- Review
Computational Workflow for Chemical Compound Analysis: From Structure Generation to Molecular Docking
- Jesus Magdiel García-Díaz,
- Asbiel Felipe Garibaldi-Ríos and
- Martha Patricia Gallegos-Arreola
- + 4 authors
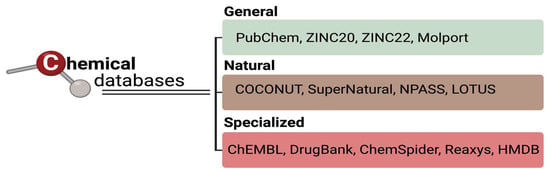
Drug discovery is a complex and expensive process in which only a small proportion of candidate molecules reach clinical approval. Computational methods, particularly computer-aided drug design (CADD), have become fundamental to accelerate and optimize early stages of discovery by integrating chemical, biological, and pharmacokinetic information into predictive models. This review outlines a complete computational workflow for chemical compound analysis, covering molecular structure generation, database selection, evaluation of absorption, distribution, metabolism, excretion and toxicity (ADMET), target prediction, and molecular docking. It focuses on freely accessible and web-based tools that enable reproducible, cost-effective, and scalable in silico studies. Key platforms such as PubChem, ChEMBL, RDKit, SwissADME, TargetNet, and SwissDock are highlighted as examples of how different resources can be integrated to support rational compound design and prioritization. The article also discusses essential methodological principles, data curation strategies, and common limitations in virtual screening and docking analyses. Finally, it explores future directions in computational drug discovery, including the incorporation of artificial intelligence, multi-omics integration, and quantum simulations, to enhance predictive accuracy and translational relevance.
13 January 2026