Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Yttria Partially Stabilized Zirconia (2.3Y-TZP) Dental Ceramics
2.2. Monitoring of the Chemical Solubility of the 2.3Y-TZP Dental Ceramics
3. Results
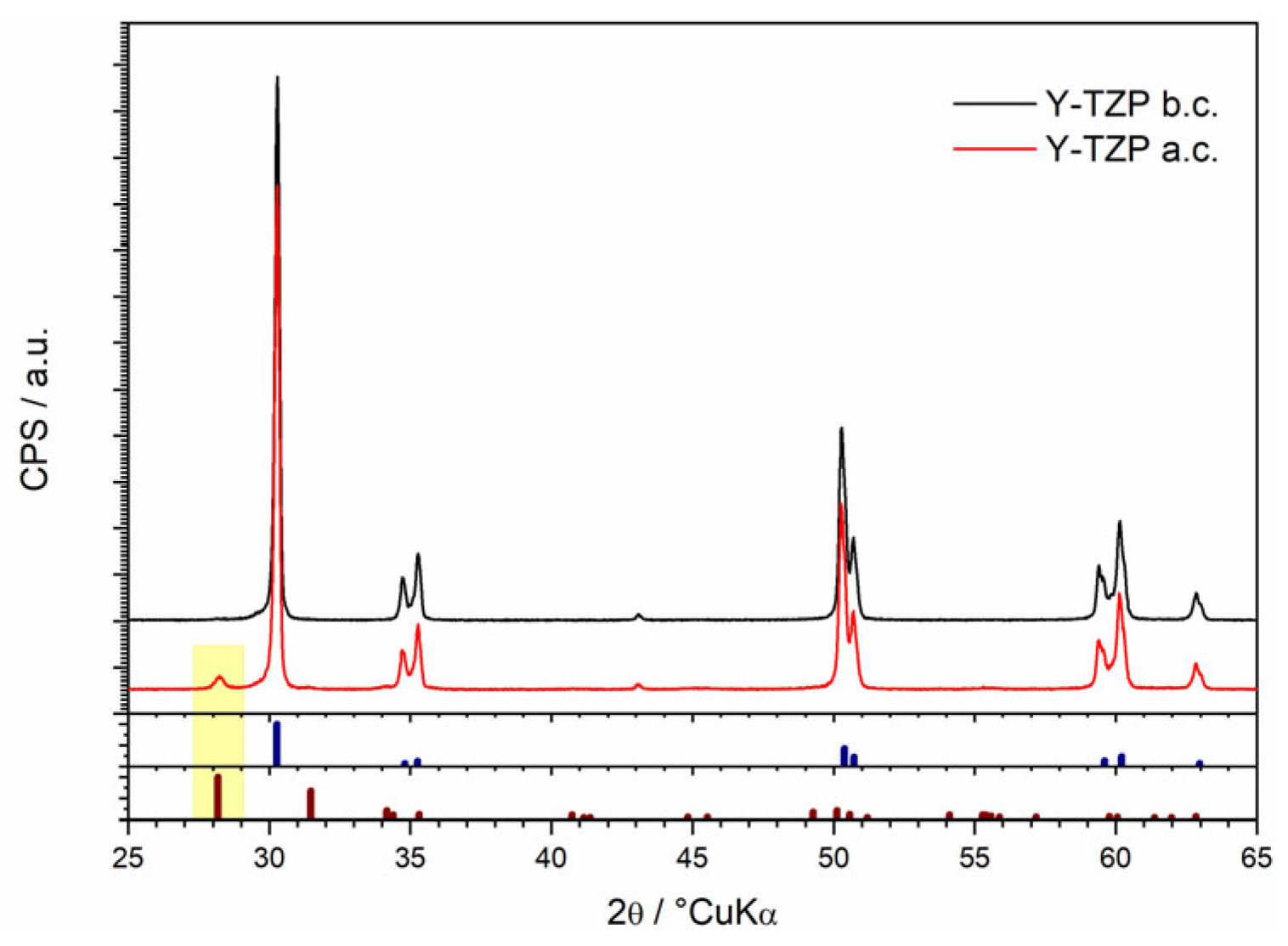
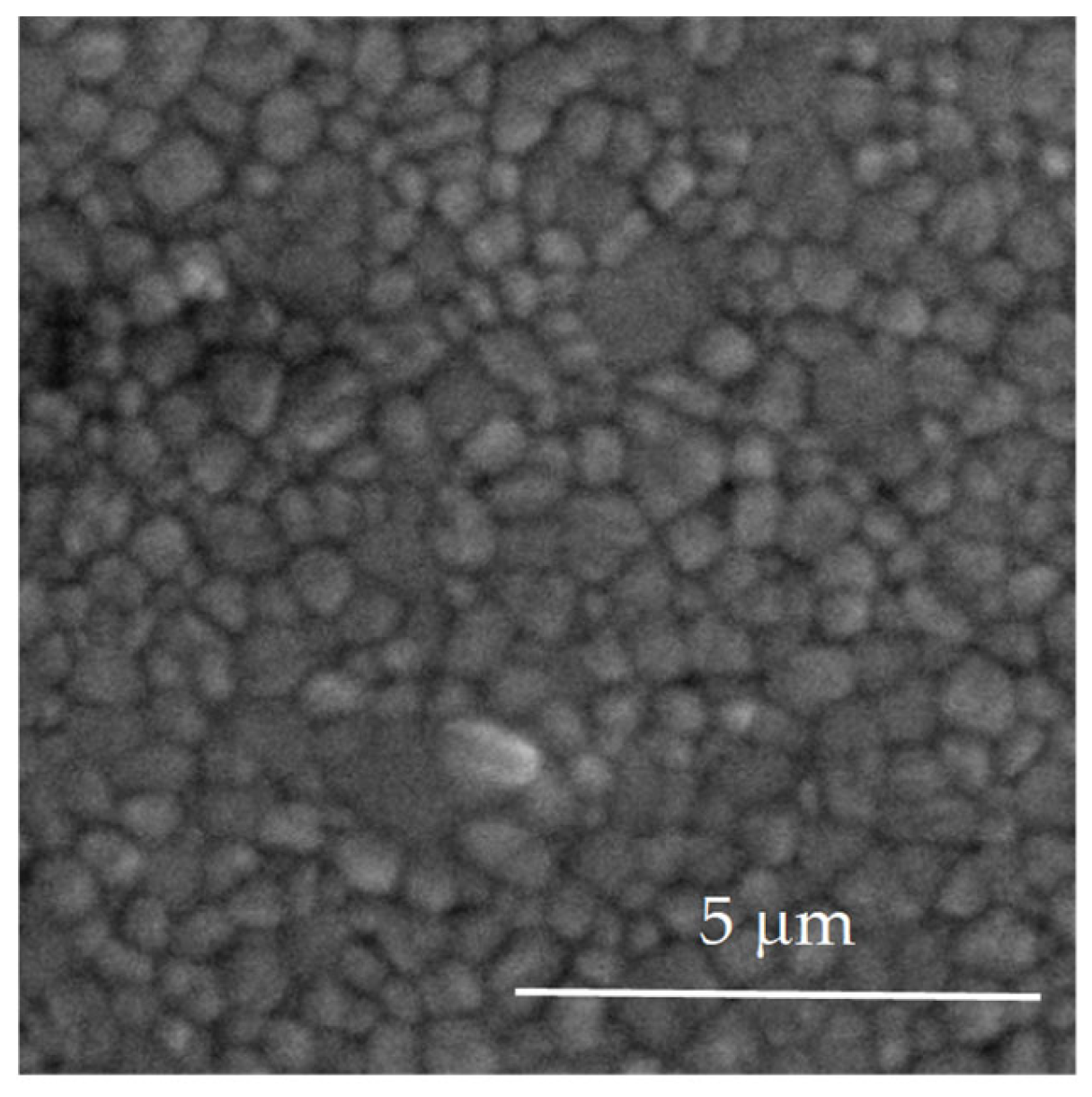
3.1. Structural and Morphological Characterization of Yttria Partially Stabilized Zirconia (2.3Y-TZP) Dental Ceramics
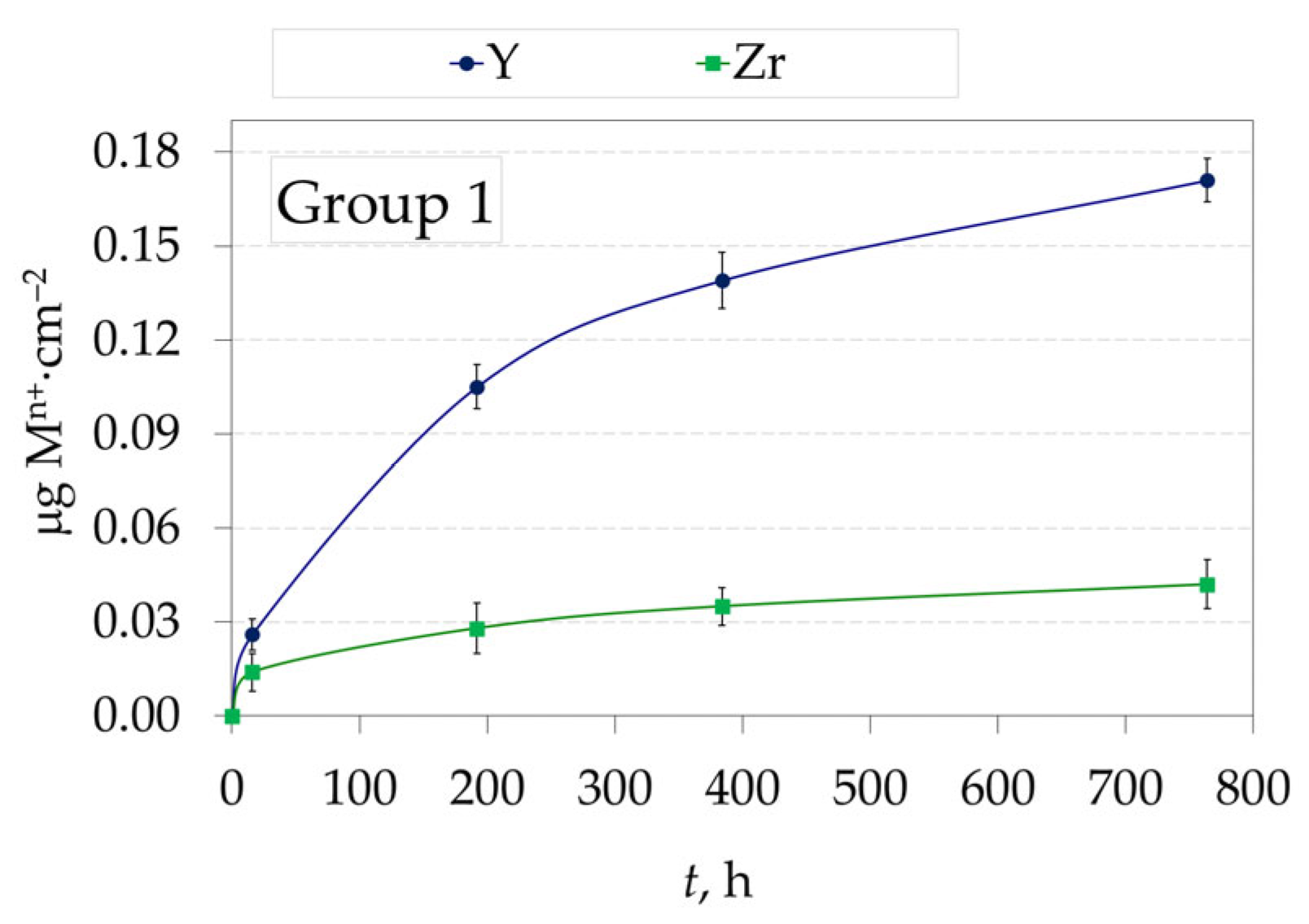
3.2. Amount of Ions Released in Testing Solution from Yttria Partially Stabilized Zirconia (2.3Y-TZP) Dental Ceramics
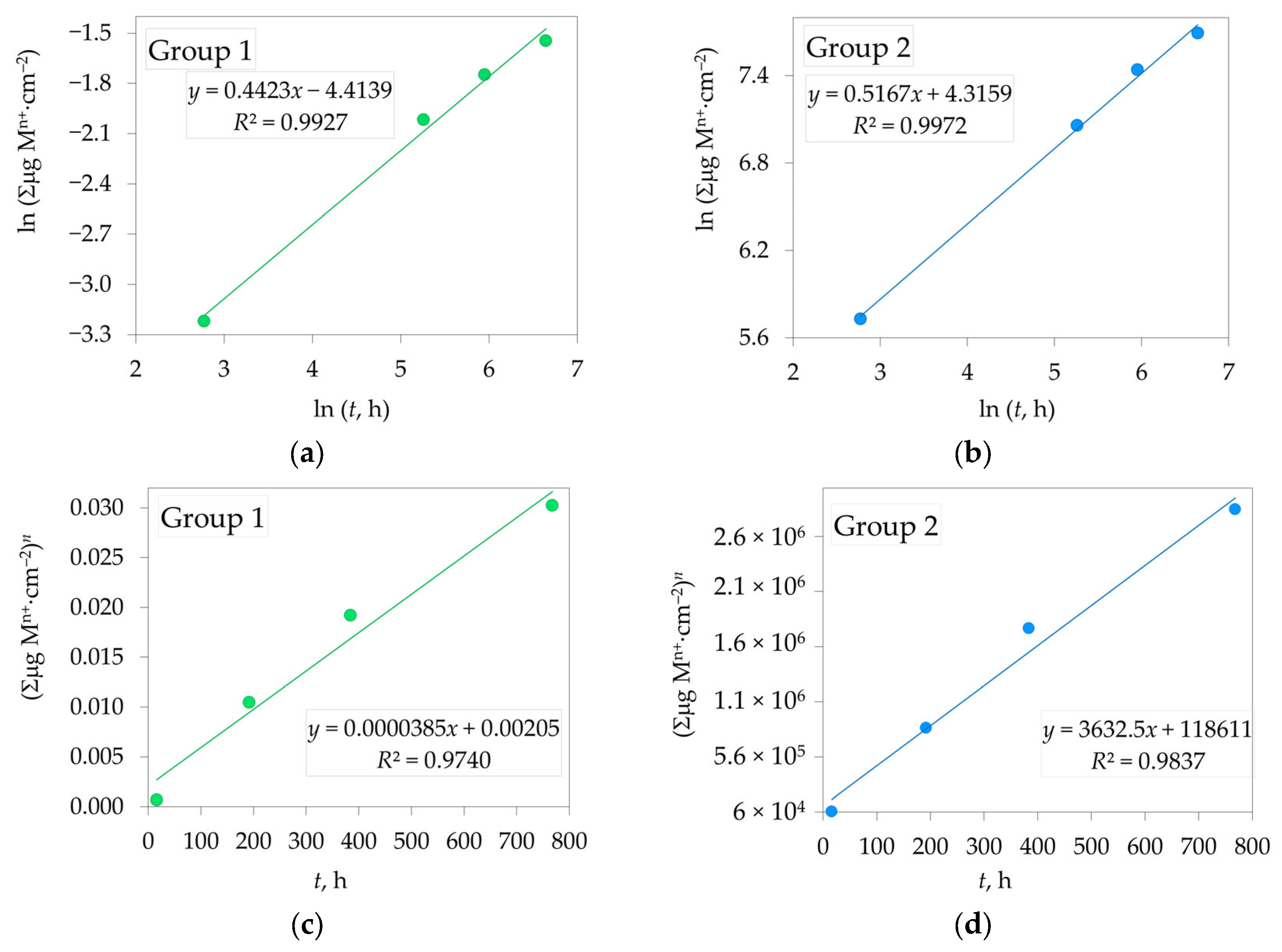
3.3. Dissolution Rate of Yttria Partially Stabilized Zirconia (2.3Y-TZP) Dental Ceramics in CH3COOH
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 12Ce-TZP | 12 mol% ceria-stabilized tetragonal zirconia polycrystal |
| 2.3Y-TZP | 2.3 mol% yttria-stabilized tetragonal zirconia polycrystal |
| 3Y-TZP | 3 mol% yttria-stabilized tetragonal zirconia polycrystal |
| AFM | Atomic force microscopy |
| c | Cubic phase |
| Ce-TZP | Ceria-stabilized tetragonal zirconia polycrystal |
| CNC | Computer numerical control machining |
| HR-ICP-MS | High-resolution inductively coupled plasma mass spectrometry |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| LTD | Low-temperature degradation |
| m | Monoclinic phase |
| PFM | Porcelain-fused-to-metal |
| SA/V | surface-area-to-volume |
| SEM | Scanning electron microscope |
| t | Tetragonal phase |
| XRD | X-ray diffraction |
| Y-TZP | Yttria partially stabilized zirconia polycrystal |
| YSZ | Yttria-stabilized zirconia polycrystal |
References
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current Status of Zirconia Restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Han, J.; Zhang, F.; Van Meerbeek, B.; Vleugels, J.; Braem, A.; Castagne, S. Laser Surface Texturing of Zirconia-Based Ceramics for Dental Applications: A Review. Mater. Sci. Eng. C 2021, 123, 112034. [Google Scholar] [CrossRef] [PubMed]
- Makhija, S.K.; Lawson, N.C.; Gilbert, G.H.; Litaker, M.S.; McClelland, J.A.; Louis, D.R.; Gordan, V.V.; Pihlstrom, D.J.; Meyerowitz, C.; Mungia, R.; et al. Dentist Material Selection for Single-Unit Crowns: Findings from the National Dental Practice-Based Research Network. J. Dent. 2016, 55, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.S.; Mohsen, C.A. Effect of Corrosion on Some Properties of Dental Ceramics. Sys. Rev. Pharm. 2021, 12, 584–588. [Google Scholar]
- Zhang, Y.; Lawn, B.R. Novel Zirconia Materials in Dentistry. J. Dent. Res. 2018, 97, 140–147. [Google Scholar] [CrossRef]
- Denry, I.; Kelly, J. State of the Art of Zirconia for Dental Applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Singh, R.G.; Lyons, K.M.; Waddell, J.N.; Li, K.C. Effect of Thermocycling on the Mechanical Properties, Inorganic Particle Release and Low Temperature Degradation of Glazed High Translucent Monolithic 3Y-TZP Dental Restorations. J. Mech. Behav. Biomed. Mater. 2022, 136, 105495. [Google Scholar] [CrossRef]
- El Yamani, A.; Soualhi, H.; Alaoui, Y.A. The Evolution of Dental Zirconia: Advancements and Applications. CODS J. Dent. 2025, 16, 20–27. [Google Scholar] [CrossRef]
- Toksoy, D.; Önöral, Ö. Optical Behavior of Zirconia Generations. Cyprus J. Med Sci. 2024, 9, 380–389. [Google Scholar] [CrossRef]
- Zhang, F.; Inokoshi, M.; Batuk, M.; Hadermann, J.; Naert, I.; Van Meerbeek, B.; Vleugels, J. Strength, Toughness and Aging Stability of Highly-Translucent Y-TZP Ceramics for Dental Restorations. Dent. Mater. 2016, 32, e327–e337. [Google Scholar] [CrossRef]
- Jitwirachot, K.; Rungsiyakull, P.; Holloway, J.A.; Jia-mahasap, W. Wear Behavior of Different Generations of Zirconia: Present Literature. Int. J. Dent. 2022, 2022, 9341616. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, J.; Ren, C.; Zhang, F.; Tang, J.; Omran, M.; Chen, G. Sintering Behaviour and Properties of Zirconia Ceramics Prepared by Pressureless Sintering. Ceram. Int. 2023, 49, 27192–27200. [Google Scholar] [CrossRef]
- Johansson, C.; Franco Tabares, S.; Larsson, C.; Papia, E. Laboratory, Clinical-Related Processing and Time-Related Factors’ Effect on Properties of High Translucent Zirconium Dioxide Ceramics Intended for Monolithic Restorations A Systematic Review. Ceramics 2023, 6, 734–797. [Google Scholar] [CrossRef]
- Kohorst, P.; Borchers, L.; Strempel, J.; Stiesch, M.; Hassel, T.; Bach, F.-W.; Hübsch, C. Low-Temperature Degradation of Different Zirconia Ceramics for Dental Applications. Acta Biomater. 2012, 8, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Jamali, M.; Ezoji, F.; Esmaeili, B.; Khafri, S. Comparative Effects of Glazing versus Polishing on Mechanical, Optical, and Surface Properties of Zirconia Ceramics with Different Translucencies. Clin. Exp. Dent. Res 2024, 10, e884. [Google Scholar] [CrossRef]
- Aksoy, G.; Polat, H.; Polat, M.; Coskun, G. Effect of Various Treatment and Glazing (Coating) Techniques on the Roughness and Wettability of Ceramic Dental Restorative Surfaces. Colloids Surf. B Biointerfaces 2006, 53, 254–259. [Google Scholar] [CrossRef]
- Farzin, M.; Ansarifard, E.; Taghva, M.; Imanpour, R. Effect of External Staining on the Optical Properties and Surface Roughness of Monolithic Zirconia of Different Thicknesses. J. Prosthet. Dent. 2021, 126, 687-e1. [Google Scholar] [CrossRef]
- Dejak, B.D.; Langot, C.; Krasowski, M.; Klich, M. Evaluation of Hardness and Wear of Conventional and Transparent Zirconia Ceramics, Feldspathic Ceramic, Glaze, and Enamel. Materials 2024, 17, 3518. [Google Scholar] [CrossRef]
- Selvaraj, U.; Koli, D.K.; Jain, V.; Nanda, A. Evaluation of the Wear of Glazed and Polished Zirconia Crowns and the Opposing Natural Teeth: A Clinical Pilot Study. J. Prosthet. Dent. 2021, 126, 52–57. [Google Scholar] [CrossRef]
- Rahardyan, P.; Peni, P. Achieving Natural Aesthetics with Zirconia Restorations in Anterior Teeth: A Case Study. World J. Adv. Res. Rev. 2025, 26, 2653–2658. [Google Scholar] [CrossRef]
- Nowicka, A.; El-Maghraby, H.F.; Švančárková, A.; Galusková, D.; Reveron, H.; Gremillard, L.; Chevalier, J.; Galusek, D. Corrosion and Low Temperature Degradation of 3Y-TZP Dental Ceramics under Acidic Conditions. J. Eur. Ceram. Soc. 2020, 40, 6114–6122. [Google Scholar] [CrossRef]
- Jakovac, M.; Živko-Babić, J.; Ćurković, L.; Aurer, A. Measurement of Ion Elution from Dental Ceramics. J. Eur. Ceram. Soc. 2006, 26, 1695–1700. [Google Scholar] [CrossRef]
- ISO 6872; Dentistry—Ceramic Materials. ISO: Geneva, Switzerland, 2024.
- Alnasser, M.; Finkelman, M.; Papathanasiou, A.; Suzuki, M.; Ghaffari, R.; Ali, A. Effect of Acidic pH on Surface Roughness of Esthetic Dental Materials. J. Prosthet. Dent. 2019, 122, 567-e1. [Google Scholar] [CrossRef]
- Hawsawi, R.A.; Miller, C.A.; Moorehead, R.D.; Stokes, C.W. Evaluation of Reproducibility of the Chemical Solubility of Dental Ceramics Using ISO 6872:2015. J. Prosthet. Dent. 2020, 124, 230–236. [Google Scholar] [CrossRef]
- Sarabi, N.; Mohammadi-Bassir, M.; Dehestani-Ardakani, F.; Labbaf, H. Effect of Hydrothermal, Chemical, and Mechanical Degradation on Flexural Strength and Phase Transformation of Ground, Glazed, and Polished Zirconia. Front. Dent. 2024, 21, 16. [Google Scholar] [CrossRef] [PubMed]
- Magnago, R.D.O.; De Lima Abreu, C.E.; Reis Silva, R.; Pais Alves, M.F.R.; Cossu, C.M.F.A.; Dos Santos, C. Ceramics and Glass-Ceramics Dental Materials: Chemical Solubility, Cytotoxicity and Mechanical Properties. Mater. Sci. Forum 2018, 912, 170–174. [Google Scholar] [CrossRef]
- Majić Renjo, M.; Ćurković, L.; Štefančić, S.; Ćorić, D. Indentation Size Effect of Y-TZP Dental Ceramics. Dent. Mater. 2014, 30, e371–e376. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Shahramian, K.; Hupa, L.; Donovan, T.E.; Vallittu, P.; Närhi, T.O. Impact of Gastric Acidic Challenge on Surface Topography and Optical Properties of Monolithic Zirconia. Dent. Mater. 2015, 31, 1445–1452. [Google Scholar] [CrossRef]
- ASTM E112-96(2004)e2; Standard Test Methods for Determining Average Grain Size. ASTM International: West Conshohocken, PA, USA, 2004.
- Arata, A.; Campos, T.M.B.; Machado, J.P.B.; Lazar, D.R.R.; Ussui, V.; Lima, N.B.; Tango, R.N. Quantitative Phase Analysis from X-Ray Diffraction in Y-TZP Dental Ceramics: A Critical Evaluation. J. Dent. 2014, 42, 1487–1494. [Google Scholar] [CrossRef]
- Gajek, M.; Rapacz-Kmita, A.; Leśniak, M.; Stodolak-Zych, E.; Dudek, M.; Sitarz, M. Influence of SrO Content on Microstructure and Crystallization of Glazes in the SiO2–Al2O3–CaO–MgO–K2O System. J. Therm. Anal. Calorim. 2019, 138, 4177–4186. [Google Scholar] [CrossRef]
- Pasiut, K.; Partyka, J.; Kozień, D.; Kronberg, T. Impact of Barium Oxide on the Structure and Surface Properties of Glass-Crystalline Glazes. Ceram. Int. 2024, 50, 47980–47990. [Google Scholar] [CrossRef]




| Element | Y2O3 | HfO2 | Al2O3 | SiO2 | Fe2O3 | Na2O | ZrO2 |
|---|---|---|---|---|---|---|---|
| wt.% | 4.1 | 4.0 | 0.34 | <0.01 | <0.01 | <0.01 | balance (91.56) |
| M, g⋅mol−1 | 225.81 | 210.49 | 101.96 | 123.22 | |||
| n, mol | 0.0182 | 0.0190 | 0.0033 | -- | -- | -- | 0.74 |
| mol.% | 2.32 | 2.33 | 0.42 | -- | -- | -- | 94.81 |
| 2.3Y-TZP | Ra, µm | Rmax, µm | Rz, µm | |||
|---|---|---|---|---|---|---|
| Before Acid Exposure | After Acid Exposure | Before Acid Exposure | After Acid Exposure | Before Acid Exposure | After Acid Exposure | |
| Group 1 | 0.005 ± 0.001 | 0.005 ± 0.001 | 0.046 ± 0.003 | 0.053 ± 0.004 | 0.043 ± 0.003 | 0.047 ± 0.005 |
| Group 2 | 0.27 ± 0.021 | 2.75 ± 0.31 | 1.33 ± 0.042 | 22.23 ± 3.05 | 1.02 ± 0.043 | 19.98 ± 1.52 |
| 2.3Y-TZP | Sa, nm | Sq, nm |
|---|---|---|
| Group 1: Before acid exposure | 7.01 | 9.35 |
| Group 1: After acid exposure | 8.97 | 10.24 |
| Group 2: Before acid exposure | 5.67 | 7.73 |
| Group 2: After acid exposure | 140.38 | 182.25 |
| 2.3Y-TZP | n | R2 | Kp, µgn·cm−2n·h−1 | R2 |
|---|---|---|---|---|
| Group 1 | 2.261 ± 0.004 | 0.99 | 3.85 × 10−5 ± 4.45 × 10−6 | 0.97 |
| Group 2 | 1.935 ± 0.015 | 0.99 | 3632.5 ± 330.7 | 0.98 |
| Model | 2.3Y-TZP | Sum of Squares | F-Value | p-Value |
|---|---|---|---|---|
| n | Group 1 | 1.672 | 274.23 | 0.0036 |
| Group 2 | 2.280 | 689.65 | 0.0014 | |
| Kp | Group 1 | 0.00046 | 74.87 | 0.0131 |
| Group 2 | 4.1 × 1012 | 120.66 | 0.0082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ćurković, L.; Štefančić, S.; Žmak, I.; Mandić, V.; Gabelica, I.; Mehulić, K. Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics. J. Funct. Biomater. 2025, 16, 374. https://doi.org/10.3390/jfb16100374
Ćurković L, Štefančić S, Žmak I, Mandić V, Gabelica I, Mehulić K. Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics. Journal of Functional Biomaterials. 2025; 16(10):374. https://doi.org/10.3390/jfb16100374
Chicago/Turabian StyleĆurković, Lidija, Sanja Štefančić, Irena Žmak, Vilko Mandić, Ivana Gabelica, and Ketij Mehulić. 2025. "Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics" Journal of Functional Biomaterials 16, no. 10: 374. https://doi.org/10.3390/jfb16100374
APA StyleĆurković, L., Štefančić, S., Žmak, I., Mandić, V., Gabelica, I., & Mehulić, K. (2025). Long-Term Chemical Solubility of 2.3Y-TZP Dental Ceramics. Journal of Functional Biomaterials, 16(10), 374. https://doi.org/10.3390/jfb16100374








