- Article
Willingness to Receive Maternal RSV Vaccination Among Pregnant Women and Those Planning Pregnancy in Southern China: A Cross-Sectional Study and Predictive Nomogram
- Xiang Meng,
- Sijie Li and
- Yonghui Zhong
- + 5 authors
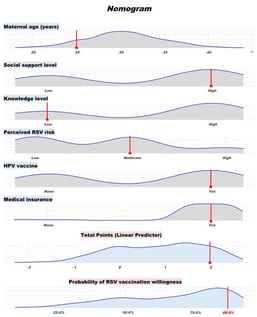
Background/Objectives: Maternal immunization against respiratory syncytial virus (RSV) is an emerging strategy to protect infants during early life when they are most vulnerable to severe RSV infection. However, little is known about the willingness to receive maternal RSV vaccination in China, where the vaccine has not yet been officially approved for marketing. This study aimed to assess the willingness to receive maternal RSV vaccination among women who are currently pregnant and those planning pregnancy in Guangzhou, and to identify the key determinants influencing vaccination willingness. Methods: A cross-sectional survey was conducted in April 2025 among 406 women at Guangzhou Women and Children’s Medical Center, China. Participants completed a self-administered questionnaire covering predisposing factors, enabling resources, health behaviors and awareness, and need factors. Logistic regression analyses were used to identify factors associated with vaccine willingness. A nomogram prediction model was constructed based on significant predictors. Results: Overall, 67.2% (n = 273) of participants reported willingness to receive maternal RSV vaccination. Younger maternal age, higher levels of social support, moderate or high perceived RSV risk, a history of HPV vaccination, and having medical insurance were independently associated with higher willingness to vaccinate. A predictive nomogram incorporating these factors demonstrated good discrimination (AUC = 0.753) and calibration. Age-stratified analysis revealed differing concerns across age groups, with vaccine safety and neonatal protection being the most cited factors influencing decision-making. Conclusions: This study provides the first evidence on maternal RSV vaccination willingness in southern China and highlights several psychosocial and demographic factors influencing vaccine intentions. The nomogram offers a practical tool to estimate individual willingness and guide targeted communication. These findings have implications for future maternal RSV vaccine application strategies in China.
8 February 2026