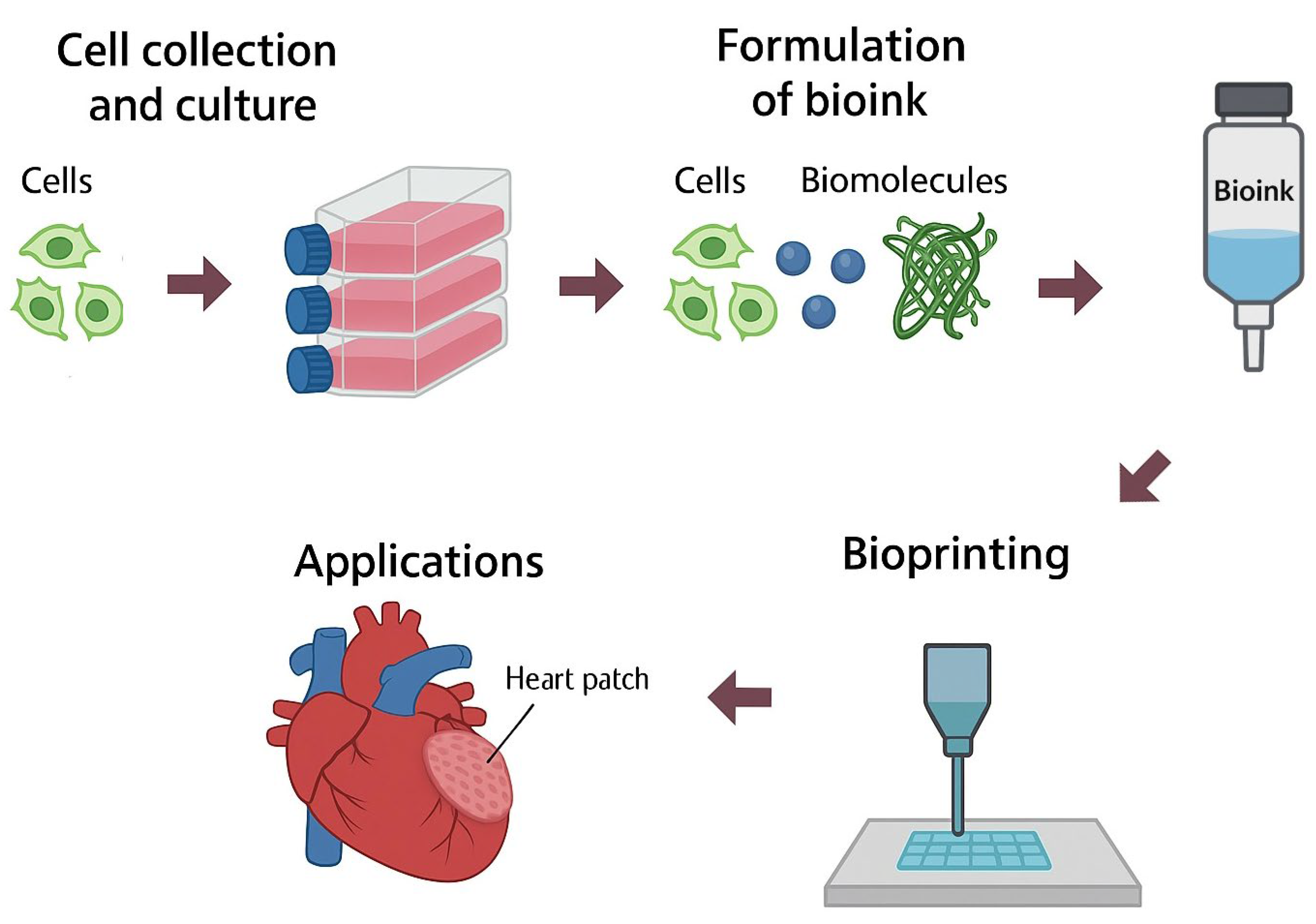
3. Pore Manufacturing
4. Pore Characteristics
4.1. Pore Size
| Type | Name | Aproximate Size (Diameter, µm) | Reference |
|---|---|---|---|
| Macromolecules | Albumin | 0.007 µm | Tojo and Kinugasa (2012) [55] |
| Cholesterol | 0.0239 µm | PDBe-KB [56] | |
| Triglyceride | 0.0015–0.002 µm (up to 1000 µm forming networks in Adipocytes) | A. Penagos et al. (2024) [57], Patterson (2009) [58] | |
| Antibodies | 0.0035 µm | Meyer-Tamaki (2013) [59] | |
| Collagen molecule | 0.0015 µm | Van den Berg (2012) [60] | |
| DNA chromatin fiber | 0.005–0.023 µm | Ou et al. (2017) [61] | |
| Cellular structures | Nucleus | 10 µm | Sun et al. (2000) [62] |
| Mitochondria (80S) | 0.5–10 µm | Duranova et al. (2020) [63] | |
| Ribosome | 0.025–0.030 µm | Khatter et al. (2015) [64] | |
| Vesicles | 0.020–0.560 µm | Chernyshev et al. (2015) [65] | |
| Exosome | 0.040–0.1 µm | Chen et al. (2019) [66] | |
| Cellular types | Osteoblast | 10–15 µm | Kassem et al. (1992) [67] |
| Chondrocyte | 12–18 µm | C. A. Pole (2019); Bush and Hall (2003) [68,69] | |
| Pancreatic ß-cell | 35 µm | Ginzberg (2015) [70] | |
| Hepatocyte | 40 µm | ||
| Keratocyte | 45 µm | ||
| Fibroblast | 85 µm | ||
| Adipocyte | 110 µm | ||
| Erythrocyte | 7–8 µm | Fabry (1981); Vömel (1980) [71,72] | |
| Lymphocyte | 6–8 µm | Bagge and Braide (1982); Kuse et al. (1985) [73,74] | |
| Macrophage | 21 µm | Krombach (1997); M. Naito (2008) [75,76] | |
| Monocyte | 9–19 µm | Wang et al. (1992) [77] | |
| Supracellular structures | Osteocyte canaliculi | 0.2–0.42 µm | Kufahl and Saha; Rath Bonivtch et al. (2007) (1990) [78,79] |
| Osteocyte lacuna | 5–6 µm | Rath Bonivtch et al. (2007) [79] | |
| Aorta | 25,000 µm | William C. Parke (2020); Connor et al. (2022); Tajeddin and Mustafaoglu (2021) [80,81,82]. | |
| Artery | 4000 µm | ||
| Arteriole | 10–100 µm | ||
| Terminal arteriole | 5–10 µm | ||
| Capillary | 5 µm | ||
| Venule | 10–200 µm | ||
| Vein | 5000 µm | ||
| Vena cava | 30,000 µm |
| Ranking | IUPAC [54] | Schwarz and Epple (1998) [83] | R.A. Perez and G. Mestres (2016) [84] | P. Habibovic et al. (2005) [85] | F. Junior Maksoud et al. (2022) [14] | M. Ebrahimi (2021) [42] |
|---|---|---|---|---|---|---|
| Macropore | >50 × 10−3 µm | >1 × 105 µm | >5 × 104 µm | - | (1–5) × 105 µm | >1 × 105 µm |
| Mesopore | (2–50) × 10−3 µm | - | - | - | - | - |
| Micropore | <2 × 10−3 µm | <1 × 103 µm | <5 × 104 µm | <1 × 104 µm | 0.1 µm–1 × 105 µm | (1– 100) × 102 µm |
| Submicropore | - | - | - | - | - | 0.1–100 µm |
| Nanopore | - | - | - | - | <0.1 µm | <0.1 µm |
| Tissue Engineered | Achivement | Pore Size | Porosity% | Material | Reference |
|---|---|---|---|---|---|
| Bone and Dental | Cortical bone regeneration | <212 μm | 27–37% | Titanium | C. Torres-Sanchez et al. (2017) [89] |
| 1–2 μm | - | Demineralised bone matrix | D. Henrich et al. (2015) [91] | ||
| Trabecular bone regeneration | 300–500 μm | 54–58% | Titanium | C. Torres-Sanchez et al. (2017) [89] | |
| Osteoconductivity, Osteogenesis and Angiogenesis | >150 μm | - | Bioceramic based scaffolds | H. Jodati et al. (2020) [86] | |
| Osteogenesis | 350 μm | - | Bioceramic based scaffolds | M. A. Velasco et al. (2015) [10] | |
| Bone marrow regeneration | 100–500 μm | 60% | b-TCP | D. Henrich et al. (2015) [91] | |
| Bone formation | 300–635 μm | 20–60% | Titanium | W. Xu et al. (2022) [92] | |
| Proliferation and differentiation of dental pulp stem cells | 251–425 μm | - | PLLA | C. M. Conde et al. (2015) [93] | |
| 65 μm | - | Collagen | Q. Zhang et al. (2022) [94] | ||
| 200–300 μm | - | Calcium Polyphosphate | F. M. Wang et al. (2006) [95] | ||
| Dental neovascularization and osteogenesis | 750–900 μm | 85% | Hydroxyapatite | P. Li et al. (2023) [96] | |
| Vascular | Vascular smooth muscle cells | 60–150 μm | - | PLLA | Y. Wang et al. (2014) [97] |
| Microvascular epithelial cells | 38–50 μm | - | PLLA | J. Zeltinger et al. (2001) [98] | |
| Cartilage | Angiogenesis and chondrocyte proliferation | 280–340 μm | 87–94% | PLGA and cell-Free Fat extract | J. Ding et al. (2024) [99] |
| Chondrogenesis | 100 μm | - | Bioceramic based scaffold | M. A. Velasco et al. (2015) [10] | |
| 150–250 μm | - | Collagen type I | Q. Zhang et al. (2014) [100] | ||
| 90–250 μm | - | Silk fibroin | K. S. Han et al. (2015) [101] | ||
| Chondrocyte proliferation | 245 μm | - | Agarose and Snail mucus | V. A. Ajisafe et al. (2024) [102] | |
| 100–300 μm | 93% | Human-Like Collagen and Bovine Serum Albumin | X. Song et al. (2017) [103] | ||
| 90–250 μm | - | Silk fibroin | K. S. Han et al. (2015) [101] | ||
| Dermal | Keratinocyte differentiation | 100–150 μm | - | Fish gellatin and Hyaluronic acid | P. Chailom et al. (2025) [104] |
| Skin wound repair | 130 μm | - | Collagen, Hyaluronic acid and Gelatin | H. M. Wang et al. (2013) [105] | |
| Fibroblast adhesion and proliferation | 100–200 μm | >85% | Silk fibroin and hair-derived keratin | N. Bhardwaj et al. (2015) [106] | |
| Neural | Peripheral axon generation | 75 μm | - | Hexamethylene diisocyanate, Poly(epsilon-caprolactone) and Dianhydro-D-sorbitol | I. Bružauskaitė et al. (2016) [107] |
| Schawn cells | 20–50 μm | - | Collagen type I | A. Bozkurt et al. (2009) [108] | |
| Direct growth of the neurons | 20–30 μm | - | Collagen coated photosensitive polyimide. | M. J. Mahoney et al. (2005) [109] | |
| Neuronal differentiation | 50–350 μm | - | Chitosan | X. Yi et al. (2011) [19] | |
| 95 μm | - | Collagen and Gycosaminoglycans | A. Kourgiantaki et al. (2020) [110] |
4.2. Porosity Percentage (%)
4.3. Porosity Connectivity
- DB is the bulk diffusion coefficient, which depends on the molecular species and the solvent, but is independent of the scaffold’s structure;
- ε is the porosity of the scaffold;
- f is the diffusion–constriction factor; accounting for pore shape and connectivity;
- τ is the tortuosity index.
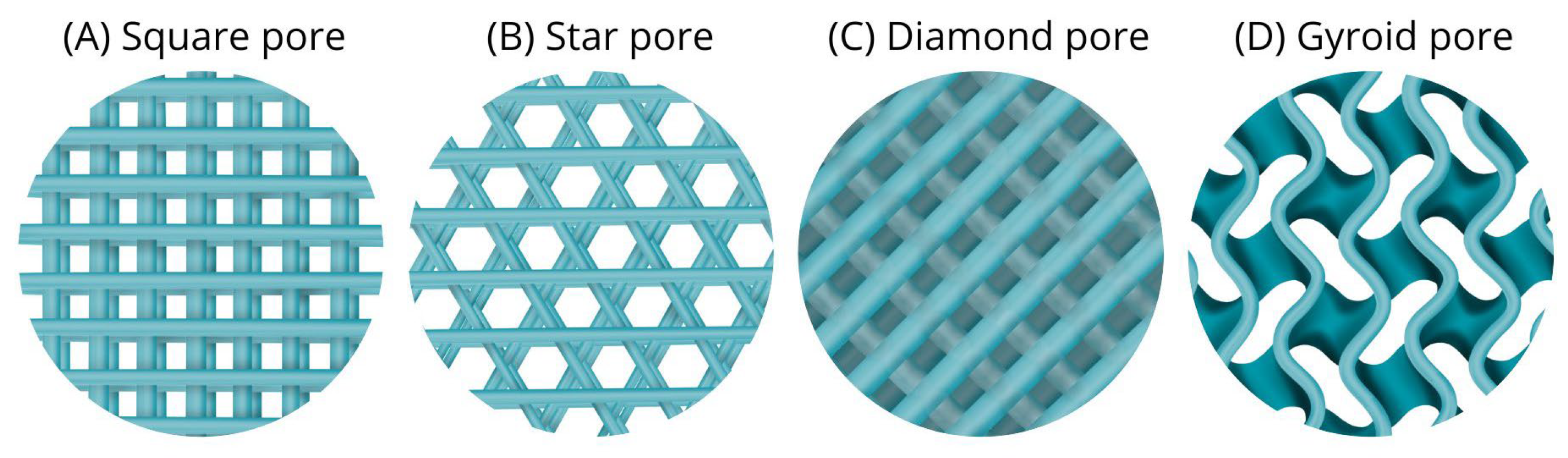
4.4. Pore Shape (Geometry)
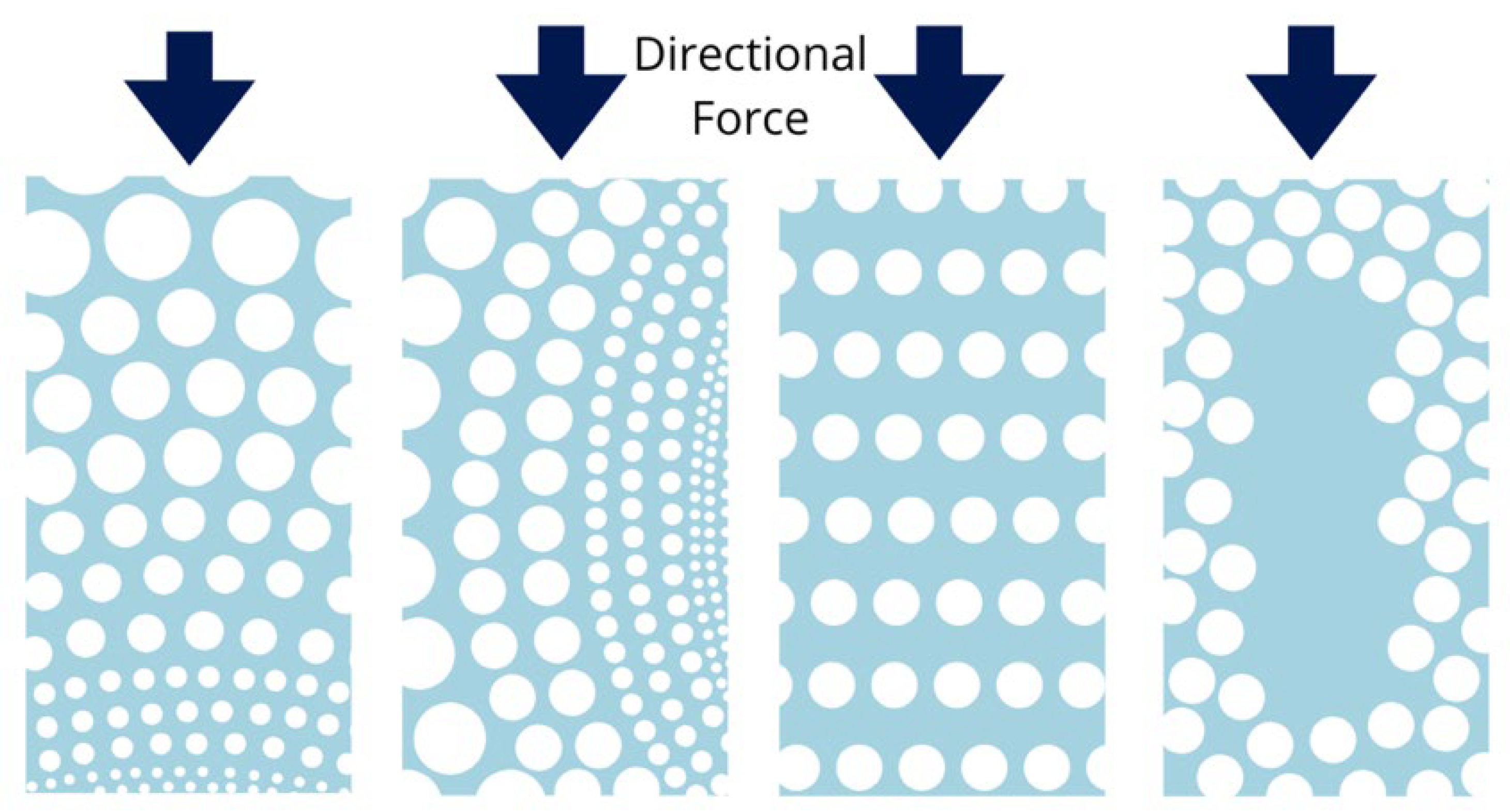
4.5. Pore Distribution
5. Porosity Assessment Techniques
5.1. Mercury Porosimetry
5.2. Liquid Displacement Method
5.3. Capillary Flow Porometry
5.4. Scanning Electron Microscopy (SEM) Analysis
5.5. Microcomputed Tomography (CT) Imaging
6. Discussion
6.1. Critical Analysis of the State of the Art
6.2. Use of Graphical Simulations in Design
6.3. Limitations
7. Conclusions
7.1. Current Limitations
- Lack of standardization in materials, pore geometries, cell types, and fabrication parameters.
- Scarcity of consistent data for tissues beyond bone and cartilage.
7.2. Required Actions
- Establish standardized methods for scaffold characterization (pore size, geometry, porosity percentage, interconnectivity, and spatial distribution).
- Develop tissue-specific porosity classifications considering cell interactions, adhesion molecule expression, and nutrient transport.
- Incorporate in silico simulations to predict scaffold performance under physiological conditions and optimize designs prior to fabrication.
7.3. Research Priorities
- Define robust correlations between scaffold microarchitecture and biological outcomes.
- Combine experimental studies with computational modeling, in vivo validation, multi-material bioprinting, and dynamic porosity approaches.
- Advance biomimetic 3D models that reproduce the mechanical and biochemical properties of native tissues for clinical translation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M. 12—Extracellular matrix: The ideal natural fibrous nanocomposite products. In Applications of Nanocomposite Materials in Orthopedics; Inamuddin, Asiri, A.M., Mohammad, A., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2019; pp. 263–286. [Google Scholar] [CrossRef]
- Hollister, S.J. Scaffold engineering: A bridge to where? Biofabrication 2009, 1, 012001. [Google Scholar] [CrossRef]
- Whitlow, J.; Paul, A.; Polini, A. Bioactive Materials: Definitions and Application in Tissue Engineering and Regeneration Therapy. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment; Marchi, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar] [CrossRef]
- Ahn, Y.; Chang, H.; Baek, J. 3D scaffolds-specific cellular mechanoresponse as a pivotal regulating factor in tissue engineering. JMST Adv. 2024, 6, 121–134. [Google Scholar] [CrossRef]
- Herrada-Manchón, H.; Fernández, M.A.; Aguilar, E. Essential Guide to Hydrogel Rheology in Extrusion 3D Printing: How to Measure It and Why It Matters? Gels 2023, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Mendoza-Cerezo, L.; Rodríguez-Rego, J.M.; Macías-García, A.; Callejas-Marín, A.; Sánchez-Guardado, L.; Marcos-Romero, A.C. Three-Dimensional Bioprinting of GelMA Hydrogels with Culture Medium: Balancing Printability, Rheology and Cell Viability for Tissue Regeneration. Polymers 2024, 16, 1437. [Google Scholar] [CrossRef]
- Shimojo, A.A.M.; Rodrigues, I.C.P.; Perez, A.G.M.; Souto, E.M.B.; Gabriel, L.P.; Webster, T. Scaffolds for Tissue Engineering: A State-of-the-Art Review Concerning Types, Properties, Materials, Processing, and Characterization. In Racing for the Surface: Antimicrobial and Interface Tissue Engineering; Li, B., Moriarty, T.F., Webster, T., Xing, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 647–676. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed Res. Int. 2015, 2015, 729076. [Google Scholar] [CrossRef]
- Stella, J.A.; D’Amore, A.; Wagner, W.R.; Sacks, M.S. On the biomechanical function of scaffolds for engineering load-bearing soft tissues. Acta Biomater. 2010, 6, 2365–2381. [Google Scholar] [CrossRef]
- Byrne, D.P.; Lacroix, D.; Planell, J.A.; Kelly, D.J.; Prendergast, P.J. Simulation of tissue differentiation in a scaffold as a function of porosity, Young’s modulus and dissolution rate: Application of mechanobiological models in tissue engineering. Biomaterials 2007, 28, 5544–5554. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, Z.; Peng, K.; Liu, K.; Xu, P.; Li, J.; Wei, X.; He, X. Integrated evaluation of biomechanical and biological properties of the biomimetic structural bone scaffold: Biomechanics, simulation analysis, and osteogenesis. Mater. Today Bio 2024, 24, 100934. [Google Scholar] [CrossRef]
- Maksoud, F.J.; de la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: A review. J. Mater. Chem. B 2022, 10, 8111–8165. [Google Scholar] [CrossRef] [PubMed]
- Aboalasaad, A.R.R.; Skenderi, Z.; Kolčavová, S.B.; Khalil, A.A.S. Analysis of Factors Affecting Thermal Comfort Properties of Woven Compression Bandages. Autex Res. J. 2020, 20, 178–185. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv. Healthc. Mater. 2022, 11, 2102087. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Kar, R.; Chwatko, M.; Shoga, E.; Cosgriff-Hernandez, E. High porosity PEG-based hydrogel foams with self-tuning moisture balance as chronic wound dressings. J. Biomed. Mater. Res. A 2023, 111, 465–477. [Google Scholar] [CrossRef]
- Pollini, M.; Striani, R.; Paladini, F.; Kiani, A.; Acocella, M.R.; Esposito Corcione, C. Nanotechnological Antibacterial and Conductive Wound Dressings for Pressure Ulcer Prevention. Nanomaterials 2024, 14, 1309. [Google Scholar] [CrossRef]
- Yi, X.; Jin, G.; Tian, M.; Mao, W.; Qin, J. Porous chitosan scaffold and ngf promote neuronal differentiation of neural stem cells in vitro. Neuro Endocrinol. Lett. 2011, 32, 705–710. [Google Scholar]
- Mendoza-Cerezo, L.; Jesús, M.R.-R.; Macías-García, A.; Marcos-Romero, A.C.; Díaz-Parralejo, A. Evolution of bioprinting and current applications. Int. J. Bioprint. 2023, 9, 742. [Google Scholar] [CrossRef]
- Tamir, T.S.; Teferi, F.B.; Hua, X.; Leng, J.; Xiong, G.; Shen, Z.; Liu, Q. A review of advances in 3D and 4D bioprinting: Toward mass individualization paradigm. J. Intell. Manuf. 2024. [Google Scholar] [CrossRef]
- Almeida, H.A.; Bártolo, P.J. Topological Optimisation of Scaffolds for Tissue Engineering. Procedia Eng. 2013, 59, 298–306. [Google Scholar] [CrossRef]
- Khajehmohammadi, M.; Azizi Tafti, R.; Nikukar, H. Effect of porosity on mechanical and biological properties of bioprinted scaffolds. J. Biomed. Mater. Res. A 2023, 111, 245–260. [Google Scholar] [CrossRef]
- Robert, L.B., Jr. Manual of symbols and terminology for physicochemical quantities and units—Appendix II. Pure Appl. Chem. 1976, 46, 71–90. [Google Scholar] [CrossRef]
- Little, M.A.; Cooper, A.I. The Chemistry of Porous Organic Molecular Materials. Adv. Funct. Mater. 2020, 30, 1909842. [Google Scholar] [CrossRef]
- Cooper, A.I. Porous Molecular Solids and Liquids. ACS Cent. Sci. 2017, 3, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tallarek, U. Effect of Intraparticle Porosity and Double Layer Overlap on Electrokinetic Mobility in Multiparticle Systems. Langmuir 2003, 19, 10901–10908. [Google Scholar] [CrossRef]
- Branton, P.; Bradley, R.H. Effects of active carbon pore size distributions on adsorption of toxic organic compounds. Adsorption 2011, 17, 293–301. [Google Scholar] [CrossRef]
- Skou, J.C. Sodium-Potassium Pump. In Membrane Transport: People and Ideas; Tosteson, D.C., Ed.; Springer: New York, NY, USA, 1989; pp. 155–185. [Google Scholar] [CrossRef]
- Schure, M.R.; Maier, R.S.; Shields, T.J.; Wunder, C.M.; Wagner, B.M. Intraparticle and interstitial flow in wide-pore superficially porous and fully porous particles. Chem. Eng. Sci. 2017, 174, 445–458. [Google Scholar] [CrossRef]
- Holst, J.R.; Trewin, A.; Cooper, A.I. Porous organic molecules. Nat. Chem. 2010, 2, 915–920. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, Z.; Duan, Z.; Zhang, C. Pore structure characteristics, modulation and its effect on concrete properties: A review. Constr. Build. Mater. 2023, 397, 132430. [Google Scholar] [CrossRef]
- Digaitis, R.; Falkman, P.; Oltner, V.; Briggner, L.-E.; Kocherbitov, V. Hydration and dehydration induced changes in porosity of starch microspheres. Carbohydr. Polym. 2022, 291, 119542. [Google Scholar] [CrossRef]
- Ura, D.P.; Stachewicz, U. The Significance of Electrical Polarity in Electrospinning: A Nanoscale Approach for the Enhancement of the Polymer Fibers’ Properties. Macromol. Mater. Eng. 2022, 307, 2100843. [Google Scholar] [CrossRef]
- Ji, D.; Lin, Y.; Guo, X.; Ramasubramanian, B.; Wang, R.; Radacsi, N.; Jose, R.; Qin, X.; Ramakrishna, S. Electrospinning of nanofibres. Nat. Rev. Methods Primer 2024, 4, 1. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in Porous Scaffold Design for Bone and Cartilage Tissue Engineering and Regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Holden, D.; Chong, S.Y.; Chen, L.; Jelfs, K.E.; Hasell, T.; Cooper, A.I. Understanding static, dynamic and cooperative porosity in molecular materials. Chem. Sci. 2016, 7, 4875–4879. [Google Scholar] [CrossRef]
- Zdravkov, B.; Čermák, J.; Šefara, M.; Janků, J. Pore Classification in the Characterization of Porous Materials: A Perspective. Open Chemistry 2007, 5, 385–395. [Google Scholar] [CrossRef]
- Mays, T.J. A new classification of pore sizes. In Studies in Surface Science and Catalysis; Llewellyn, P.L., Rodriquez-Reinoso, F., Rouqerol, J., Seaton, N., Eds.; Characterization of Porous Solids VII; Elsevier: Amsterdam, The Netherlands, 2007; Volume 160, pp. 57–62. [Google Scholar]
- Haugen, H.J.; Bertoldi, S. 2—Characterization of morphology—3D and porous structure. In Characterization of Polymeric Biomaterials; Tanzi, M.C., Farè, S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 21–53. [Google Scholar] [CrossRef]
- Gibson, L.J. Cellular Solids. MRS Bull. 2003, 28, 270–274. [Google Scholar] [CrossRef]
- Ebrahimi, M. Porosity parameters in biomaterial science: Definition, impact, and challenges in tissue engineering. Front. Mater. Sci. 2021, 15, 352–373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cheng, D.; Xu, S.; Du, C.; Xie, L.; Zhao, W. Applications of electrospun scaffolds with enlarged pores in tissue engineering. Biomater. Sci. 2022, 10, 1423–1447. [Google Scholar] [CrossRef]
- Macchetta, A.; Turner, I.G.; Bowen, C.R. Fabrication of HA/TCP scaffolds with a graded and porous structure using a camphene-based freeze-casting method. Acta Biomater. 2009, 5, 1319–1327. [Google Scholar] [CrossRef]
- Lee, E.-J.; Koh, Y.-H.; Yoon, B.-H.; Kim, H.-E.; Kim, H.-W. Highly porous hydroxyapatite bioceramics with interconnected pore channels using camphene-based freeze casting. Mater. Lett. 2007, 61, 2270–2273. [Google Scholar] [CrossRef]
- Fiume, E.; Ciavattini, S.; Verné, E.; Baino, F. Foam Replica Method in the Manufacturing of Bioactive Glass Scaffolds: Out-of-Date Technology or Still Underexploited Potential? Materials 2021, 14, 2795. [Google Scholar] [CrossRef]
- Otsuki, B.; Takemoto, M.; Fujibayashi, S.; Neo, M.; Kokubo, T.; Nakamura, T. Pore throat size and connectivity determine bone and tissue ingrowth into porous implants: Three-dimensional micro-CT based structural analyses of porous bioactive titanium implants. Biomaterials 2006, 27, 5892–5900. [Google Scholar] [CrossRef]
- Naghavi, S.A.; Tamaddon, M.; Marghoub, A.; Wang, K.; Babamiri, B.B.; Hazeli, K.; Xu, W.; Lu, X.; Sun, C.; Wang, L.; et al. Mechanical Characterisation and Numerical Modelling of TPMS-Based Gyroid and Diamond Ti6Al4V Scaffolds for Bone Implants: An Integrated Approach for Translational Consideration. Bioengineering 2022, 9, 504. [Google Scholar] [CrossRef]
- Widantha, K.W. Immersion Behavior Study of Hydroxyapatite Scaffolds Derived from Bovine Sources In Acidic, Basic, and Neutral Solutions. Front. Adv. Appl. Sci. Eng. 2024, 2, 68–76. [Google Scholar] [CrossRef]
- Will, J.; Melcher, R.; Treul, C.; Travitzky, N.; Kneser, U.; Polykandriotis, E.; Horch, R.; Greil, P. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J. Mater. Sci. Mater. Med. 2008, 19, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, T.; Tao, M.; Wang, Y.; Yao, X.; Zhu, C.; Xin, F.; Jiang, M. Development of Recombinant Human Collagen-Based Porous Scaffolds for Skin Tissue Engineering: Enhanced Mechanical Strength and Biocompatibility. Polymers 2025, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Tierney, C.M.; Haugh, M.G.; Liedl, J.; Mulcahy, F.; Hayes, B.; O’Brien, F.J. The effects of collagen concentration and crosslink density on the biological, structural and mechanical properties of collagen-GAG scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. Mater. 2009, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Darus, F.; Isa, R.M.; Mamat, N.; Jaafar, M. Techniques for fabrication and construction of three-dimensional bioceramic scaffolds: Effect on pores size, porosity and compressive strength. Ceram. Int. 2018, 44, 18400–18407. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Tojo, A.; Kinugasa, S. Mechanisms of Glomerular Albumin Filtration and Tubular Reabsorption. Int. J. Nephrol. 2012, 2012, 481520. [Google Scholar] [CrossRef]
- PDBe—Knowledge Base. Available online: https://www.ebi.ac.uk/pdbe/pdbe-kb/ (accessed on 10 December 2024).
- Penagos, I.A.; De Witte, F.; Rimaux, T.; Chèvremont, W.; Pintelon, I.; Dewettinck, K.; Van Bockstaele, F. Multiscale analysis of triglycerides using X-ray scattering: Implementing a shape-dependent model for CNP characterization. Soft Matter 2024, 20, 5071–5085. [Google Scholar] [CrossRef]
- Patterson, H.B.W. Chapter 1—Basic Components and Procedures. In Bleaching and Purifying Fats and Oils, 2nd ed.; List, G.R., Ed.; AOCS Press: Champaign, IL, USA, 2009; pp. 1–52. [Google Scholar]
- Meyer-Tamaki, K.B. Chapter 21—Preclinical Development of Monoclonal Antibodies. In A Comprehensive Guide to Toxicology in Preclinical Drug Development; Faqi, A.S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 489–516. [Google Scholar] [CrossRef]
- Van den Berg, F. 4.3–Extracellular matrix. In Fascia: The Tensional Network of the Human Body; Schleip, R., Findley, T.W., Chaitow, L., Huijing, P.A., Eds.; Churchill Livingstone: Oxford, UK, 2012; pp. 165–170. [Google Scholar] [CrossRef]
- Ou, H.D.; Phan, S.; Deerinck, T.J.; Thor, A.; Ellisman, M.H.; O’Shea, C.C. ChromEMT: Visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 2017, 357, eaag0025. [Google Scholar] [CrossRef]
- Sun, H.B.; Shen, J.; Yokota, H. Size-Dependent Positioning of Human Chromosomes in Interphase Nuclei. Biophys. J. 2000, 79, 184–190. [Google Scholar] [CrossRef]
- Duranova, H.; Valkova, V.; Knazicka, Z.; Olexikova, L.; Vasicek, J. Mitochondria: A worthwhile object for ultrastructural qualitative characterization and quantification of cells at physiological and pathophysiological states using conventional transmission electron microscopy. Acta Histochem. 2020, 122, 151646. [Google Scholar] [CrossRef] [PubMed]
- Khatter, H.; Myasnikov, A.G.; Natchiar, S.K.; Klaholz, B.P. Structure of the human 80S ribosome. Nature 2015, 520, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, V.S.; Rachamadugu, R.; Tseng, Y.H.; Belnap, D.M.; Jia, Y.; Branch, K.J.; Butterfield, A.E.; Pease, L.F.; Bernard, P.S.; Skliar, M. Size and shape characterization of hydrated and desiccated exosomes. Anal. Bioanal. Chem. 2015, 407, 3285–3301. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Sung, C.W.-H.; Chen, C.; Cheng, C.-M.; Lin, D.P.-C.; Huang, C.-T.; Hsu, M.-Y. Advances in exosomes technology. Clin. Chim. Acta 2019, 493, 14–19. [Google Scholar] [CrossRef]
- Kassem, M.; Rungby, J.; Mosekilde, L.; Eriksen, E.F. Ultrastructure of human osteoblasts and associated matrix in culture. APMIS 1992, 100, 490–497. [Google Scholar] [CrossRef]
- Poole, C.A. Articular cartilage chondrons: Form, function and failure. J. Anat. 1997, 191, 1–13. [Google Scholar] [CrossRef]
- Bush, P.G.; Hall, A.C. The volume and morphology of chondrocytes within non-degenerate and degenerate human articular cartilage. Osteoarthr. Cartil. 2003, 11, 242–251. [Google Scholar] [CrossRef]
- Ginzberg, M.B.; Kafri, R.; Kirschner, M. On being the right (cell) size. Science 2015, 348, 1245075. [Google Scholar] [CrossRef]
- Fabry, M.E.; Kaul, D.K.; Raventos, C.; Baez, S.; Rieder, R.; Nagel, R.L. Some aspects of the pathophysiology of homozygous Hb CC erythrocytes. J. Clin. Investig. 1981, 67, 1284–1291. [Google Scholar] [CrossRef]
- Vömel, T.; Platt, D.; Strobelt, W. Diameters of erythrocytes of different ages measured by scanning electron-microscopy. Mech. Ageing Dev. 1980, 13, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Bagge, U.; Braide, M. Leukocyte Plugging of Capillaries in Vivo. In White Blood Cells: Morphology and Rheology as Related to Function; Bagge, U., Born, G.V.R., Gaehtgens, P., Eds.; Springer: Dordrecht, The Netherlands, 1982; pp. 89–98. [Google Scholar] [CrossRef]
- Kuse, R.; Schuster, S.; Schübbe, H.; Dix, S.; Hausmann, K. Blood lymphocyte volumes and diameters in patients with chronic lymphocytic leukemia and normal controls. Blut 1985, 50, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Krombach, F.; Munzing, S.E.A.; Allmeling, A.; Gerlach, J.T.; Behr, J.; Dorger, M. Cell size of alveolar macrophages: An interspecies comparison. Environ. Health Perspect. 1997, 105, 1261–1263. [Google Scholar] [CrossRef]
- Naito, M. Macrophage differentiation and function in health and disease. Pathol. Int. 2008, 58, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Mak, K.L.; Chen, L.Y.; Chou, M.P.; Ho, C.K. Heterogeneity of human blood monocyte: Two subpopulations with different sizes, phenotypes and functions. Immunology 1992, 77, 298–303. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1421624/ (accessed on 17 December 2024).
- Kufahl, R.H.; Saha, S. A theoretical model for stress-generated fluid flow in the canaliculi-lacunae network in bone tissue. J. Biomech. 1990, 23, 171–180. [Google Scholar] [CrossRef]
- Rath Bonivtch, A.; Bonewald, L.F.; Nicolella, D.P. Tissue strain amplification at the osteocyte lacuna: A microstructural finite element analysis. J. Biomech. 2007, 40, 2199–2206. [Google Scholar] [CrossRef]
- William, C. Parke Fluid mechanics Applied to Biosystems. In Fluid Mechanics Applied to Biosystems; Springer: Cham, Switzerland, 2020; pp. 77–111. [Google Scholar] [CrossRef]
- O’Connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the multiscale complexity of vascular networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef]
- Tajeddin, A.; Mustafaoglu, N. Design and Fabrication of Organ-on-Chips: Promises and Challenges. Micromachines 2021, 12, 1443. [Google Scholar] [CrossRef]
- Schwarz, K.; Epple, M. Hierarchically structured polyglycolide—A biomaterial mimicking natural bone. Macromol. Rapid Commun. 1998, 19, 613–617. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.A.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Jodati, H.; Yılmaz, B.; Evis, Z. A review of bioceramic porous scaffolds for hard tissue applications: Effects of structural features. Ceram. Int. 2020, 46, 15725–15739. [Google Scholar] [CrossRef]
- Song, J.E.; Tripathy, N.; Cha, S.R.; Jeon, S.H.; Kwon, S.Y.; Suh, D.S.; Khang, G. Three-dimensional duck’s feet collagen/PLGA scaffold for chondrification: Role of pore size and porosity. J. Biomater. Sci. Polym. Ed. 2018, 29, 932–941. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Torres-Sanchez, C.; Al Mushref, F.R.A.; Norrito, M.; Yendall, K.; Liu, Y.; Conway, P.P. The effect of pore size and porosity on mechanical properties and biological response of porous titanium scaffolds. Mater. Sci. Eng. C 2017, 77, 219–228. [Google Scholar] [CrossRef]
- Contreras Raggio, J.I.; Arancibia, C.T.; Millán, C.; Ploeg, H.-L.; Aiyangar, A.; Vivanco, J.F. Height-to-Diameter Ratio and Porosity Strongly Influence Bulk Compressive Mechanical Properties of 3D-Printed Polymer Scaffolds. Polymers 2022, 14, 5017. [Google Scholar] [CrossRef]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of Bone Marrow Mononuclear Cells on Biomaterials for Bone Tissue Engineering In Vitro. BioMed Res. Int. 2015, 2015, 762407. [Google Scholar] [CrossRef]
- Xu, W.; Yu, A.; Jiang, Y.; Li, Y.; Zhang, C.; Singh, H.; Liu, B.; Hou, C.; Zhang, Y.; Tian, S.; et al. Gyroid-based functionally graded porous titanium scaffolds for dental application: Design, simulation and characterizations. Mater. Des. 2022, 224, 111300. [Google Scholar] [CrossRef]
- Conde, C.M.; Demarco, F.F.; Casagrande, L.; Alcazar, J.C.; Nör, J.E.; Tarquinio, S.B.C. Influence of Poly-L-Lactic Acid Scaffold’s Pore Size on the Proliferation and Differentiation of Dental Pulp Stem Cells. Braz. Dent. J. 2015, 26, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, C.; Liu, L.; Wen, S.; Wang, X. Effect of 3-dimensional Collagen Fibrous Scaffolds with Different Pore Sizes on Pulp Regeneration. J. Endod. 2022, 48, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-M.; Qiu, K.; Hu, T.; Wan, C.-X.; Zhou, X.-D.; Gutmann, J.L. Biodegradable porous calcium polyphosphate scaffolds for the three-dimensional culture of dental pulp cells. Int. Endod. J. 2006, 39, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, X.; Zhou, X.; Xun, Q.; Zheng, J.; Mu, Y.; Liao, J. A novel porous hydroxyapatite scaffold (pHAMG) enhances angiogenesis and osteogenesis around dental implants by regulating the immune microenvironment. Clin. Oral Investig. 2023, 27, 6879–6889. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Jiao, J.; Liu, Z.; Zhou, Z.; Zhao, C.; Chang, L.-J.; Chen, Y.E.; Ma, P.X.; Yang, B. Engineering vascular tissue with functional smooth muscle cells derived from human iPS cells and nanofibrous scaffolds. Biomaterials 2014, 35, 8960–8969. [Google Scholar] [CrossRef] [PubMed]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of Pore Size and Void Fraction on Cellular Adhesion, Proliferation, and Matrix Deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef]
- Ding, J.; Wei, C.; Xu, Y.; Dai, W.; Chen, R. 3D printing of Ceffe-infused scaffolds for tailored nipple-like cartilage development. BMC Biotechnol. 2024, 24, 25. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, H.; Kawazoe, N.; Chen, G. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014, 10, 2005–2013. [Google Scholar] [CrossRef]
- Han, K.-S.; Song, J.E.; Tripathy, N.; Kim, H.; Moon, B.M.; Park, C.H.; Khang, G. Effect of pore sizes of silk scaffolds for cartilage tissue engineering. Macromol. Res. 2015, 23, 1091–1097. [Google Scholar] [CrossRef]
- Ajisafe, V.A.; Raichur, A.M. Snail Mucus-Enhanced Adhesion of Human Chondrocytes on 3D Porous Agarose Scaffolds. ACS Appl. Mater. Interfaces 2024, 16, 11324–11335. [Google Scholar] [CrossRef]
- Song, X.; Zhu, C.; Fan, D.; Mi, Y.; Li, X.; Fu, R.Z.; Duan, Z.; Wang, Y.; Feng, R.R. A Novel Human-Like Collagen Hydrogel Scaffold with Porous Structure and Sponge-Like Properties. Polymers 2017, 9, 638. [Google Scholar] [CrossRef]
- Chailom, P.; Pattarakankul, T.; Palaga, T.; Hoven, V.P. Fish Gelatin-Hyaluronic Acid Scaffold for Construction of an Artificial Three-Dimensional Skin Model. ACS Omega 2025, 10, 8172–8181. [Google Scholar] [CrossRef]
- Wang, H.-M.; Chou, Y.-T.; Wen, Z.-H.; Wang, Z.-R.; Chen, C.-H.; Ho, M.-L. Novel Biodegradable Porous Scaffold Applied to Skin Regeneration. PLoS ONE 2013, 8, e56330. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Sow, W.T.; Devi, D.; Ng, K.W.; Mandal, B.B.; Cho, N.J. Silk fibroin–keratin based 3D scaffolds as a dermal substitute for skin tissue engineering. Integr. Biol. 2015, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, A.; Deumens, R.; Beckmann, C.; Olde Damink, L.; Schügner, F.; Heschel, I.; Sellhaus, B.; Weis, J.; Jahnen-Dechent, W.; Brook, G.A.; et al. In vitro cell alignment obtained with a Schwann cell enriched microstructured nerve guide with longitudinal guidance channels. Biomaterials 2009, 30, 169–179. [Google Scholar] [CrossRef]
- Mahoney, M.J.; Chen, R.R.; Tan, J.; Mark Saltzman, W. The influence of microchannels on neurite growth and architecture. Biomaterials 2005, 26, 771–778. [Google Scholar] [CrossRef]
- Kourgiantaki, A.; Tzeranis, D.S.; Karali, K.; Georgelou, K.; Bampoula, E.; Psilodimitrakopoulos, S.; Yannas, I.V.; Stratakis, E.; Sidiropoulou, K.; Charalampopoulos, I.; et al. Neural stem cell delivery via porous collagen scaffolds promotes neuronal differentiation and locomotion recovery in spinal cord injury. Npj Regen. Med. 2020, 5, 12. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, F.; Coathup, M.; Shen, W.; Wu, D. Effect of Porosity and Pore Shape on the Mechanical and Biological Properties of Additively Manufactured Bone Scaffolds. Adv. Healthc. Mater. 2023, 12, 2301111. [Google Scholar] [CrossRef]
- Wang, G.; Shen, L.; Zhao, J.; Liang, H.; Xie, D.; Tian, Z.; Wang, C. Design and Compressive Behavior of Controllable Irregular Porous Scaffolds: Based on Voronoi-Tessellation and for Additive Manufacturing. ACS Biomater. Sci. Eng. 2018, 4, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Karimipour-Fard, P.; Behravesh, A.H.; Jones-Taggart, H.; Pop-Iliev, R.; Rizvi, G. Effects of design, porosity and biodegradation on mechanical and morphological properties of additive-manufactured triply periodic minimal surface scaffolds. J. Mech. Behav. Biomed. Mater. 2020, 112, 104064. [Google Scholar] [CrossRef] [PubMed]
- Roy, T.D.; Simon, J.L.; Ricci, J.L.; Rekow, E.D.; Thompson, V.P.; Parsons, J.R. Performance of degradable composite bone repair products made via three-dimensional fabrication techniques. J. Biomed. Mater. Res. A 2003, 66A, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef]
- Lu, J.X.; Flautre, B.; Anselme, K.; Hardouin, P.; Gallur, A.; Descamps, M.; Thierry, B. Role of interconnections in porous bioceramics on bone recolonization in vitro and in vivo. J. Mater. Sci. Mater. Med. 1999, 10, 111–120. [Google Scholar] [CrossRef]
- Griffon, D.J.; Sedighi, M.R.; Schaeffer, D.V.; Eurell, J.A.; Johnson, A.L. Chitosan scaffolds: Interconnective pore size and cartilage engineering. Acta Biomater. 2006, 2, 313–320. [Google Scholar] [CrossRef]
- Ashworth, J.C.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Cell Invasion in Collagen Scaffold Architectures Characterized by Percolation Theory. Adv. Healthc. Mater. 2015, 4, 1317–1321. [Google Scholar] [CrossRef]
- da Silva, M.T.Q.S.; do Rocio Cardoso, M.; Veronese, C.M.P.; Mazer, W. Tortuosity: A brief review. Mater. Today Proc. 2022, 58, 1344–1349. [Google Scholar] [CrossRef]
- Gabrieli, R.; Schiavi, A.; Baino, F. Determining the Permeability of Porous Bioceramic Scaffolds: Significance, Overview of Current Methods and Challenges Ahead. Materials 2024, 17, 5522. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef]
- Guo, J.-T.; Wei, Y.-Q.; Chen, S.-L.; Sun, W.; Fan, T.-T.; Xu, M.-R.; Zhang, C.-C. Measurement of pore diffusion factor of porous solid materials. Pet. Sci. 2022, 19, 1897–1904. [Google Scholar] [CrossRef]
- Kolan, K.C.R.; Huang, Y.-W.; Semon, J.A.; Leu, M.C. 3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects. Int. J. Bioprint. 2020, 6, 274. [Google Scholar] [CrossRef]
- Joshi, S.R.; Pendyala, G.S.; Shah, P.; Mopagar, V.P.; Padmawar, N.; Padubidri, M. Scaffolds––The Ground for Regeneration: A Narrative Review. J. Int. Soc. Prev. Community Dent. 2020, 10, 692–699. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Chong, P.L.; Zohourkari, I.; Roy, S.; Merdji, A.; Linda Gnanasagaran, C.; Faraji, F.; Moey, L.K.; Yazdi, M.H. Mechanical influence of tissue scaffolding design with different geometries using finite element study. Proc. Inst. Mech. Eng. 2023, 237, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Montazerian, H.; Davoodi, E.; Asadi-Eydivand, M.; Kadkhodapour, J.; Solati-Hashjin, M. Porous scaffold internal architecture design based on minimal surfaces: A compromise between permeability and elastic properties. Mater. Des. 2017, 126, 98–114. [Google Scholar] [CrossRef]
- Feng, J.; Fu, J.; Yao, X.; He, Y. Triply periodic minimal surface (TPMS) porous structures: From multi-scale design, precise additive manufacturing to multidisciplinary applications. Int. J. Extrem. Manuf. 2022, 4, 022001. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Zhang, L.; Zhang, S.; Hsi Fuh, J.Y.; Lu, W.F. Triply Periodic Minimal Surfaces Sheet Scaffolds for Tissue Engineering Applications: An Optimization Approach toward Biomimetic Scaffold Design. ACS Appl. Bio Mater. 2018, 1, 259–269. [Google Scholar] [CrossRef]
- Blanquer, S.B.G.; Grijpma, D.W. Triply Periodic Minimal Surfaces (TPMS) for the Generation of Porous Architectures Using Stereolithography. In Computer-Aided Tissue Engineering: Methods and Protocols; Rainer, A., Moroni, L., Eds.; Springer: New York, NY, USA, 2021; pp. 19–30. [Google Scholar] [CrossRef]
- Bock, M.; Tyagi, A.K.; Kreft, J.-U.; Alt, W. Generalized Voronoi Tessellation as a Model of Two-dimensional Cell Tissue Dynamics. Bull. Math. Biol. 2010, 72, 1696–1731. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, J.; Yao, D.; Wei, Y. Mechanical and permeability properties of porous scaffolds developed by a Voronoi tessellation for bone tissue engineering. J. Mater. Chem. B 2022, 10, 9699–9712. [Google Scholar] [CrossRef]
- Afshar, M.; Pourkamali Anaraki, A.; Montazerian, H. Compressive characteristics of radially graded porosity scaffolds architectured with minimal surfaces. Mater. Sci. Eng. C 2018, 92, 254–267. [Google Scholar] [CrossRef]
- Ke, D.; Bose, S. Effects of pore distribution and chemistry on physical, mechanical, and biological properties of tricalcium phosphate scaffolds by binder-jet 3D printing. Addit. Manuf. 2018, 22, 111–117. [Google Scholar] [CrossRef]
- Tien, N.D.; Geng, T.; Coelho, D.; Reseland, J.E.; Lyngstadaas, S.P.; Blaker, J.J.; Haugen, H.J. Multilayer gradient chitosan fiber scaffolds for skin tissue regeneration with enhanced mechanical strength and cellular infiltration. React. Funct. Polym. 2025, 214, 106276. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-Dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Tissue Eng Part B Rev 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Mayer, R.P.; Stowe, R.A. Mercury porosimetry—Breakthrough pressure for penetration between packed spheres. J. Colloid Sci. 1965, 20, 893–911. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun Poly(ε-caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef]
- Hu, Y.; Grainger, D.W.; Winn, S.R.; Hollinger, J.O. Fabrication of poly(α-hydroxy acid) foam scaffolds using multiple solvent systems. J. Biomed. Mater. Res. 2002, 59, 563–572. [Google Scholar] [CrossRef]
- Min, B.-M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef]
- Ghasemi-Mobarakeh, L.; Semnani, D.; Morshed, M. A novel method for porosity measurement of various surface layers of nanofibers mat using image analysis for tissue engineering applications. J. Appl. Polym. Sci. 2007, 106, 2536–2542. [Google Scholar] [CrossRef]
- León y León, C.A. New perspectives in mercury porosimetry. Adv. Colloid Interface Sci. 1998, 76–77, 341–372. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q.; Wang, C.; Lu, N.; Wang, S.; Bei, J. Fabrication and biocompatibility of cell scaffolds of poly(L-lactic acid) and poly(L-lactic-co-glycolic acid). Polym. Adv. Technol. 2002, 13, 227–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S. Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers. Appl. Sci. 2021, 11, 5096. [Google Scholar] [CrossRef]
- Peinador, R.I.; Calvo, J.I.; Ben Aim, R. Comparison of Capillary Flow Porometry (CFP) and Liquid Extrusion Porometry (LEP) Techniques for the Characterization of Porous and Face Mask Membranes. Appl. Sci. 2020, 10, 5703. [Google Scholar] [CrossRef]
- Lo Re, G.; Lopresti, F.; Petrucci, G.; Scaffaro, R. A facile method to determine pore size distribution in porous scaffold by using image processing. Micron 2015, 76, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Hojat, N.; Gentile, P.; Ferreira, A.M.; Šiller, L. Automatic pore size measurements from scanning electron microscopy images of porous scaffolds. J. Porous Mater. 2023, 30, 93–101. [Google Scholar] [CrossRef]
- Jenkins, D.; Salhadar, K.; Ashby, G.; Mishra, A.; Cheshire, J.; Beltran, F.; Grunlan, M.; Andrieux, S.; Stubenrauch, C.; Cosgriff-Hernandez, E. PoreScript: Semi-automated pore size algorithm for scaffold characterization. Bioact. Mater. 2022, 13, 1–8. [Google Scholar] [CrossRef]
- Rouhollahi, A.; Ilegbusi, O.; Foroosh, H. Segmentation and Pore Structure Estimation in SEM Images of Tissue Engineering Scaffolds Using Genetic Algorithm. Ann. Biomed. Eng. 2021, 49, 1033–1045. [Google Scholar] [CrossRef]
- Bals, J.; Epple, M. Artificial Scanning Electron Microscopy Images Created by Generative Adversarial Networks from Simulated Particle Assemblies. Adv. Intell. Syst. 2023, 5, 2300004. [Google Scholar] [CrossRef]
- Hashimoto, T.; Thompson, G.E.; Zhou, X.; Withers, P.J. 3D imaging by serial block face scanning electron microscopy for materials science using ultramicrotomy. Ultramicroscopy 2016, 163, 6–18. [Google Scholar] [CrossRef]
- Bartoš, M.; Suchý, T.; Foltán, R. Note on the use of different approaches to determine the pore sizes of tissue engineering scaffolds: What do we measure? Biomed. Eng. Online 2018, 17, 110. [Google Scholar] [CrossRef]
- Vásárhelyi, L.; Kónya, Z.; Kukovecz, Á.; Vajtai, R. Microcomputed tomography–based characterization of advanced materials: A review. Mater. Today Adv. 2020, 8, 100084. [Google Scholar] [CrossRef]
- Li, N.; Duan, X.; Ding, X.F.; Zhu, N.; Chen, X. Characterization of hydrogel-scaffold mechanical properties and microstructure by using synchrotron propagation-based imaging. J. Mech. Behav. Biomed. Mater. 2025, 163, 106844. [Google Scholar] [CrossRef]
- Sabree, I.; Gough, J.E.; Derby, B. Mechanical properties of porous ceramic scaffolds: Influence of internal dimensions. Ceram. Int. 2015, 41, 8425–8432. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, X.F.; Jiang, H.T.; Yu, C.L. Preparation of Porous Hydroxyapatite-Zirconia Composite Scaffolds by Combination of Gel-Casting and Polymer Sponge Methods. Adv. Mater. Res. 2010, 105–106, 616–619. [Google Scholar] [CrossRef]
- Cho, Y.S.; Choi, S.; Lee, S.-H.; Kim, K.K.; Cho, Y.-S. Assessments of polycaprolactone/hydroxyapatite composite scaffold with enhanced biomimetic mineralization by exposure to hydroxyapatite via a 3D-printing system and alkaline erosion. Eur. Polym. J. 2019, 113, 340–348. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Vahid, A.; Ataee, A.; Wen, C. High strength porous PLA gyroid scaffolds manufactured via fused deposition modeling for tissue-engineering applications. Smart Mater. Med. 2021, 2, 15–25. [Google Scholar] [CrossRef]
- Zhao, H.; Li, L.; Ding, S.; Liu, C.; Ai, J. Effect of porous structure and pore size on mechanical strength of 3D-printed comby scaffolds. Mater. Lett. 2018, 223, 21–24. [Google Scholar] [CrossRef]
- Long, T.; Yang, J.; Shi, S.-S.; Guo, Y.-P.; Ke, Q.-F.; Zhu, Z.-A. Fabrication of three-dimensional porous scaffold based on collagen fiber and bioglass for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 1455–1464. [Google Scholar] [CrossRef]
- Al-Munajjed, A.A.; Hien, M.; Kujat, R.; Gleeson, J.P.; Hammer, J. Influence of pore size on tensile strength, permeability and porosity of hyaluronan-collagen scaffolds. J. Mater. Sci. Mater. Med. 2008, 19, 2859–2864. [Google Scholar] [CrossRef]
- Singh, G.; Soundarapandian, S. Bone-like structure by modified freeze casting. Sci. Rep. 2020, 10, 7914. [Google Scholar] [CrossRef]
- Top, N.; Gökçe, H.; Şahin, I. Additive Manufacturing of Bio-Inspired Microstructures for Bone Tissue Engineering. Exp. Tech. 2023, 47, 1213–1227. [Google Scholar] [CrossRef]
- Altunbek, M.; Afghah, S.F.; Fallah, A.; Acar, A.A.; Koc, B. Design and 3D Printing of Personalized Hybrid and Gradient Structures for Critical Size Bone Defects. ACS Appl. Bio Mater. 2023, 6, 1873–1885. [Google Scholar] [CrossRef]
- Cosgriff-Hernandez, E.; Timmins, L.H. Model-Directed Design of Tissue Engineering Scaffolds. ACS Biomater. Sci. Eng. 2022, 8, 4622–4624. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mohol, S.S.; Sharma, V. A computational approach from design to degradation of additively manufactured scaffold for bone tissue engineering application. Rapid Prototyp. J. 2022, 28, 1956–1967. [Google Scholar] [CrossRef]
- Foroughi, A.H.; Razavi, M.J. Multi-objective Shape Optimization of Bone Scaffolds: Enhancement of Mechanical Properties and Permeability. Acta Biomater. 2022, 146, 317–340. [Google Scholar] [CrossRef]
- Khajehmohammadi, M.; Bakhtiary, N.; Davari, N.; Sarkari, S.; Tolabi, H.; Li, D.; Ghalandari, B.; Yu, B.; Ghorbani, F. Bioprinting of cell-laden protein-based hydrogels: From cartilage to bone tissue engineering. Int. J. Bioprint. 2023, 9, 1089. [Google Scholar] [CrossRef]
- Hossain, M.S.; Bergstrom, D.J.; Chen, X.B. Computational modelling of the scaffold-free chondrocyte regeneration: A two-way coupling between the cell growth and local fluid flow and nutrient concentration. Biomech. Model. Mechanobiol. 2015, 14, 1217–1225. [Google Scholar] [CrossRef]
- Foroughi, A.H.; Valeri, C.; Razavi, M.J. A review of computational optimization of bone scaffold architecture: Methods, challenges, and perspectives. Prog. Biomed. Eng. 2024, 7, 012003. [Google Scholar] [CrossRef]
- Virijević, K.; Živanović, M.N.; Nikolić, D.; Milivojević, N.; Pavić, J.; Morić, I.; Šenerović, L.; Dragačević, L.; Thurner, P.J.; Rufin, M.; et al. AI-Driven Optimization of PCL/PEG Electrospun Scaffolds for Enhanced In Vivo Wound Healing. ACS Appl. Mater. Interfaces 2024, 16, 22989–23002. [Google Scholar] [CrossRef]
- Jung, H.; Meile, C. Pore-Scale Numerical Investigation of Evolving Porosity and Permeability Driven by Biofilm Growth. Transp. Porous Media 2021, 139, 203–221. [Google Scholar] [CrossRef]
- Chui, B.W.; Wright, N.J.; Ly, J.; Maginnis, D.A.; Haniff, T.M.; Blaha, C.; Fissell, W.H.; Roy, S. A Scalable, Hierarchical Rib Design for Larger-Area, Higher-Porosity Nanoporous Membranes for the Implantable Bio-Artificial Kidney. J. Microelectromech. Syst. 2020, 29, 762–768. [Google Scholar] [CrossRef]
- Obayemi, J.D.; Jusu, S.M.; Salifu, A.A.; Ghahremani, S.; Tadesse, M.; Uzonwanne, V.O.; Soboyejo, W.O. Degradable porous drug-loaded polymer scaffolds for localized cancer drug delivery and breast cell/tissue growth. Mater. Sci. Eng. C 2020, 112, 110794. [Google Scholar] [CrossRef] [PubMed]
- Fay, C.D. Computer-Aided Design and Manufacturing (CAD/CAM) for Bioprinting. In 3D Bioprinting: Principles and Protocols; Crook, J.M., Ed.; Springer: New York, NY, USA, 2020; pp. 27–41. [Google Scholar] [CrossRef]
- Mikaeeli Kangarshahi, B.; Naghib, S.M.; Rabiee, N. 3D printing and computer-aided design techniques for drug delivery scaffolds in tissue engineering. Expert Opin. Drug Deliv. 2024, 21, 1615–1636. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, F. Recent Trends in Bioprinting. Procedia Manuf. 2019, 32, 95–101. [Google Scholar] [CrossRef]
- Shin, J.; Lee, Y.; Li, Z.; Hu, J.; Park, S.S.; Kim, K. Optimized 3D Bioprinting Technology Based on Machine Learning: A Review of Recent Trends and Advances. Micromachines 2022, 13, 363. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Schuurman, W.; Wijnberg, H.M.; Prins, H.-J.; van Weeren, P.R.; Malda, J.; Alblas, J.; Dhert, W.J.A. Biofabrication of Osteochondral Tissue Equivalents by Printing Topologically Defined, Cell-Laden Hydrogel Scaffolds. Tissue Eng. Part C Methods 2012, 18, 33–44. [Google Scholar] [CrossRef]
- Daly, A.C. Granular Hydrogels in Biofabrication: Recent Advances and Future Perspectives. Adv. Healthc. Mater. 2024, 13, 2301388. [Google Scholar] [CrossRef]



| TPMS | Voronoi-Tessellation | |
|---|---|---|
| Distribution of the units | Periodic and continuous repetition. | Random and discrete distribution. |
| Pore interconnection | Highly interconnected. | Heterogeneous, haphazard interconnection. |
| Mechanical resistance | High and highly controllable. | Depends on local seed distributions. |
| Applications | Bone scaffolds, filters, lightweight structures. Allows control of pore geometries, curvature levels and distribution. | Modeling of trabecular bone, soft tissues. Allows us to offer elaborated biomimetic micropatterns. |
| Material | Pore Size (Diameter μm) | Porosity (%) | Young’s Modulus (GPa) | Compressive Strength (MPa) | Reference |
|---|---|---|---|---|---|
| Hydroxyapatite | 300 | 42 | - | 4.7 | I. Sabree et al. (2025) [156] |
| Hydroxyapatite | 200–400 | 71 | - | 5 | L. L. Wang et al. (2010) [157] |
| Hydroxyapatite (40 wt%) + PCL (60 wt%) | 354 | 45 | - | 38.7 | Y. S. Cho et al. (2019) [158] |
| PLA | 200–1000 | 73 | 134.8 | 4.6 | M. Alizadeh-Osgouei et al. (2021) [159] |
| PLA | 1200–1300 | 73 | 108 | 2.7 | |
| PLA | 1000 | 40 | - | 51.3 | H. Zhao et al. (2018) [160] |
| PLA | 1000 | 70 | - | 5.1 | |
| Collagen + bioglass | 40–200 | 81 | 0.35 | 5.8 | T. Long et al. (2015) [161] |
| Hyaluronan (70%) + Collagen (30%) | 302.5 | 94% | 7 × 10−5 | - | A. Al-Munajjed et al. (2015) [162] |
| Hyaluronan (70%) + Collagen (30%) | 402.5 | 94% | 8.5 × 10−5 | - | |
| Hyaluronan (70%) + Collagen (30%) | 525 | 95% | 9 × 10−5 | - | |
| Collagen (0.25 wt%) + Chondroitin-6-sulphate (0.044 wt%) | 58 | 98.9 | 3 × 10−7 | - | C. M. Tierney et al. (2009) [52] |
| Collagen (0.5 wt%) + Chondroitin-6-sulphate (0.044 wt%) | 92.95 | 99.3 | 5 × 10−7 | - | |
| Collagen (1 wt%) + Chondroitin-6-sulphate (0.044 wt%) | 111.02 | 98.8 | 9.4 × 10−7 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picado-Tejero, D.; Mendoza-Cerezo, L.; Rodríguez-Rego, J.M.; Carrasco-Amador, J.P.; Marcos-Romero, A.C. Recent Advances in 3D Bioprinting of Porous Scaffolds for Tissue Engineering: A Narrative and Critical Review. J. Funct. Biomater. 2025, 16, 328. https://doi.org/10.3390/jfb16090328
Picado-Tejero D, Mendoza-Cerezo L, Rodríguez-Rego JM, Carrasco-Amador JP, Marcos-Romero AC. Recent Advances in 3D Bioprinting of Porous Scaffolds for Tissue Engineering: A Narrative and Critical Review. Journal of Functional Biomaterials. 2025; 16(9):328. https://doi.org/10.3390/jfb16090328
Chicago/Turabian StylePicado-Tejero, David, Laura Mendoza-Cerezo, Jesús M. Rodríguez-Rego, Juan P. Carrasco-Amador, and Alfonso C. Marcos-Romero. 2025. "Recent Advances in 3D Bioprinting of Porous Scaffolds for Tissue Engineering: A Narrative and Critical Review" Journal of Functional Biomaterials 16, no. 9: 328. https://doi.org/10.3390/jfb16090328
APA StylePicado-Tejero, D., Mendoza-Cerezo, L., Rodríguez-Rego, J. M., Carrasco-Amador, J. P., & Marcos-Romero, A. C. (2025). Recent Advances in 3D Bioprinting of Porous Scaffolds for Tissue Engineering: A Narrative and Critical Review. Journal of Functional Biomaterials, 16(9), 328. https://doi.org/10.3390/jfb16090328









