The Remineralizing and Desensitizing Potential of Hydroxyapatite in Dentistry: A Narrative Review of Recent Clinical Evidence
Abstract
1. Introduction
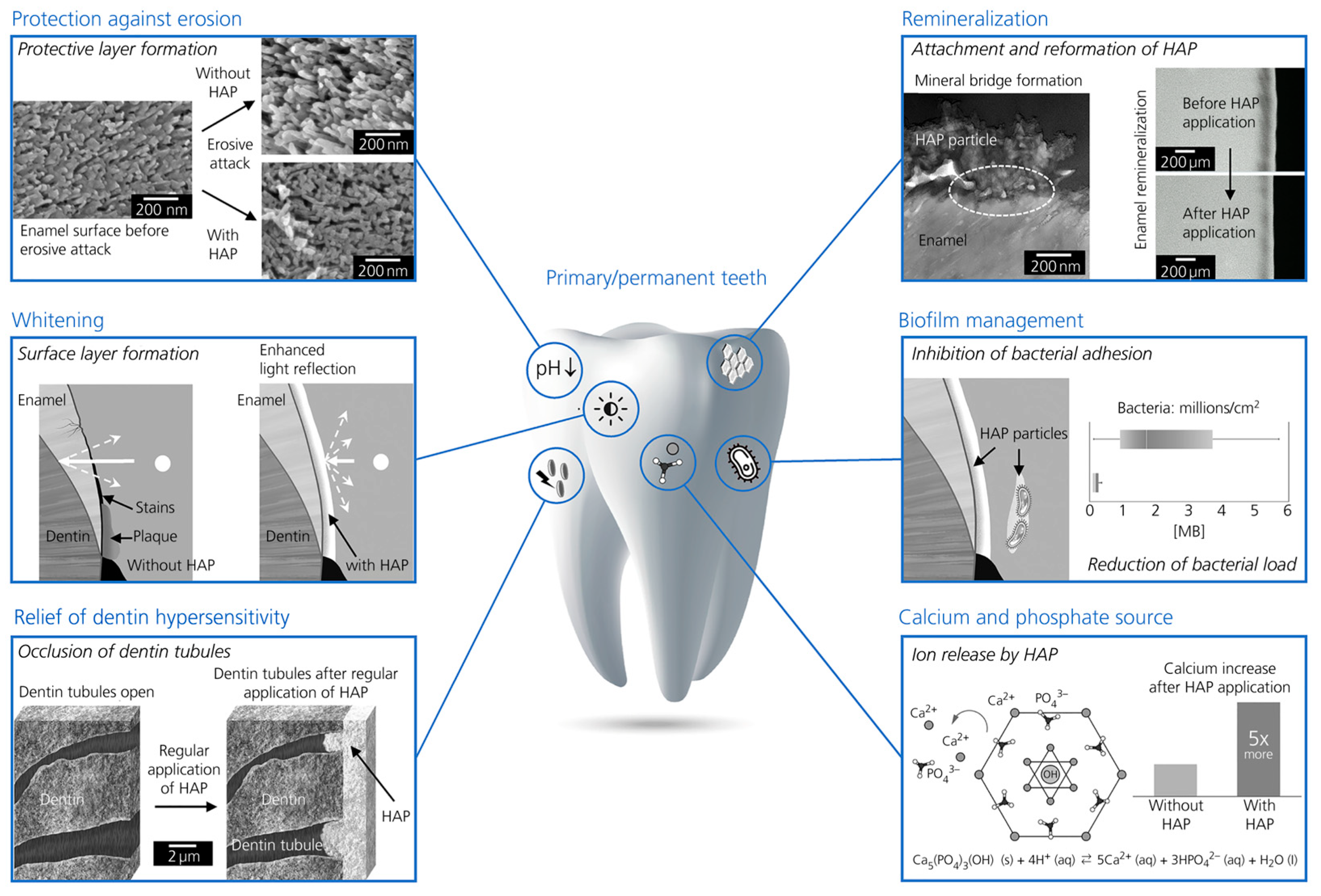
- Research Question
- Search strategy
- P (Population): Children and adults receiving non-invasive dental care for the purposes of remineralization, caries prevention, or management of dentin hypersensitivity.
- I (Intervention): Hydroxyapatite-containing products (e.g., toothpaste, gel, lotion, chewing gum).
- C (Comparison): Alternative agents, placebo, or no intervention.
- O (Outcome): Improvement in enamel or dentin remineralization, reduction in caries incidence, or decrease in dentin hypersensitivity.
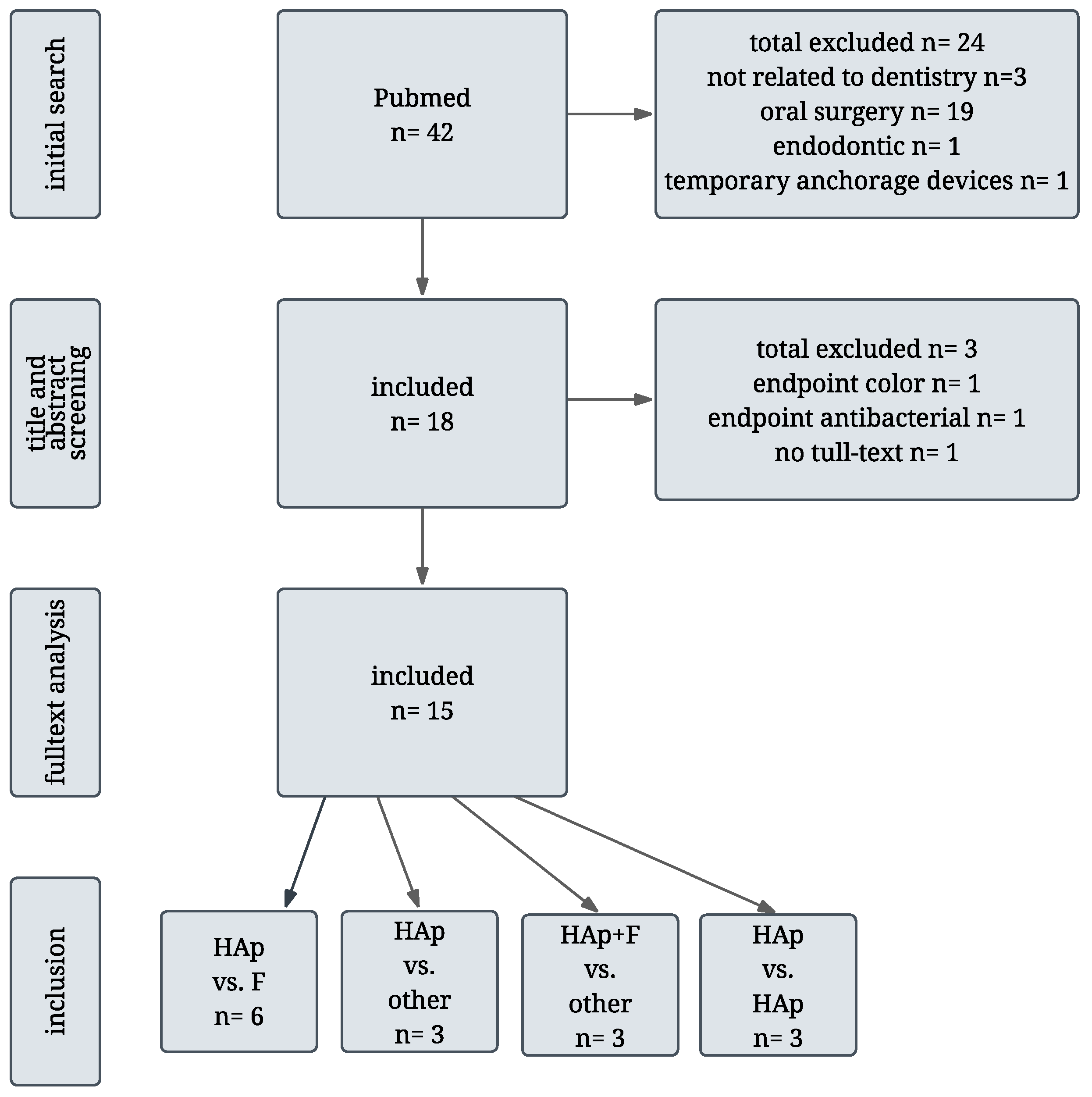
2. Literature Search and Study Selection
Overview and Categorization of Included Studies
- Hydroxyapatite vs. Fluoride
- Hydroxyapatite vs. other agents
- Hydroxyapatite + Fluoride vs. other
- Hydroxyapatite vs. Hydroxyapatite
3. Interpretation and Implications
3.1. Comparative Efficacy of Hydroxyapatite and Fluoride
3.2. Hydroxyapatite in the Management of Molar–Incisor Hypomineralization
3.3. Advantages of Combination Therapies
3.4. Limitations of the Current Evidence
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MIH | molar-incisor hypomineralization |
| Fl | fluoride |
| CPP-ACP | casein phosphopeptide–amorphous calcium phosphate |
| HAp | hydroxyapatite |
References
- Jordan, A.R.; Meyer-Lueckel, H.; Kuhr, K.; Sasunna, D.; Bekes, K.; Schiffner, U. Caries experience and care in Germany: Results of the 6th German Oral Health Study (DMS • 6). Quintessence Int. 2025, 56, S30–S39. [Google Scholar] [CrossRef]
- Uribe, S.E.; Innes, N.; Maldupa, I. The global prevalence of early childhood caries: A systematic review with meta-analysis using the WHO diagnostic criteria. Int. J. Paediatr. Dent. 2021, 31, 817–830. [Google Scholar] [CrossRef]
- Weerheijm, K.L.; Duggal, M.; Mejàre, I.; Papagiannoulis, L.; Koch, G.; Martens, L.C.; Hallonsten, A.L. Judgement criteria for molar incisor hypomineralisation (MIH) in epidemiologic studies: A summary of the European meeting on MIH held in Athens, 2003. Eur. J. Paediatr. Dent. 2003, 4, 110–113. [Google Scholar]
- Bekes, K.; Steffen, R.; Krämer, N. Update of the molar incisor hypomineralization: Würzburg concept. Eur. Arch. Paediatr. Dent. 2023, 24, 807–813. [Google Scholar] [CrossRef]
- Edunoori, R.; Dasari, A.K.; Chagam, M.R.; Velpula, D.R.; Kakuloor, J.S.; Renuka, G. Comparison of the efficacy of Icon resin infiltration and Clinpro XT varnish on remineralization of white spot lesions: An in-vitro study. J. Orthod. Sci. 2022, 11, 12. [Google Scholar] [CrossRef]
- Qadri, G.; Alkilzy, M.; Franze, M.; Hoffmann, W.; Splieth, C. School-based oral health education increases caries inequalities. Community Dent. Health 2018, 35, 153–159. [Google Scholar] [CrossRef]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2003, 2003, CD002278. [Google Scholar] [CrossRef] [PubMed]
- ten Cate, J.M.; Featherstone, J.D. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Rošin-Grget, K.; Peroš, K.; Sutej, I.; Bašić, K. The cariostatic mechanisms of fluoride. Acta Med. Acad. 2013, 42, 179–188. [Google Scholar] [CrossRef]
- Schiffner, U. Verwendung von Fluoriden zur Kariesprävention [Use of fluorides for caries prevention]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2021, 64, 830–837. (In German) [Google Scholar] [CrossRef] [PubMed]
- Armfield, J.M. When public action undermines public health: A critical examination of antifluoridationist literature. Aust. N. Z. Health Policy 2007, 4, 25. [Google Scholar] [CrossRef]
- Philip, N.; Walsh, L. The potential ecological effects of casein phosphopeptide-amorphous calcium phosphate in dental caries prevention. Aust. Dent. J. 2019, 64, 66–71. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan-Wong, K.; Enax, J.; Meyer, F.; Ganss, B. The use of hydroxyapatite toothpaste to prevent dental caries. Odontology 2022, 110, 223–230. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Farah, R.; Liu, J.A.; Phillips, T.S.; Perozo, B.I.; Kataoka, Y.; Meyer, F.; Enax, J. Remineralization of molar incisor hypomineralization (MIH) with a hydroxyapatite toothpaste: An in-situ study. BDJ Open 2022, 8, 33. [Google Scholar] [CrossRef]
- Pasini, M.; Giuca, M.R.; Scatena, M.; Gatto, R.; Caruso, S. Molar incisor hypomineralization treatment with casein phosphopeptide and amorphous calcium phosphate in children. Minerva Stomatol. 2018, 67, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Manchery, N.; John, J.; Nagappan, N.; Subbiah, G.K.; Premnath, P. Remineralization potential of dentifrice containing nanohydroxyapatite on artificial carious lesions of enamel: A comparative in vitro study. Dent. Res. J. 2019, 16, 310–317. [Google Scholar] [CrossRef]
- Pepla, E.; Besharat, L.K.; Palaia, G.; Tenore, G.; Migliau, G. Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: A review of literature. Ann. Stomatol. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Enax, J.; Fabritius, H.O.; Fabritius-Vilpoux, K.; Amaechi, B.T.; Meyer, F. Modes of action and clinical efficacy of particulate hydroxyapatite in preventive oral health care–state of the art. Open Dent. J. 2019, 13, 274–287. [Google Scholar] [CrossRef]
- Dussa, S.; C, S.; Kiran Kumar, P.; Saritha, T. Qualitative and quantitative evaluation of enamel surface roughness and remineralization after interproximal reduction: An in vivo study. Am. J. Orthod. Dentofac. Orthop. 2024, 166, 227–234. [Google Scholar] [CrossRef]
- Amaechi, B.T.; AbdulAzees, P.A.; Alshareif, D.O.; Shehata, M.A.; Lima, P.P.C.S.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V. Comparative efficacy of a hydroxyapatite and a fluoride toothpaste for prevention and remineralization of dental caries in children. BDJ Open 2019, 5, 18. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Tao, J.; Xu, X.; Mao, C.; Gu, X.; Tang, R. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J. Mater. Chem. 2008, 18, 4079–4084. [Google Scholar] [CrossRef]
- Bordea, I.R.; Candrea, S.; Alexescu, G.T.; Bran, S.; Băciuț, M.; Băciuț, G.; Lucaciu, O.; Dinu, C.M.; Todea, D.A. Nano-hydroxyapatite use in dentistry: A systematic review. Drug Metab. Rev. 2020, 52, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Spivak, J.M.; Hasharoni, A. Use of hydroxyapatite in spine surgery. Eur. Spine J. 2001, 10 (Suppl. S2), S197–S204. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Lee, D.; Koo, K.T.; Seol, Y.J.; Lee, Y.M.; Lee, J. A randomized controlled trial of immediate implant placement comparing hydroxyapatite nano-coated and uncoated sandblasted/acid-etched implants using a digital surgical guide. Int. J. Implant. Dent. 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Arghami, A.; Simmons, D.; St Germain, J.; Maney, P. Immediate and early loading of hydrothermally treated, hydroxyapatite-coated dental implants: A 7-year prospective randomized clinical study. Int. J. Implant. Dent. 2021, 7, 21. [Google Scholar] [CrossRef]
- Sokolowski, A.; Theisen, K.; Arefnia, B.; Payer, M.; Lorenzoni, M.; Sokolowski, A. A randomized clinical trial of phycogenic materials for sinus grafting with hydroxyapatite versus biphasic calcium phosphate: 2 years clinical outcomes. Clin. Oral Implants. Res. 2024, 35, 155–166. [Google Scholar] [CrossRef]
- Mittal, N.; Baranwal, H.C.; Kumar, P.; Gupta, S. Assessment of pulp sensibility in the mature necrotic teeth using regenerative endodontic therapy with various scaffolds—Randomised clinical trial. Indian J. Dent. Res. 2021, 32, 216–220. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Alshareif, D.O.; Azees, P.A.A.; Shehata, M.A.; Lima, P.P.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V.; Bagheri, A.; Okoye, L.O. Anti-caries evaluation of a nano-hydroxyapatite dental lotion for use after toothbrushing: An in situ study. J. Dent. 2021, 115, 103863. [Google Scholar] [CrossRef]
- Esparza-Villalpando, V.; Fernandez-Hernandez, E.; Rosales-Berber, M.; Torre-Delgadillo, G.; Garrocho-Rangel, A.; Pozos-Guillén, A. Clinical Efficacy of Two Topical Agents for the Remineralization of Enamel White Spot Lesions in Primary Teeth. Pediatr. Dent. 2021, 43, 95–101. [Google Scholar]
- Schestakow, A.; Pütz, N.; Guth, M.S.; Eisenmenger, T.A.; Dudek, J.; Hannig, M. Influence of a hydroxyapatite suspension on 48-h dental biofilm formation in-situ. Arch. Oral Biol. 2022, 136, 105388. [Google Scholar] [CrossRef]
- Hassani, A.R.; Baladi, M.; Amiri, M.; Hamze, F.; Salavati-Niasari, M.; Sharifi, M.; Hanna, R. Effectiveness of plant-mediated synthesis of hydroxyapatite nano-particles impregnated in Pistachio oleogum resin on mineral contents of human teeth. An in-situ single-blind controlled study. J. Mech. Behav. Biomed. Mater. 2023, 148, 106155. [Google Scholar] [CrossRef] [PubMed]
- Anil, A.; Ibraheem, W.I.; Meshni, A.A.; Preethanath, R.S.; Anil, S. Nano-Hydroxyapatite (nHAp) in the Remineralization of Early Dental Caries: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 5629. [Google Scholar] [CrossRef]
- Pawinska, M.; Paszynska, E.; Limeback, H.; Amaechi, B.T.; Fabritius, H.O.; Ganss, B.; O’Hagan-Wong, K.; Schulze zur Wiesche, E.; Meyer, F.; Enax, E. Hydroxyapatite as an active ingredient in oral care: An international symposium report. Biomim. Nanobiomaterials 2024, 13, 1–14. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Enax, J.; Meyer, F.; Schulze Zur Wiesche, E.; May, T.W.; Amaechi, B.T.; Limeback, H.; Hernik, A.; Otulakowska-Skrzynska, J.; et al. Caries-preventing effect of a hydroxyapatite-toothpaste in adults: A 18-month double-blinded randomized clinical trial. Front. Public Health 2023, 11, 1199728. [Google Scholar] [CrossRef]
- Paszynska, E.; Pawinska, M.; Gawriolek, M.; Kaminska, I.; Otulakowska-Skrzynska, J.; Marczuk-Kolada, G.; Rzatowski, S.; Sokolowska, K.; Olszewska, A.; Schlagenhauf, U.; et al. Impact of a toothpaste with microcrystalline hydroxyapatite on the occurrence of early childhood caries: A 1-year randomized clinical trial. Sci. Rep. 2021, 11, 2650. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Montasser, M.A.; Abd El Latief, M.H.; Modica, G.G.; Scribante, A. Home Oral Care with Biomimetic Hydroxyapatite vs. Conventional Fluoridated Toothpaste for the Remineralization and Desensitizing of White Spot Lesions: Randomized Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 8676. [Google Scholar] [CrossRef] [PubMed]
- Gümüştaş, B.; Dikmen, B. Effectiveness of remineralization agents on the prevention of dental bleaching induced sensitivity: A randomized clinical trial. Int. J. Dent. Hyg. 2022, 20, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Musiol, J.; Erdwey, D.; Esteves-Oliveira, M.; Apel, C.; Meyer-Lueckel, H. Re- and demineralization characteristics of dentin depending on fluoride application and baseline characteristics in situ. J. Dent. 2020, 94, 103305. [Google Scholar] [CrossRef]
- Scribante, A.; Pascadopoli, M.; Bergomi, P.; Licari, A.; Marseglia, G.L.; Bizzi, F.M.; Butera, A. Evaluation of two different remineralising toothpastes in children with drug-controlled asthma and allergic rhinitis: A randomised clinical trial. Eur. J. Paediatr. Dent. 2024, 25, 137–142. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Trapani, B.; Gallo, S.; Radu, M.; Scribante, A. Biomimetic hydroxyapatite paste for molar-incisor hypomineralization: A randomized clinical trial. Oral Dis. 2023, 29, 2789–2798. [Google Scholar] [CrossRef]
- Grocholewicz, K.; Matkowska-Cichocka, G.; Makowiecki, P.; Droździk, A.; Ey-Chmielewska, H.; Dziewulska, A.; Tomasik, M.; Trybek, G.; Janiszewska-Olszowska, J. Effect of nano-hydroxyapatite and ozone on approximal initial caries: A randomized clinical trial. Sci. Rep. 2020, 10, 11192. [Google Scholar] [CrossRef] [PubMed]
- Campus, G.; Cocco, F.; Wierichs, R.J.; Wolf, T.G.; Salerno, C.; Arghittu, A.; Dettori, M.; Cagetti, M.G. Effects of Hydroxyapatite-Containing Toothpastes on Some Caries-Related Variables: A Randomised Clinical Trial. Int. Dent. J. 2024, 74, 754–761. [Google Scholar] [CrossRef]
- Cagetti, M.G.; Cocco, F.; Wierichs, R.J.; Wolf, T.G.; Salerno, C.; Arghittu, A.; Campus, G. Efficacy of HAF toothpastes in primary and permanent dentitions. A 2-years triple-blind RCT. J. Dent. 2022, 121, 104049. [Google Scholar] [CrossRef]
- Hihara, H.; Izumita, K.; Kawata, T.; Akatsuka, R.; Tagaino, R.; Kitaoka, A.; Kayaba, C.; Ikeda, K.; Sasaki, K. A novel treatment based on powder jet deposition technique for dentin hypersensitivity: A randomized controlled trial. BMC Oral Health 2023, 23, 695, Erratum in BMC Oral Health 2023, 23, 941. https://doi.org/10.1186/s12903-023-03664-x. [Google Scholar] [CrossRef]
- Guntermann, L.; Rohrbach, A.; Schäfer, E.; Dammaschke, T. Remineralization and protection from demineralization: Effects of a hydroxyapatite-containing, a fluoride-containing and a fluoride- and hydroxyapatite-free toothpaste on human enamel in vitro. Head Face Med. 2022, 18, 26. [Google Scholar] [CrossRef]
- Grewal, N.; Sharma, N.; Kaur, N. Surface remineralization potential of nano-hydroxyapatite, sodium monofluorophosphate, and amine fluoride containing dentifrices on primary and permanent enamel surfaces: An in vitro study. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 158–166. [Google Scholar] [CrossRef]
- Thornton-Evans, G.; Junger, M.L.; Lin, M.; Wei, L.; Espinoza, L.; Beltran-Aguilar, E. Use of Toothpaste and Toothbrushing Patterns Among Children and Adolescents—United States, 2013–2016. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 87–90. [Google Scholar] [CrossRef]
- Erdal, S.; Buchanan, S.N. A quantitative look at fluorosis, fluoride exposure, and intake in children using a health risk assessment approach. Environ. Health Perspect. 2005, 113, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Creeth, J.; Bosma, M.L.; Govier, K. How much is a ‘pea-sized amount’? A study of dentifrice dosing by parents in three countries. Int. Dent. J. 2013, 63 (Suppl. S2), 25–30. [Google Scholar] [CrossRef]
- Miranda, G.H.N.; Alvarenga, M.O.P.; Ferreira, M.K.M.; Puty, B.; Bittencourt, L.O.; Fagundes, N.C.F.; Pessan, J.P.; Buzalaf, M.A.R.; Lima, R.R. A systematic review and meta-analysis of the association between fluoride exposure and neurological disorders. Sci. Rep. 2021, 11, 22659. [Google Scholar] [CrossRef] [PubMed]
- Epple, M. Review of potential health risks associated with nanoscopic calcium phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef]
- Unnikrishnan, V.; Venugopal, A.; Sivadasan, S.B.; Boniface Fernandez, F.; Arumugam, S.; R, H.V.P.; Parayanthala Valappil, M. Cellular and sub-chronic toxicity of hydroxyapatite porous beads loaded with antibiotic in rabbits, indented for chronic osteomyelitis. Int. J. Pharm. 2022, 616, 121535. [Google Scholar] [CrossRef] [PubMed]
- Wierichs, R.J.; Wolf, T.G.; Campus, G.; Carvalho, T.S. Efficacy of nano-hydroxyapatite on caries prevention-a systematic review and meta-analysis. Clin. Oral Investig. 2022, 26, 3373–3381. [Google Scholar] [CrossRef]
- Sundfeld, D.; da Silva, L.; Kluppel, O.J.; Santin, G.C.; de Oliveira, R.; Pacheco, R.R.; Pini, N. Molar Incisor Hypomineralization: Etiology, Clinical Aspects, and a Restorative Treatment Case Report. Oper. Dent. 2020, 45, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Bandeira Lopes, L.; Machado, V.; Botelho, J.; Haubek, D. Molar-incisor hypomineralization: An umbrella review. Acta Odontol. Scand. 2021, 79, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Linner, T.; Khazaei, Y.; Bücher, K.; Pfisterer, J.; Hickel, R.; Kühnisch, J. Hypersensitivity in teeth affected by molar-incisor hypomineralization (MIH). Sci. Rep. 2021, 11, 17922. [Google Scholar] [CrossRef]
- Ehlers, V.; Reuter, A.K.; Kehl, E.B.; Enax, J.; Meyer, F.; Schlecht, J.; Schmidtmann, I.; Deschner, J. Efficacy of a Toothpaste Based on Microcrystalline Hydroxyapatite on Children with Hypersensitivity Caused by MIH: A Randomised Controlled Trial. Oral Health Prev. Dent. 2021, 19, 647–658. [Google Scholar] [CrossRef]
- Lelli, M.; Putignano, A.; Marchetti, M.; Foltran, I.; Mangani, F.; Procaccini, M.; Roveri, N.; Orsini, G. Remineralization and repair of enamel surface by biomimetic Zn-carbonate hydroxyapatite containing toothpaste: A comparative in vivo study. Front. Physiol. 2014, 5, 333. [Google Scholar] [CrossRef]
- Enax, J.; Amaechi, B.T.; Farah, R.; Liu, J.A.; Schulze Zur Wiesche, E.; Meyer, F. Remineralization Strategies for Teeth with Molar Incisor Hypomineralization (MIH): A Literature Review. Dent. J. 2023, 11, 80. [Google Scholar] [CrossRef]
- Cardoso-Martins, I.; Pessanha, S.; Coelho, A.; Arantes-Oliveira, S.; Marques, P.F. Evaluation of the Efficacy of CPP-ACP Remineralizing Mousse in Molar-Incisor Hypomineralized Teeth Using Polarized Raman and Scanning Electron Microscopy-An In Vitro Study. Biomedicines 2022, 10, 3086. [Google Scholar] [CrossRef]
- Cardoso-Martins, I.; Arantes-Oliveira, S.; Coelho, A.; Pessanha, S.; FMarques, P. Evaluation of the Efficacy of CPP-ACP Remineralizing Mousse in MIH White and Yellow Opacities-In Vitro Vickers Microhardness Analysis. Dent. J. 2022, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Mielczarek, A.; Gedrange, T.; Michalik, J. An in vitro evaluation of the effect of fluoride products on white spot lesion remineralization. Am. J. Dent. 2015, 28, 51–56. [Google Scholar] [PubMed]
| Author and Year | Objective | Methods | Result | Conclusion | Review Comment |
|---|---|---|---|---|---|
| Paszynska et al. [34] 2023 | Toothpaste comparison: HAp vs. NaF | 189 adults; non-inferiority trial in terms of no increase in DMFS over 18 months | 89.3% (HAp) vs. 87.4% (NaF) showed no increase in DMFS | HAp is a safe, effective, and non-inferior alternative to NaF | HAp is as effective as NaF in preventing caries |
| Paszynska et al. [35] 2021 | Toothpaste comparison: HAp vs. Fl | 207 children; non-inferiority trial in terms of caries progression over 12 months | 72.7% (HAp) vs. 74.2% (Fl) showed caries progression; HAp non-inferior to fluoride | HAp is not inferior to Fl in preventing caries in primary dentition | HAp is as effective as Fl in caries prevention |
| Butera et al. [36] 2022 | Toothpaste comparison in white spot lesions: HAp vs. NaF | 40 adults; SAI, VAS and BEWE examination over 90 days | SAI and VAS significantly decreased in HAp group; no significant change in BEWE in either group | HAp more effective than NaF in reducing dental hypersensitivity | HAp outperforms NaF in reducing dental hypersensitivity |
| Gümüştaş et Dikmen [37] 2022 | Comparison of HAp (solution) vs. NaF (gel) vs. other before in-office bleaching to reduce sensitivity | 64 adults; agent applied before bleaching with 38% H2O2; sensitivity (VAS) and color change measured | No difference in bleaching efficacy; HAp and NaF significantly reduced sensitivity vs. other | HAp and NaF reduce bleaching-induced sensitivity without affecting whitening effect | HAp as effective as NaF in reducing sensitivity |
| Wierichs et al. [38] 2020 | Toothpaste comparison: HAp vs. F | 20 adults; 4 toothpastes (HAp, NaF 0, 1100, 5000 ppm) each tested over 4 weeks using mandibular appliances; mineral loss and lesion depth measured on bovine enamel and dentin | HAp and NaF0 showed demineralization; NaF1100 and NaF5000 showed significant remineralization, especially in highly demineralized dentin | Only NaF toothpastes showed dose-dependent remineralizing effects; HAp was not effective | HAp does not prevent demineralization; only NaF effective in remineralization |
| Dussa et al. [19] 2024 | Remineralization effects after IPR with HAp vs. NaF | 25 patients; 5 groups (HAp weekly, NaF monthly, 3 controls); enamel assessed via Ca/P ratio, Vickers microhardness, and surface roughness | HAp resulted in highest remineralization (close to untreated enamel). NaF showed a slightly weaker trend but also promoted enamel remineralization | HAp was slightly more effective than NaF; however, application frequency differed (HAp weekly, NaF monthly) | HAp may be more suitable post-IPR due to frequent application without risk of fluoride overexposure |
| Author and Year | Objective | Methods | Result | Conclusion | Review Comment |
|---|---|---|---|---|---|
| Scribante et al. [39] 2024 | Toothpaste comparison: zinc-HAp (with monthly HAp gel) vs. calcium sodium phosphosilicate | 40 children with asthma/allergic rhinitis; Indices assessed over 6 months: SAI, PI, pH, DMFT, BEWE, SI | SAI and PI significantly reduced in both groups; no significant intergroup differences overall | Both groups showed similar clinical improvement | since calcium sodium phosphosilicate forms HAp, comparable effects are expected |
| Butera et al. [40] 2022 | Evaluate zinc-HAp paste for desensitizing and remineralizing MIH-affected teeth | 25 children with MIH lesions; zinc-HAp paste applied to 2 teeth, 2 untreated control teeth (split-mouth); HAp-free toothpaste used in both groups; PCR, MIH-TNI, SAI assessed over 9 months | PCR improved in both groups; SAI and MIH-TNI improved significantly in zinc-HAp group, but showed only minor changes in control group | Zinc-HAp reduced sensitivity and improved MIH-TNI | Zinc-HAp paste shows desensitizing effect and supports remineralization in MIH treatment |
| Grocholewicz et al. [41] 2020 | Compare remineralization of initial approximal caries using HAp gel, ozone therapy, or both | 92 adults; all used fluoride toothpaste; 6-month treatment with HAp gel, ozone, or both; follow-up over 2 years | After 1 year: remineralization in 36.5% (HAp), 52.8% (ozone), 69.3% (combined); caries reversal in 18.0%, 38.0%, and 45.4%, respectively, over 2 years | All treatments showed remineralizing effects; combination was most effective but required continued application | HAp + ozone showed best short-term effect; ozone outperforms HAp; long-term benefit depends on consistent use |
| Author and Year | Objective | Methods | Result | Conclusion | Review Comment |
|---|---|---|---|---|---|
| Campus et al. [42] 2024 | Toothpaste comparison: HAF vs. F | 610 children; 4 toothpastes (2 HAF, 2 fluoride); plaque pH and microbiological analyses over 24 months | All groups showed increased minimum pH; HAF had the greatest increase and significantly fewer cariogenic bacteria | HAF toothpaste shows promising effects, but evidence remains inconclusive | HAF may outperform F alone |
| Cagetti et al. [43] 2022 | Toothpaste comparison: HAF vs. Fl in caries prevention | 610 children; 4 toothpastes (2 HAF, 2 Fl); ICDAS scoring over 24 months | HAF significantly reduced caries progression in both primary and permanent dentition; risk reduction 38–39% | HAF shows greater risk reduction for caries compared to Fl over 2 years | HAF may outperform Fl alone |
| Esparza- Villalpando et al. [29] 2021 | Topical agent comparison for white spot lesions in primary enamel: Fl + HAp vs. Fl + CPP-ACP vs. NaF | 130 children; topical application of Fl + HAp, Fl + CPP-ACP, NaF; UF measured over 3 weeks; regular toothpaste use unknown | UF decreased in all groups; Fl + HAp and Fl + CPP-ACP more effective than NaF; no significant difference between Fl + HAp and Fl + CPP-ACP | Fl + HAp and Fl + CPP-ACP are more effective than NaF in remineralizing | Additives like HAp or CPP-ACP outperform NaF alone |
| Author and Year | Objective | Methods | Result | Conclusion | Review Comment |
|---|---|---|---|---|---|
| Hihara et al. [44] 2023 | Compare HAp layer applied by powder jet vs. CaP paste that forms HAp in situ for dentin hypersensitivity | 35 adults; split-mouth design; sensitivity assessed via VAS (air/scratch pain) over 12 weeks | Both groups showed similar improvement (air pain ~69%, scratch pain ~81%) | HAp reduced dentin hypersensitivity | Both HAp treatments reduced hypersensitivity; no significant difference between application types |
| Amaechi et al. [28] 2021 | Assess whether HAp lotion enhances the effect of HAp toothpaste | 30 adults; HAp toothpaste followed by either HAp or placebo lotion; 14-day treatment per phase using intraoral appliance with enamel blocks; mineral loss measured | Both groups showed significant remineralization; HAp lotion group showed greater effect (58.4% vs. 37.7%) | HAp toothpaste is effective; additional HAp lotion further enhances remineralization | HAp inhibits demineralization; additional HAp lotion boosts the effect |
| Hassani et al. [31] 2023 | Compare the effect of plant-based vs. synthetic HAp in gum for remineralization | 2 adults; Ca2+ and P content measured after chewing using enamel specimens in intraoral appliances | Synthetic HAp group showed significantly higher Ca2+ levels; plant-based HAp also increased Ca2+; no difference in P levels | HAp gum enhances enamel remineralization; synthetic HAp more effective than plant-based HAp | Synthetic HAp outperforms plant-based HAp in delivering Ca2+ for remineralization via chewing gum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naim, J.; Sen, S. The Remineralizing and Desensitizing Potential of Hydroxyapatite in Dentistry: A Narrative Review of Recent Clinical Evidence. J. Funct. Biomater. 2025, 16, 325. https://doi.org/10.3390/jfb16090325
Naim J, Sen S. The Remineralizing and Desensitizing Potential of Hydroxyapatite in Dentistry: A Narrative Review of Recent Clinical Evidence. Journal of Functional Biomaterials. 2025; 16(9):325. https://doi.org/10.3390/jfb16090325
Chicago/Turabian StyleNaim, Jusef, and Sinan Sen. 2025. "The Remineralizing and Desensitizing Potential of Hydroxyapatite in Dentistry: A Narrative Review of Recent Clinical Evidence" Journal of Functional Biomaterials 16, no. 9: 325. https://doi.org/10.3390/jfb16090325
APA StyleNaim, J., & Sen, S. (2025). The Remineralizing and Desensitizing Potential of Hydroxyapatite in Dentistry: A Narrative Review of Recent Clinical Evidence. Journal of Functional Biomaterials, 16(9), 325. https://doi.org/10.3390/jfb16090325






