Integrating 3D Printing and Additive Manufacturing into Personalized Medicine for Pharmaceuticals: Opportunities, Limitations, and Future Perspectives
Abstract
1. Introduction
2. Overview of Additive Manufacturing Technologies
3. Advantages of Additive Manufacturing
3.1. Simple Manufacturing Process
3.2. Improved Bioavailability
3.3. Easily Portable
3.4. Easy to Scale
3.5. Continuous Manufacturing
3.6. Dose Personalization
3.7. Patient-Centric Design
3.8. Complex Geometries and Release Profiles
3.9. Accelerated Development Timelines
3.10. Reduction in Material Waste
3.11. On-Demand and Decentralized Production
3.12. Artificial Intelligence (AI) and Machine Learning (ML)
4. Limitations and Challenges
4.1. Limited Materials
4.2. Material Degradation
4.3. Stability and Shelf-Life
4.4. Risk of Product Liability
4.5. Cyber Risk (Fake Pills)
4.6. Safety and Efficacy
4.7. Regulatory Landscape
4.8. Post-Processing
4.9. Mass Production
5. Recent Advancements
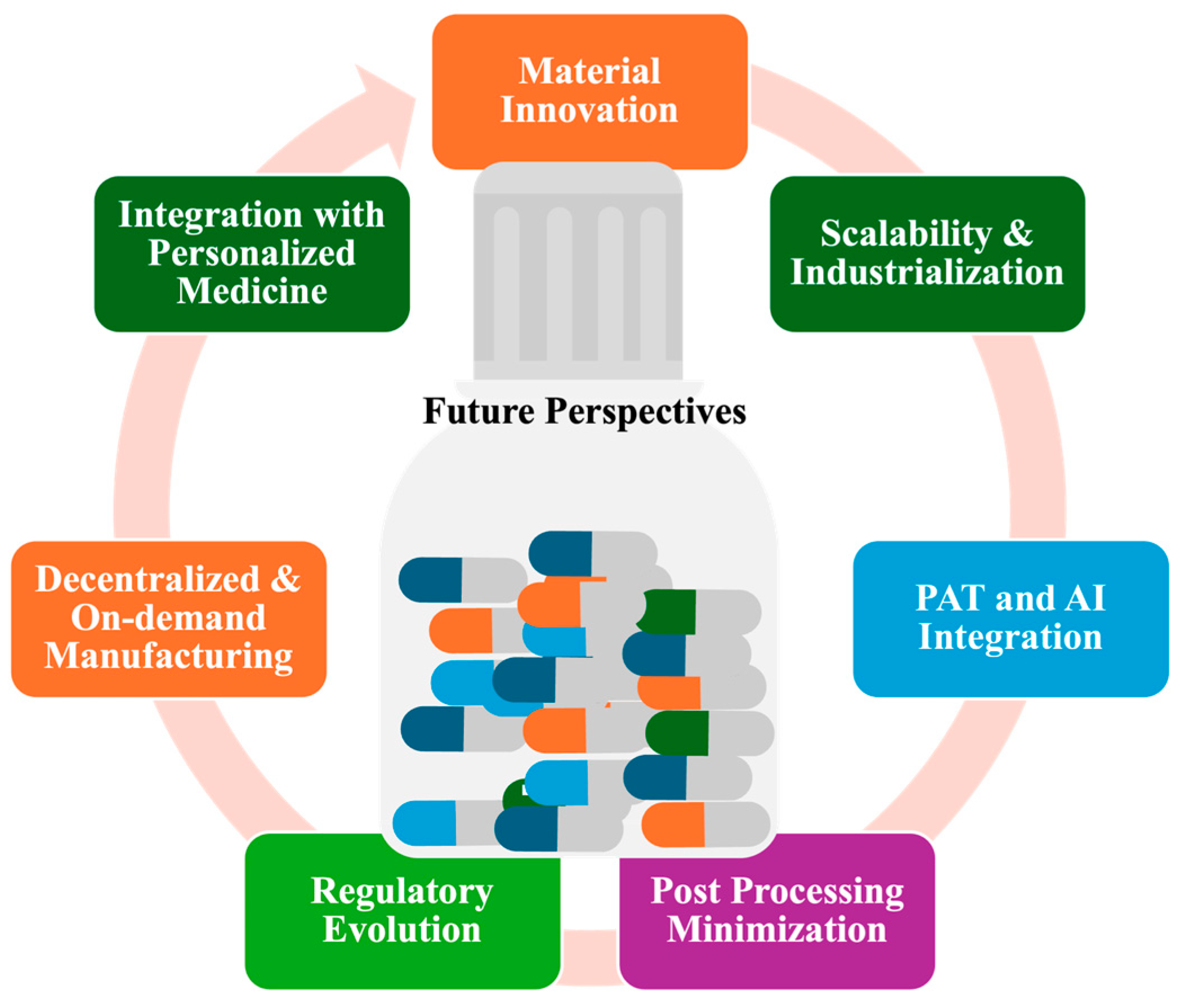
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muhindo, D.; Elkanayati, R.; Srinivasan, P.; Repka, M.A.; Ashour, E.A. Recent Advances in the Applications of Additive Manufacturing (3D Printing) in Drug Delivery: A Comprehensive Review. AAPS PharmSciTech 2023, 24, 57. [Google Scholar] [CrossRef]
- Chang, S.Y.; Jin, J.; Yan, J.; Dong, X.; Chaudhuri, B.; Nagapudi, K.; Ma, A.W.K. Development of a Pilot-Scale HuskyJet Binder Jet 3D Printer for Additive Manufacturing of Pharmaceutical Tablets. Int. J. Pharm. 2021, 605, 120791. [Google Scholar] [CrossRef] [PubMed]
- Narkevich, I.A.; Flisyuk, E.V.; Terent’eva, O.A.; Semin, A.A. Additive Manufacturing Technologies for Pharmaceutics. Pharm. Chem. J. 2018, 51, 1025–1029. [Google Scholar] [CrossRef]
- Rahman, Z.; Barakh Ali, S.F.; Ozkan, T.; Charoo, N.A.; Reddy, I.K.; Khan, M.A. Additive Manufacturing with 3D Printing: Progress from Bench to Bedside. AAPS J. 2018, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Y.; Kwok, P.C.L.; Kang, L. Pharmaceutical Applications of 3D Printing. Addit. Manuf. 2020, 34, 101209. [Google Scholar] [CrossRef]
- Bock, S.; Rades, T.; Rantanen, J.; Scherließ, R. Additive Manufacturing in Respiratory Sciences—Current Applications and Future Prospects. Adv. Drug Deliv. Rev. 2022, 186, 114341. [Google Scholar] [CrossRef]
- Hirshfield, L.; Içten, E.; Giridhar, A.; Nagy, Z.K.; Reklaitis, G.V. Real-Time Process Management Strategy for Dropwise Additive Manufacturing of Pharmaceutical Products. J. Pharm. Innov. 2015, 10, 140–155. [Google Scholar] [CrossRef]
- Sen, K.; West, T.G.; Chaudhuri, B. History and Present Scenario of Additive Manufacturing in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer Nature: London, UK, 2023; pp. 1–44. ISBN 9789819924042. [Google Scholar]
- Vyas, J.; Singh, S.; Shah, I.; Prajapati, B.G. Potential Applications and Additive Manufacturing Technology-Based Considerations of Mesoporous Silica: A Review. AAPS PharmSciTech 2024, 25, 6. [Google Scholar] [CrossRef]
- Mbodji, A.; Cruz, K.M.; Gómez, A.A.; Vlaar, C.P.; Duconge, J.; Monbaliu, J.-C.M.; Cersonsky, R.K.; Yu, L.; Zhang, G.G.; Coquerel, G.; et al. Controlled API Crystallization during Additive Manufacturing of Solid Dosage Form for Flexible Integrated Pharmaceutical Manufacturing. Int. J. Pharm. 2025, 685, 126197. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Recent Advancements in Additive Manufacturing Techniques Employed in the Pharmaceutical Industry: A Bird’s Eye View. Ann. 3D Print. Med. 2022, 8, 100081. [Google Scholar] [CrossRef]
- Lee, J.; Song, C.; Noh, I.; Rhee, Y.S. Applications of the Design of Additive Manufacturing (DfAM) in the Development of Pharmaceutical Dosage Forms. J. Pharm. Investig. 2024, 54, 175–193. [Google Scholar] [CrossRef]
- Samaro, A.; Janssens, P.; Vanhoorne, V.; Van Renterghem, J.; Eeckhout, M.; Cardon, L.; De Beer, T.; Vervaet, C. Screening of Pharmaceutical Polymers for Extrusion-Based Additive Manufacturing of Patient-Tailored Tablets. Int. J. Pharm. 2020, 586, 119591. [Google Scholar] [CrossRef]
- Muehlenfeld, C.; Duffy, P.; Yang, F.; Zermeño Pérez, D.; El-Saleh, F.; Durig, T. Excipients in Pharmaceutical Additive Manufacturing: A Comprehensive Exploration of Polymeric Material Selection for Enhanced 3D Printing. Pharmaceutics 2024, 16, 317. [Google Scholar] [CrossRef]
- Rahman, M.H.; Liza, N.Y.; Hossain, K.R.; Kalambhe, D.R.; Shyeed, M.A.; Noor, D.H. Additive Manufacturing in Nano Drug Delivery Systems. Pharm. Sci. Adv. 2024, 2, 100036. [Google Scholar] [CrossRef]
- Bianchi, M.; Pegoretti, A.; Fredi, G. An Overview of Poly(Vinyl Alcohol) and Poly(Vinyl Pyrrolidone) in Pharmaceutical Additive Manufacturing. J. Vinyl Addit. Technol. 2023, 29, 223–239. [Google Scholar] [CrossRef]
- Içten, E.; Purohit, H.S.; Wallace, C.; Giridhar, A.; Taylor, L.S.; Nagy, Z.K.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Amorphous and Self Emulsifying Drug Delivery Systems. Int. J. Pharm. 2017, 524, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Radcliffe, A.J.; Hilden, J.L.; Nagy, Z.K.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products Using Particle Suspensions. J. Pharm. Sci. 2019, 108, 914–928. [Google Scholar] [CrossRef]
- Healy, A.V.; Fuenmayor, E.; Doran, P.; Geever, L.M.; Higginbotham, C.L.; Lyons, J.G. Additive Manufacturing of Personalized Pharmaceutical Dosage Forms via Stereolithography. Pharmaceutics 2019, 11, 645. [Google Scholar] [CrossRef]
- Lind, J.; Kälvemark Sporrong, S.; Kaae, S.; Rantanen, J.; Genina, N. Social Aspects in Additive Manufacturing of Pharmaceutical Products. Expert. Opin. Drug Deliv. 2017, 14, 927–936. [Google Scholar] [CrossRef]
- Goyanes, A.; Fina, F.; Martorana, A.; Sedough, D.; Gaisford, S.; Basit, A.W. Development of Modified Release 3D Printed Tablets (Printlets) with Pharmaceutical Excipients Using Additive Manufacturing. Int. J. Pharm. 2017, 527, 21–30. [Google Scholar] [CrossRef]
- Gioumouxouzis, C.I.; Karavasili, C.; Fatouros, D.G. Recent Advances in Pharmaceutical Dosage Forms and Devices Using Additive Manufacturing Technologies. Drug Discov. Today 2019, 24, 636–643. [Google Scholar] [CrossRef]
- Hirshfield, L.; Giridhar, A.; Taylor, L.S.; Harris, M.T.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Solvent-Based Dosage Forms. J. Pharm. Sci. 2014, 103, 496–506. [Google Scholar] [CrossRef]
- Trivedi, M.; Jee, J.; Silva, S.; Blomgren, C.; Pontinha, V.M.; Dixon, D.L.; Van Tassel, B.; Bortner, M.J.; Williams, C.; Gilmer, E.; et al. Additive Manufacturing of Pharmaceuticals for Precision Medicine Applications: A Review of the Promises and Perils in Implementation. Addit. Manuf. 2018, 23, 319–328. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive Manufacturing Technologies with Emphasis on Stereolithography 3D Printing in Pharmaceutical and Medical Applications: A Review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef] [PubMed]
- Içten, E.; Giridhar, A.; Taylor, L.S.; Nagy, Z.K.; Reklaitis, G.V. Dropwise Additive Manufacturing of Pharmaceutical Products for Melt-Based Dosage Forms. J. Pharm. Sci. 2015, 104, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Vo, A.Q.; Feng, X.; Bandari, S.; Repka, M.A. Pharmaceutical Additive Manufacturing: A Novel Tool for Complex and Personalized Drug Delivery Systems. AAPS PharmSciTech 2018, 19, 3388–3402. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in Pharmaceutical Additive Manufacturing: A Balancing Act between Printability and Product Performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef]
- Zhang, J.; Thakkar, R.; Kulkarni, V.R.; Zhang, Y.; Lu, A.; Maniruzzaman, M. Investigation of the Fused Deposition Modeling Additive Manufacturing I: Influence of Process Temperature on the Quality and Crystallinity of the Dosage Forms. AAPS PharmSciTech 2021, 22, 258. [Google Scholar] [CrossRef]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling Hot Melt Extrusion and Fused Deposition Modeling: Critical Properties for Successful Performance. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low Temperature Fused Deposition Modeling (FDM) 3D Printing of Thermolabile Drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef]
- Joo, Y.; Shin, I.; Ham, G.; Abuzar, S.M.; Hyun, S.M.; Hwang, S.J. The Advent of a Novel Manufacturing Technology in Pharmaceutics: Superiority of Fused Deposition Modeling 3D Printer. J. Pharm. Investig. 2020, 50, 131–145. [Google Scholar] [CrossRef]
- Alhijjaj, M.; Nasereddin, J.; Belton, P.; Qi, S. Impact of Processing Parameters on the Quality of Pharmaceutical Solid Dosage Forms Produced by Fused Deposition Modeling (FDM). Pharmaceutics 2019, 11, 633. [Google Scholar] [CrossRef] [PubMed]
- Feuerbach, T.; Kock, S.; Thommes, M. Characterisation of Fused Deposition Modeling 3D Printers for Pharmaceutical and Medical Applications. Pharm. Dev. Technol. 2018, 23, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.R.P.; Sa-Barreto, L.L.; Gratieri, T.; Gelfuso, G.M.; Cunha-Filho, M. The Digital Pharmacies Era: How 3D Printing Technology Using Fused Deposition Modeling Can Become a Reality. Pharmaceutics 2019, 11, 128. [Google Scholar] [CrossRef]
- Long, J.; Gholizadeh, H.; Lu, J.; Bunt, C.; Seyfoddin, A. Application of Fused Deposition Modelling (FDM) Method of 3D Printing in Drug Delivery. Curr. Pharm. Des. 2016, 23, 433–439. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Shi, X. Development of Methylcellulose-Based Sustained-Release Dosage by Semisolid Extrusion Additive Manufacturing in Drug Delivery System. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 257–268. [Google Scholar] [CrossRef]
- Levine, V.R.; Paulsson, M.; Strømme, M.; Quodbach, J.; Lindh, J. Off-the-Shelf Medication Transformed: Custom-Dosed Metoprolol Tartrate Tablets via Semisolid Extrusion Additive Manufacturing and the Perception of This Technique in a Hospital Context. Int. J. Pharm. X 2024, 8, 100277. [Google Scholar] [CrossRef]
- Funk, N.L.; Leão, J.; de Oliveira, T.V.; Beck, R.C.R. Semi-Solid Extrusion (SSE) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer Nature: London, UK, 2023; pp. 171–200. ISBN 9789819924042. [Google Scholar]
- Bom, S.; Cavaco, A.; Martins, A.M.; Ribeiro, H.M.; Marto, J. Beyond the Hype: The Potential and Challenges of Semi-Solid Extrusion 3D Printing in Pharmaceutical Applications through the Lens of Portuguese 3D Experts. Int. J. Pharm. 2025, 680, 125760. [Google Scholar] [CrossRef]
- Cerveto, T.; Denis, L.; Stoops, M.; Lechanteur, A.; Jérôme, C.; Leenhardt, J.; Flynn, S.; Goyanes, A.; Mazet, R.; Annereau, M.; et al. The Promising Role of Semi-Solid Extrusion Technology in Custom Drug Formulation for Pediatric Medicine. Int. J. Bioprint 2024, 10, 4063. [Google Scholar] [CrossRef]
- Jabbari, A.; Abrinia, K. A Metal Additive Manufacturing Method: Semi-Solid Metal Extrusion and Deposition. Int. J. Adv. Manuf. Technol. 2018, 94, 3819–3828. [Google Scholar] [CrossRef]
- Teoh, X.Y.; Zhang, B.; Belton, P.; Chan, S.Y.; Qi, S. The Effects of Solid Particle Containing Inks on the Printing Quality of Porous Pharmaceutical Structures Fabricated by 3D Semi-Solid Extrusion Printing. Pharm. Res. 2022, 39, 1267–1279. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Pan, H.; Fang, D.; Qiao, S.; Wang, S.; Pan, W. Fabrication of High Drug Loading Levetiracetam Tablets Using Semi-Solid Extrusion 3D Printing. J. Drug Deliv. Sci. Technol. 2020, 57, 101683. [Google Scholar] [CrossRef]
- Nyavanandi, D.; Mandati, P.; Vidiyala, N.; Parupathi, P.; Kolimi, P.; Mamidi, H.K. Enhancing Patient-Centric Drug Development: Coupling Hot Melt Extrusion with Fused Deposition Modeling and Pressure-Assisted Microsyringe Additive Manufacturing Platforms with Quality by Design. Pharmaceutics 2024, 17, 14. [Google Scholar] [CrossRef]
- Carou-Senra, P.; Rodríguez-Pombo, L.; Awad, A.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Inkjet Printing of Pharmaceuticals. Adv. Mater. 2024, 36, 2309164. [Google Scholar] [CrossRef]
- Daly, R.; Harrington, T.S.; Martin, G.D.; Hutchings, I.M. Inkjet Printing for Pharmaceutics—A Review of Research and Manufacturing. Int. J. Pharm. 2015, 494, 554–567. [Google Scholar] [CrossRef]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Gonçalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-Based 3D Inkjet Printing of an Oral Pharmaceutical Dosage Form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet Printing for Pharmaceutical Applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Scoutaris, N.; Ross, S.; Douroumis, D. Current Trends on Medical and Pharmaceutical Applications of Inkjet Printing Technology. Pharm. Res. 2016, 33, 1799–1816. [Google Scholar] [CrossRef]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical Applications of Powder-Based Binder Jet 3D Printing Process—A Review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-Laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Scoutaris, N.; Alexander, M.R.; Gellert, P.R.; Roberts, C.J. Inkjet Printing as a Novel Medicine Formulation Technique. J. Control. Release 2011, 156, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Müllertz, A.; Rantanen, J. Additive Manufacturing of Solid Products for Oral Drug Delivery Using Binder Jetting Three-Dimensional Printing. AAPS PharmSciTech 2022, 23, 196. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Wu, J.; Duan, S.; Wang, X.; Hong, X.; Han, X.; Li, C.; Kang, D.; Wang, Z.; et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics 2022, 14, 2589. [Google Scholar] [CrossRef]
- Wang, Y.; Genina, N.; Müllertz, A.; Rantanen, J. Binder Jetting 3D Printing in Fabricating Pharmaceutical Solid Products for Precision Medicine. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 325–332. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Madla, C.M.; Basit, A.W.; Gaisford, S. Binder Jet Printing in Pharmaceutical Manufacturing. In AAPS Advances in the Pharmaceutical Sciences Series; Springer: Berlin/Heidelberg, Germany, 2018; Volume 31, pp. 41–54. [Google Scholar]
- Antic, A.; Zhang, J.; Amini, N.; Morton, D.A.V.; Hapgood, K.P. Screening Pharmaceutical Excipient Powders for Use in Commercial 3D Binder Jetting Printers. Adv. Powder Technol. 2021, 32, 2469–2483. [Google Scholar] [CrossRef]
- Kozakiewicz-Latała, M.; Nartowski, K.P.; Dominik, A.; Malec, K.; Gołkowska, A.M.; Złocińska, A.; Rusińska, M.; Szymczyk-Ziółkowska, P.; Ziółkowski, G.; Górniak, A.; et al. Binder Jetting 3D Printing of Challenging Medicines: From Low Dose Tablets to Hydrophobic Molecules. Eur. J. Pharm. Biopharm. 2022, 170, 144–159. [Google Scholar] [CrossRef]
- Liravi, F.; Vlasea, M. Powder Bed Binder Jetting Additive Manufacturing of Silicone Structures. Addit. Manuf. 2018, 21, 112–124. [Google Scholar] [CrossRef]
- Deshmane, S.; Kendre, P.; Mahajan, H.; Jain, S. Stereolithography 3D Printing Technology in Pharmaceuticals: A Review. Drug Dev. Ind. Pharm. 2021, 47, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Ravi, P.; Patel, P. Stereolithography (SLA) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer: Singapore, 2023; pp. 97–123. [Google Scholar] [CrossRef]
- Biswas, A.; Kumar Singh, A.; Das, D. Stereolithography-Based Polymer Additive Manufacturing Process for Microfluidics Devices. In Advances in Additive Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 237–268. [Google Scholar] [CrossRef]
- Xu, X.; Robles-Martinez, P.; Madla, C.M.; Joubert, F.; Goyanes, A.; Basit, A.W.; Gaisford, S. Stereolithography (SLA) 3D Printing of an Antihypertensive Polyprintlet: Case Study of an Unexpected Photopolymer-Drug Reaction. Addit. Manuf. 2020, 33, 101071. [Google Scholar] [CrossRef]
- Robles Martinez, P.; Basit, A.W.; Gaisford, S. The History, Developments and Opportunities of Stereolithography. In 3D Printing of Pharmaceuticals; Springer: Berlin/Heidelberg, Germany, 2018; Volume 31, pp. 55–79. [Google Scholar]
- Curti, C.; Kirby, D.J.; Russell, C.A. Stereolithography Apparatus Evolution: Enhancing Throughput and Efficiency of Pharmaceutical Formulation Development. Pharmaceutics 2021, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Choudhury, D.; Yadav, V.; Murty, U.S.N.; Banerjee, S. 3D Printing of Nanocomposite Pills through Desktop Vat Photopolymerization (Stereolithography) for Drug Delivery Reasons. 3D Print. Med. 2022, 8, 3. [Google Scholar] [CrossRef]
- Triacca, A.; Pitzanti, G.; Mathew, E.; Conti, B.; Dorati, R.; Lamprou, D.A. Stereolithography 3D Printed Implants: A Preliminary Investigation as Potential Local Drug Delivery Systems to the Ear. Int. J. Pharm. 2022, 616, 121529. [Google Scholar] [CrossRef]
- Choudhury, D.; Sharma, P.K.; Suryanarayana Murty, U.; Banerjee, S. Stereolithography-Assisted Fabrication of 3D Printed Polymeric Film for Topical Berberine Delivery: In-Vitro, Ex-Vivo and In-Vivo Investigations. J. Pharm. Pharmacol. 2022, 74, 1477–1488. [Google Scholar] [CrossRef]
- Charoo, N.A.; Barakh Ali, S.F.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective Laser Sintering 3D Printing—An Overview of the Technology and Pharmaceutical Applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Tikhomirov, E.; Åhlén, M.; Di Gallo, N.; Strømme, M.; Kipping, T.; Quodbach, J.; Lindh, J. Selective Laser Sintering Additive Manufacturing of Dosage Forms: Effect of Powder Formulation and Process Parameters on the Physical Properties of Printed Tablets. Int. J. Pharm. 2023, 635, 122780. [Google Scholar] [CrossRef]
- Kulinowski, P.; Malczewski, P.; Pesta, E.; Łaszcz, M.; Mendyk, A.; Polak, S.; Dorożyński, P. Selective Laser Sintering (SLS) Technique for Pharmaceutical Applications—Development of High Dose Controlled Release Printlets. Addit. Manuf. 2021, 38, 101761. [Google Scholar] [CrossRef]
- Karanwad, T.; Lekurwale, S.; Banerjee, S. Selective Laser Sintering (SLS) in Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer Nature: London, UK, 2023; pp. 125–169. ISBN 9789819924042. [Google Scholar]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective Laser Sintering (SLS) 3D Printing of Medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef]
- Balasankar, A.; Anbazhakan, K.; Arul, V.; Mutharaian, V.N.; Sriram, G.; Aruchamy, K.; Oh, T.H.; Ramasundaram, S. Recent Advances in the Production of Pharmaceuticals Using Selective Laser Sintering. Biomimetics 2023, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Kuofie, H.; Scoble, J.; Boulton, S.; Douroumis, D. Selective Laser Sintering for Printing Pharmaceutical Dosage Forms. J. Drug Deliv. Sci. Technol. 2023, 86, 104699. [Google Scholar] [CrossRef]
- Choudhury, D.; Ponneganti, S.; Radhakrishnanand, P.; Murty, U.S.; Banerjee, S. Selective Laser Sintering Additive Manufacturing of Solid Oral Dosage Form: Effect of Laser Power and Hatch Spacing on the Physico-Technical Behaviour of Sintered Printlets. Appl. Mater. Today 2023, 35, 101943. [Google Scholar] [CrossRef]
- Thakkar, R.; Jara, M.O.; Swinnea, S.; Pillai, A.R.; Maniruzzaman, M. Impact of Laser Speed and Drug Particle Size on Selective Laser Sintering 3d Printing of Amorphous Solid Dispersions. Pharmaceutics 2021, 13, 1149. [Google Scholar] [CrossRef]
- Giubilini, A.; Bondioli, F.; Messori, M.; Nyström, G.; Siqueira, G. Advantages of Additive Manufacturing for Biomedical Applications of Polyhydroxyalkanoates. Bioengineering 2021, 8, 29. [Google Scholar] [CrossRef]
- Li, C.; Pisignano, D.; Zhao, Y.; Xue, J. Advances in Medical Applications of Additive Manufacturing. Engineering 2020, 6, 1222–1231. [Google Scholar] [CrossRef]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A New Chapter in Pharmaceutical Manufacturing: 3D-Printed Drug Products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Desu, P.K.; Maddiboyina, B.; Vanitha, K.; Rao Gudhanti, S.N.K.; Anusha, R.; Jhawat, V. 3D Printing Technology in Pharmaceutical Dosage Forms: Advantages and Challenges. Curr. Drug Targets 2021, 22, 1901–1914. [Google Scholar] [CrossRef]
- Wostry, M.; Scherließ, R. Possibilities and Advantages of Additive Manufacturing in Dry Powder Formulations for Inhalation. Eur. J. Pharm. Sci. 2023, 190, 106583. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Khosravi, F.; Neisiany, R.E.; Singh, S.; Ramakrishna, S. Future of Additive Manufacturing in Healthcare. Curr. Opin. Biomed. Eng. 2021, 17, 100255. [Google Scholar] [CrossRef]
- Nyavanandi, D.; Mandati, P.; Narala, S.; Alzahrani, A.; Kolimi, P.; Vemula, S.K.; Repka, M.A. Twin Screw Melt Granulation: A Single Step Approach for Developing Self-Emulsifying Drug Delivery System for Lipophilic Drugs. Pharmaceutics 2023, 15, 2267. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Vidiyala, N.; Sunkishala, P.; Parupathi, P.; Mandati, P.; Mantena, S.K.; Kasu, R.R.R.; Nyavanandi, D. High Drug Loading of Amorphous Solid Dispersion by Hot Melt Extrusion: The Role of Magnesium Aluminometasilicate (Neusilin® US2). Sci. Pharm. 2025, 93, 30. [Google Scholar] [CrossRef]
- Wang, J.; Shao, C.; Wang, Y.; Sun, L.; Zhao, Y. Microfluidics for Medical Additive Manufacturing. Engineering 2020, 6, 1244–1257. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A Review of Fabrication Polymer Scaffolds for Biomedical Applications Using Additive Manufacturing Techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Kawish, S.M.; Sharma, S.; Almalki, W.H.; Alghamdi, S.; Afzal, O.; Kazmi, I.; Altamimi, A.S.A.; Al-Abbasi, F.A.; Beg, S.; Ahmad, F.J. Additive Manufacturing and Printing Approaches for the Development of Pharmaceutical Dosage Forms with Improved Biopharmaceutical Attributes. Curr. Drug Metab. 2022, 23, 616–629. [Google Scholar] [CrossRef]
- Jamróz, W.; Szafraniec, J.; Kurek, M.; Jachowicz, R. 3D Printing in Pharmaceutical and Medical Applications—Recent Achievements and Challenges. Pharm. Res. 2018, 35, 176. [Google Scholar] [CrossRef]
- Zhang, B.; Gleadall, A.; Belton, P.; Mcdonagh, T.; Bibb, R.; Qi, S. New Insights into the Effects of Porosity, Pore Length, Pore Shape and Pore Alignment on Drug Release from Extrusionbased Additive Manufactured Pharmaceuticals. Addit. Manuf. 2021, 46, 102196. [Google Scholar] [CrossRef]
- Lima, A.L.; Gross, I.P.; Cunha-Filho, M. Pharmaceutical Technologies and Applications over Additive Manufacturing. In Additive Manufacturing Materials and Technology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 267–289. [Google Scholar] [CrossRef]
- Warsi, M.H.; Yusuf, M.; Al Robaian, M.; Khan, M.; Muheem, A.; Khan, S. 3D Printing Methods for Pharmaceutical Manufacturing: Opportunity and Challenges. Curr. Pharm. Des. 2018, 24, 4949–4956. [Google Scholar] [CrossRef]
- Bishnoi, M.; Ankita; Mody, N.; Jain, A. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices. In Medical Additive Manufacturing: Concepts and Fundamentals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 619–647. ISBN 9780323953832. [Google Scholar]
- Li, W.; Ekren, B.Y.; Aktas, E. Additive Manufacturing and Its Impact on Pharmaceutical Supply Chains. In Medical Additive Manufacturing: Concepts and Fundamentals; Elsevier: Amsterdam, The Netherlands, 2024; pp. 683–712. ISBN 9780323953832. [Google Scholar]
- Içten, E.; Nagy, Z.K.; Reklaitis, G.V. Process Control of a Dropwise Additive Manufacturing System for Pharmaceuticals Using Polynomial Chaos Expansion Based Surrogate Model. Comput. Chem. Eng. 2015, 83, 221–231. [Google Scholar] [CrossRef]
- Sundarkumar, V.; Nagy, Z.K.; Reklaitis, G.V. Small-Scale Continuous Drug Product Manufacturing Using Dropwise Additive Manufacturing and Three Phase Settling for Integration with Upstream Drug Substance Production. J. Pharm. Sci. 2022, 111, 2330–2340. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, 1901994. [Google Scholar] [CrossRef] [PubMed]
- Jose, P.A.; GV, P.C. 3D Printing of Pharmaceuticals—A Potential Technology in Developing Personalized Medicine. Asian J. Pharm. Res. Dev. 2018, 6, 46–54. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. Extrusion Based Additive Manufacturing of Medicines. AIP Conf. Proc. 2024, 3007, 100046. [Google Scholar] [CrossRef]
- Rahman, Z.; Charoo, N.A.; Mohamed, E.M.; Kuttolamadom, M.; Khan, M.A. Regulatory Perspective of Additive Manufacturing in the Field of Pharmaceuticals. In Additive Manufacturing in Pharmaceuticals; Springer: Singapore, 2023; pp. 327–348. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface Modification of Biomaterials and Biomedical Devices Using Additive Manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J. Additive Manufacturing (3d Printing). In Polymers for Oral Drug Delivery Technologies; Elsevier Science Ltd.: Amsterdam, The Netherlands, 2025; pp. 675–701. ISBN 9780443137747. [Google Scholar]
- Ahangar, P.; Cooke, M.E.; Weber, M.H.; Rosenzweig, D.H. Current Biomedical Applications of 3D Printing and Additive Manufacturing. Appl. Sci. 2019, 9, 1713. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Hernandez, J.R. Antimicrobial Polymers for Additive Manufacturing. Int. J. Mol. Sci. 2019, 20, 1210. [Google Scholar] [CrossRef]
- Pawar, A.; Karanwad, T.; Banerjee, S. Tuneable Dissolution Profile of Tinidazole through Thermoplastic Polymer Composites in Low Temperature 3D Printing Settings for Pharmaceutical Additive Manufacturing Applications. Biomed. Mater. 2025, 20, 035029. [Google Scholar] [CrossRef]
- Bouguéon, G.; Kauss, T.; Dessane, B.; Barthélémy, P.; Crauste-Manciet, S. Micro- and Nano-Formulations for Bioprinting and Additive Manufacturing. Drug Discov. Today 2019, 24, 163–178. [Google Scholar] [CrossRef]
- Mathew, E.; Pitzanti, G.; Larrañeta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Vidiyala, N.; Parupathi, P.; Sunkishala, P.; Muppavarapu, C.S.; Gujja, A.; Kanagala, P.; Meduri, S.K.; Nyavanandi, D. Artificial Intelligence: A New Era in Prostate Cancer Diagnosis and Treatment. Int. J. Pharm. 2025, 683, 126024. [Google Scholar] [CrossRef] [PubMed]
- Vidiyala, N.; Sunkishala, P.; Parupathi, P.; Nyavanandi, D. The Role of Artificial Intelligence in Drug Discovery and Pharmaceutical Development: A Paradigm Shift in the History of Pharmaceutical Industries. AAPS PharmSciTech 2025, 26, 133. [Google Scholar] [CrossRef] [PubMed]
- Colorado, H.A.; Gutierrez-Velasquez, E.I.; Gil, L.D.; de Camargo, I.L. Exploring the Advantages and Applications of Nanocomposites Produced via Vat Photopolymerization in Additive Manufacturing: A Review. Adv. Compos. Hybrid. Mater. 2024, 7, 1. [Google Scholar] [CrossRef]
- Kantaros, A.; Petrescu, F.I.T.; Abdoli, H.; Diegel, O.; Chan, S.; Iliescu, M.; Ganetsos, T.; Munteanu, I.S.; Ungureanu, L.M. Additive Manufacturing for Surgical Planning and Education: A Review. Appl. Sci. 2024, 14, 2550. [Google Scholar] [CrossRef]
- Kopp, S.P.; Medvedev, V.; Tangermann-Gerk, K.; Wöltinger, N.; Rothfelder, R.; Graßl, F.; Heinrich, M.R.; Januskaite, P.; Goyanes, A.; Basit, A.W.; et al. Electrophotographic 3D Printing of Pharmaceutical Films. Addit. Manuf. 2023, 73, 103707. [Google Scholar] [CrossRef]
- Goyal, V.; Kumar, A.; Verma, G.C. Role of Additive Manufacturing in Biomedical Application. In Additive Manufacturing: Advanced Materials and Design Techniques; CRC Press: Boca Raton, FL, USA, 2023; pp. 117–131. [Google Scholar] [CrossRef]
- Yi, H.; Guo, X.; Chang, F.; Cao, H.; An, J.; Chua, C. Ink-Jetting-Based Conformal Additive Manufacturing: Advantages, Opportunities, and Challenges. Int. J. Extrem. Manuf. 2025, 7, 032002. [Google Scholar] [CrossRef]
- Tyagi, N.; Bhardwaj, V.; Sharma, D.; Tomar, R.; Chaudhary, V.; Khanuja, M.; Singh, M.K.; Sharma, G. 3D Printing Technology in the Pharmaceutical and Biomedical Applications: A Critical Review. Biomed. Mater. Devices 2024, 2, 178–190. [Google Scholar] [CrossRef]
- Phalke, P.; Balivada, S.; Murkute, S. Additive Manufacturing Technologies in Tissue Engineering and Drug Delivery System. Int. J. Health Technol. Innov. 2022, 1, 14–23. [Google Scholar] [CrossRef]
- Methani, M.M.; Cesar, P.F.; de Paula Miranda, R.B.; Morimoto, S.; Özcan, M.; Revilla-León, M. Additive Manufacturing in Dentistry: Current Technologies, Clinical Applications, and Limitations. Curr. Oral. Health Rep. 2020, 7, 327–334. [Google Scholar] [CrossRef]
- Zhao, C.; Lv, Q.; Wu, W. Application and Prospects of Hydrogel Additive Manufacturing. Gels 2022, 8, 297. [Google Scholar] [CrossRef]
- Razzaghi, M.; Bakhtiari, S.S.E.; Charest, G.; Fortin, D.; Akbari, M. Microneedle Arrays for Brain Drug Delivery: The Potential of Additive Manufacturing. Progress. Trans. Can. Soc. Mech. Eng. 2025, 49, 175–191. [Google Scholar] [CrossRef]
- da Silva, L.R.R.; Sales, W.F.; dos Anjos Rodrigues Campos, F.; de Sousa, J.A.G.; Davis, R.; Singh, A.; Coelho, R.T.; Borgohain, B. A Comprehensive Review on Additive Manufacturing of Medical Devices. Progress. Addit. Manuf. 2021, 6, 517–553. [Google Scholar] [CrossRef]
- Praveena, B.A.; Lokesh, N.; Buradi, A.; Santhosh, N.; Praveena, B.L.; Vignesh, R. A Comprehensive Review of Emerging Additive Manufacturing (3D Printing Technology): Methods, Materials, Applications, Challenges, Trends and Future Potential. Mater. Today Proc. 2022, 52, 1309–1313. [Google Scholar] [CrossRef]
- Ali, M.A.; Rajabi, M.; Sudhir Sali, S. Additive Manufacturing Potential for Medical Devices and Technology. Curr. Opin. Chem. Eng. 2020, 28, 127–133. [Google Scholar] [CrossRef]
- Salifu, S.; Ogunbiyi, O.; Olubambi, P.A. Potentials and Challenges of Additive Manufacturing Techniques in the Fabrication of Polymer Composites. Int. J. Adv. Manuf. Technol. 2022, 122, 577–600. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, P.; Mokasdar, A.; Hou, L. Additive Manufacturing and Its Societal Impact: A Literature Review. Int. J. Adv. Manuf. Technol. 2013, 67, 1191–1203. [Google Scholar] [CrossRef]
- Skrodzka, M.; Cieślak, A.; Łabowska, M.B.; Detyna, J.; Michalak, I. Bio-Based Additive Manufacturing: An Overview. In Additive Manufacturing Materials and Technology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 291–316. [Google Scholar] [CrossRef]
- Ramola, M.; Yadav, V.; Jain, R. On the Adoption of Additive Manufacturing in Healthcare: A Literature Review. J. Manuf. Technol. Manag. 2019, 30, 48–69. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive Manufacturing of Tissues and Organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Sheoran, A.J.; Kumar, H.; Arora, P.K.; Moona, G. Bio-Medical Applications of Additive Manufacturing: A Review. Procedia Manuf. 2020, 51, 663–670. [Google Scholar] [CrossRef]
- Mathur, V.; Dsouza, V.; Srinivasan, V.; Vasanthan, K.S. Volumetric Additive Manufacturing for Cell Printing: Bridging Industry Adaptation and Regulatory Frontiers. ACS Biomater. Sci. Eng. 2025, 11, 156–181. [Google Scholar] [CrossRef]
- Laskowska, D.; Mitura, K.; Ziółkowska, E.; Bałasz, B. Additive Manufacturing Methods, Materials and Medical Applications—The Review. J. Mech. Energy Eng. 2021, 5, 15–30. [Google Scholar] [CrossRef]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-Generation Surgical Meshes for Drug Delivery and Tissue Engineering Applications: Materials, Design and Emerging Manufacturing Technologies. Biodes Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [PubMed]
- Puryear, J.R.; Yoon, J.K.; Kim, Y.T. Advanced Fabrication Techniques of Microengineered Physiological Systems. Micromachines 2020, 11, 730. [Google Scholar] [CrossRef]
- Chua, K.; Khan, I.; Malhotra, R.; Zhu, D. Additive Manufacturing and 3D Printing of Metallic Biomaterials. Eng. Regen. 2021, 2, 288–299. [Google Scholar] [CrossRef]
- Touri, M.; Kabirian, F.; Saadati, M.; Ramakrishna, S.; Mozafari, M. Additive Manufacturing of Biomaterials—The Evolution of Rapid Prototyping. Adv. Eng. Mater. 2019, 21, 1800511. [Google Scholar] [CrossRef]
- van Tienderen, G.S.; Berthel, M.; Yue, Z.; Cook, M.; Liu, X.; Beirne, S.; Wallace, G.G. Advanced Fabrication Approaches to Controlled Delivery Systems for Epilepsy Treatment. Expert. Opin. Drug Deliv. 2018, 15, 915–925. [Google Scholar] [CrossRef]
- Ladani, L.; Palmieri, M. Review of the Use of Metals in Biomedical Applications: Biocompatibility, Additive Manufacturing Technologies, and Standards and Regulations. Metals 2024, 14, 1039. [Google Scholar] [CrossRef]
- Pérez, M.; García-Collado, A.; Carou, D.; Medina-Sánchez, G.; Dorado-Vicente, R. 3D Printing of Pharmaceutical Products. In Additive Manufacturing; Elsevier: Amsterdam, The Netherlands, 2021; pp. 569–597. ISBN 9780128184110. [Google Scholar]
- Long, J.; Nand, A.; Ray, S. Application of Spectroscopy in Additive Manufacturing. Materials 2021, 14, 203. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tariq, A.; Arif, Z.U.; Khalid, M.Y.; Hossain, M.; Rasool, P.I.; Umer, R.; Ramakrishna, S. Recent Advances in the Additive Manufacturing of Stimuli-Responsive Soft Polymers. Adv. Eng. Mater. 2023, 25, 2301074. [Google Scholar] [CrossRef]
- Singh, A.B. Transforming Healthcare: A Review of Additive Manufacturing Applications in the Healthcare Sector. Eng. Proc. 2024, 72, 2. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Wu, J.; Zhang, X.; Su, R.; Ma, L.; Sun, Q.; He, R. Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 1164–1189. [Google Scholar] [CrossRef]
- Muehlenfeld, C.; Roberts, S.A. 3D/4D Printing in Additive Manufacturing: Process Engineering and Novel Excipients. In 3D and 4D Printing in Biomedical Applications: Process Engineering and Additive Manufacturing; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–23. [Google Scholar] [CrossRef]
- Alghamdi, S.S.; John, S.; Choudhury, N.R.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Omigbodun, F.T.; Olawumi, M.A. Medical Additive Manufacturing Then, Now, and Will. In Medical Additive Manufacturing: Concepts and Fundamentals Additive Manufacturing Materials and Technologies; Elsevier: Amsterdam, The Netherlands, 2024; pp. 381–400. [Google Scholar] [CrossRef]
- Veeman, D.; Sai, M.S.; Sureshkumar, P.; Jagadeesha, T.; Natrayan, L.; Ravichandran, M.; Mammo, W.D. Additive Manufacturing of Biopolymers for Tissue Engineering and Regenerative Medicine: An Overview, Potential Applications, Advancements, and Trends. Int. J. Polym. Sci. 2021, 2021, 4907027. [Google Scholar] [CrossRef]
- Srivastava, M.; Rathee, S. Additive Manufacturing: Recent Trends, Applications and Future Outlooks. Prog. Addit. Manuf. 2022, 7, 261–287. [Google Scholar] [CrossRef]
- Amaya-Rivas, J.L.; Perero, B.S.; Helguero, C.G.; Hurel, J.L.; Peralta, J.M.; Flores, F.A.; Alvarado, J.D. Future Trends of Additive Manufacturing in Medical Applications: An Overview. Heliyon 2024, 10, e26641. [Google Scholar] [CrossRef]
- Mohanadas, H.P.; Nair, V.; Doctor, A.A.; Faudzi, A.A.M.; Tucker, N.; Ismail, A.F.; Ramakrishna, S.; Saidin, S.; Jaganathan, S.K. A Systematic Analysis of Additive Manufacturing Techniques in the Bioengineering of In Vitro Cardiovascular Models. Ann. Biomed. Eng. 2023, 51, 2365–2383. [Google Scholar] [CrossRef]
- Yuvaraj, A.R.; Jayadurka, J.; Elavarasi, E.; Srinivasan, R. Applications and Advancement of 3D Printing in Pharmaceutical Manufacturing and Drug Delivery: Review Article. J. Pharma Insights Res. 2024, 2, 023–029. [Google Scholar] [CrossRef]
- Pathak, K.; Saikia, R.; Das, A.; Das, D.; Islam, M.A.; Pramanik, P.; Parasar, A.; Borthakur, P.P.; Sarmah, P.; Saikia, M.; et al. 3D Printing in Biomedicine: Advancing Personalized Care through Additive Manufacturing. Open Explor. 2023, 4, 1135–1167. [Google Scholar] [CrossRef]
- Guddati, S.; Kiran, A.S.K.; Leavy, M.; Ramakrishna, S. Recent Advancements in Additive Manufacturing Technologies for Porous Material Applications. Int. J. Adv. Manuf. Technol. 2019, 105, 193–215. [Google Scholar] [CrossRef]
- Kulkarni, V.R.; Saha, T.; Raj Giri, B.; Lu, A.; Das, S.C.; Maniruzzaman, M. Recent Advancements in Pharmaceutical 3D Printing Industry. J. Drug Deliv. Sci. Technol. 2024, 100, 106072. [Google Scholar] [CrossRef]
- Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y.; Han, H.K. Pharmaceutical Applications of 3D Printing Technology: Current Understanding and Future Perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar] [CrossRef]
- Salama, A.H. Recent Advances in 3D and 4D Printing in Pharmaceutical Technology: Applications, Challenges, and Future Perspectives. Futur. J. Pharm. Sci. 2025, 11, 107. [Google Scholar] [CrossRef]
- Lepowsky, E.; Tasoglu, S. 3D Printing for Drug Manufacturing: A Perspective on the Future of Pharmaceuticals. Int. J. Bioprint 2017, 4, 119. [Google Scholar] [CrossRef]
- Thompson, M.S. Current Status and Future Roles of Additives in 3D Printing—A Perspective. J. Vinyl Addit. Technol. 2022, 28, 3–16. [Google Scholar] [CrossRef]
- Nizam, M.; Purohit, R.; Taufik, M. 3D Printing in Healthcare: A Review on Drug Printing, Challenges and Future Perspectives. Mater. Today Commun. 2024, 40, 110199. [Google Scholar] [CrossRef]
- Klenam, D.E.P.; Bamisaye, O.S.; Williams, I.E.; Van Der Merwe, J.W.; Bodunrin, M.O. Global Perspective and African Outlook on Additive Manufacturing Research—An Overview. Manuf. Rev. 2022, 9, 35. [Google Scholar] [CrossRef]
- Soni, S.J. Revolution of 3d Printing in Pharmaceuticals: Innovations, Applications, and Future Perspectives. Int. J. Health Care Biol. Sci. 2024, 6–12. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Stabrauskiene, J.; Kazlauskaite, J.A.; Bernatonyte, U.; Kopustinskiene, D.M. The Future of Medicine: How 3D Printing Is Transforming Pharmaceuticals. Pharmaceutics 2025, 17, 390. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Puri, A.; Gholap, A.D.; Chaudhari, Y.; Nagime, P.V.; Singh, S.; Mohite, P.; Puri, A.; Gholap, A.D.; Chaudhari, Y.; et al. Regulatory Aspects, Future Prospects, and Challenges Associated with Biomaterials-Based Additive Manufacturing for Tissue Engineering. In Biomaterial-Based Additive Manufacturing in Tissue Engineering and Regeneration; Springer: Cham, Switzerland, 2025; pp. 531–551. ISBN 978-3-031-96070-3. [Google Scholar]
- Garg, U.; Jain, N.; Kaul, S.; Rai, V.K.; Pandey, M.; Nagaich, U.; Dua, K. The Emerging Role of 3D-Printing in Ocular Drug Delivery: Challenges, Current Status, and Future Prospects. J. Drug Deliv. Sci. Technol. 2022, 76, 103798. [Google Scholar] [CrossRef]
- Agrawal, R.; Garg, A.; Deshmukh, R. A Snapshot of Current Updates and Future Prospects of 3D Printing in Medical and Pharmaceutical Science. Curr. Pharm. Des. 2023, 29, 604–619. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D Printing Technologies for Drug Delivery Devices: Current Status and Future Perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef]
- Malheiro, V.; Duarte, J.; Veiga, F.; Mascarenhas-Melo, F. Exploiting Pharma 4.0 Technologies in the Non-Biological Complex Drugs Manufacturing: Innovations and Implications. Pharmaceutics 2023, 15, 2545. [Google Scholar] [CrossRef]
- Feng, S.; Repka, M.A. Future Prospects Including Novel Polymeric Excipients for 3D Printing of Pharmaceutical and Biomedical Applications. In 3D Printing; Springer: Cham, Switzerland, 2024; Volume 44, pp. 273–286. ISBN 978-3-031-46015-9. [Google Scholar]
- Shah, S.W.A.; Xu, Q.; Ullah, M.W.; Zahoor; Sethupathy, S.; Morales, G.M.; Sun, J.; Zhu, D. Lignin-Based Additive Materials: A Review of Current Status, Challenges, and Future Perspectives. Addit. Manuf. 2023, 74, 103711. [Google Scholar] [CrossRef]
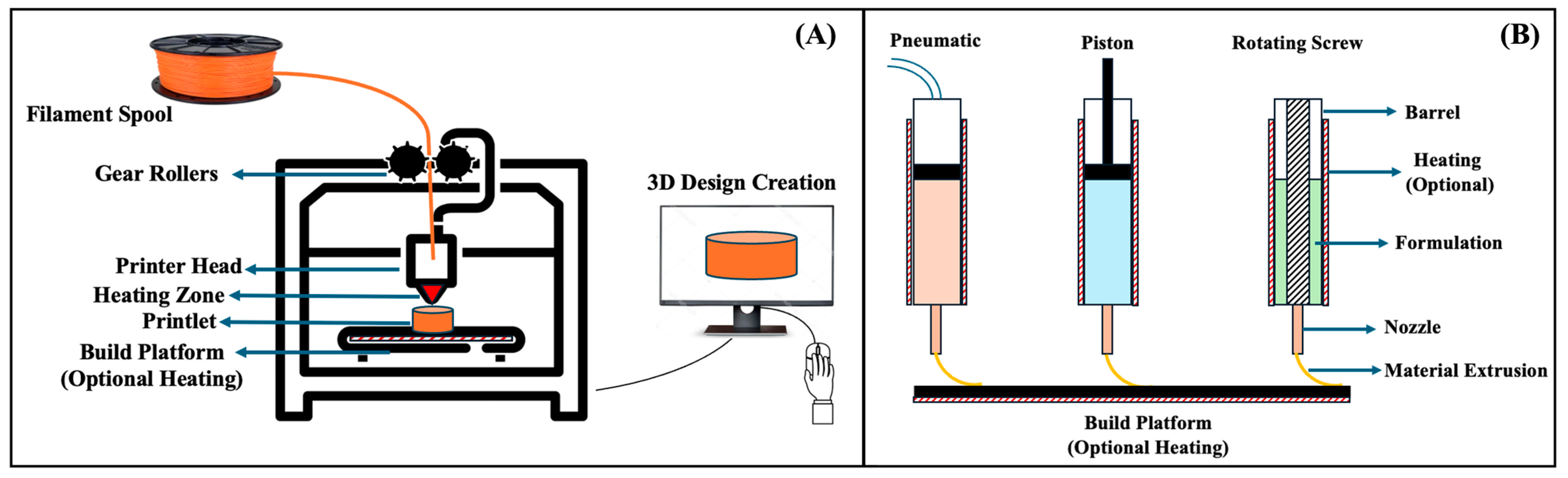
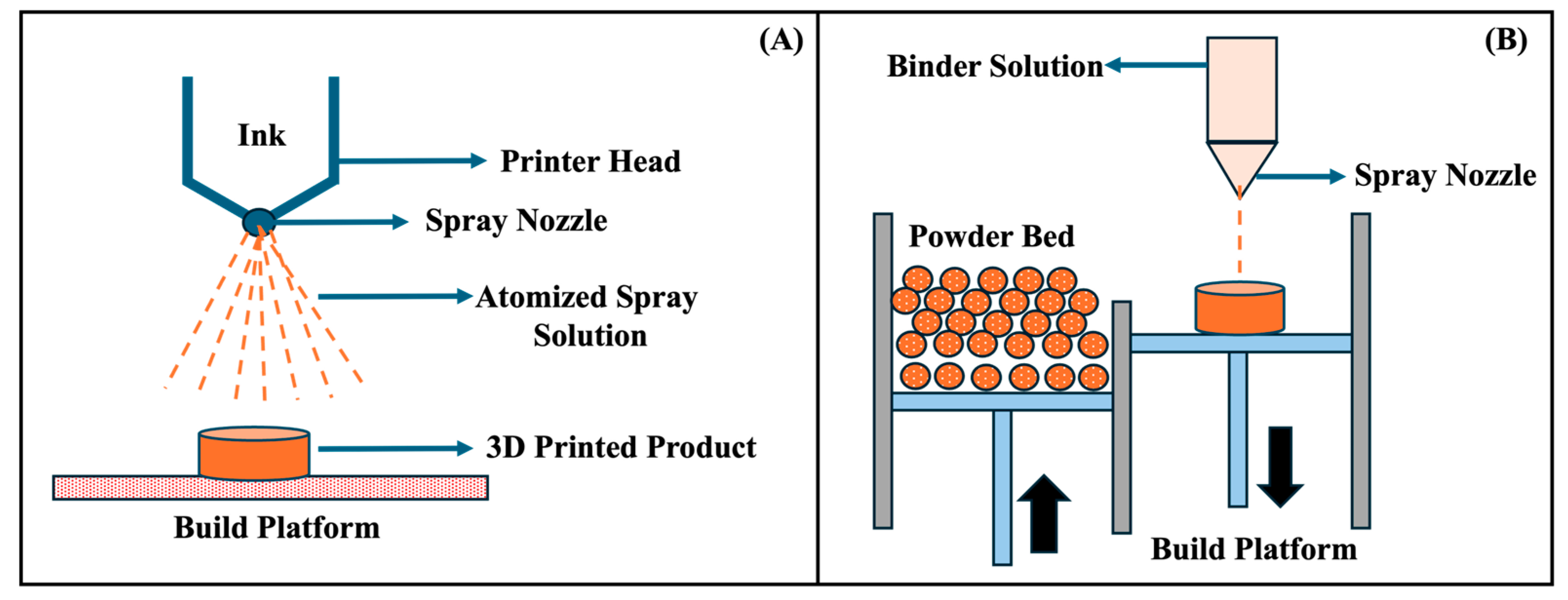
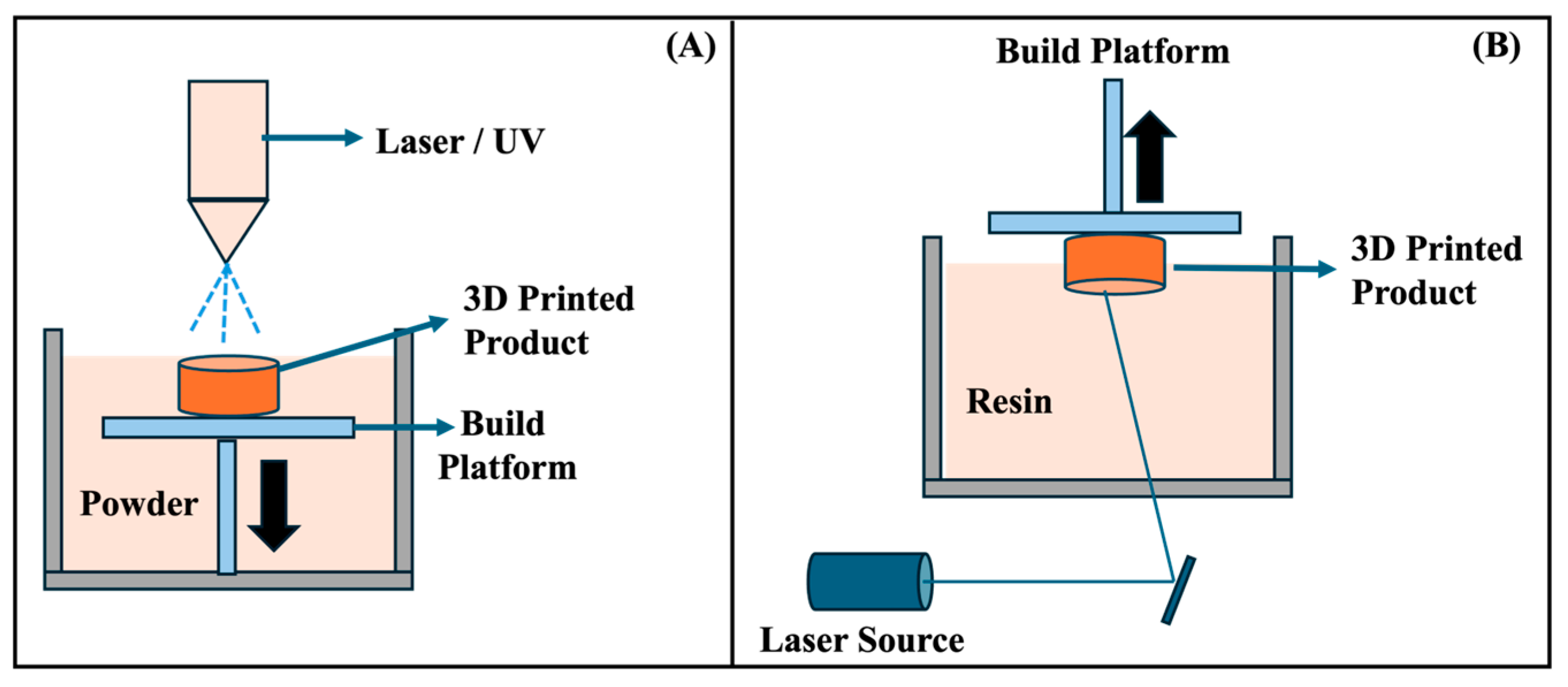
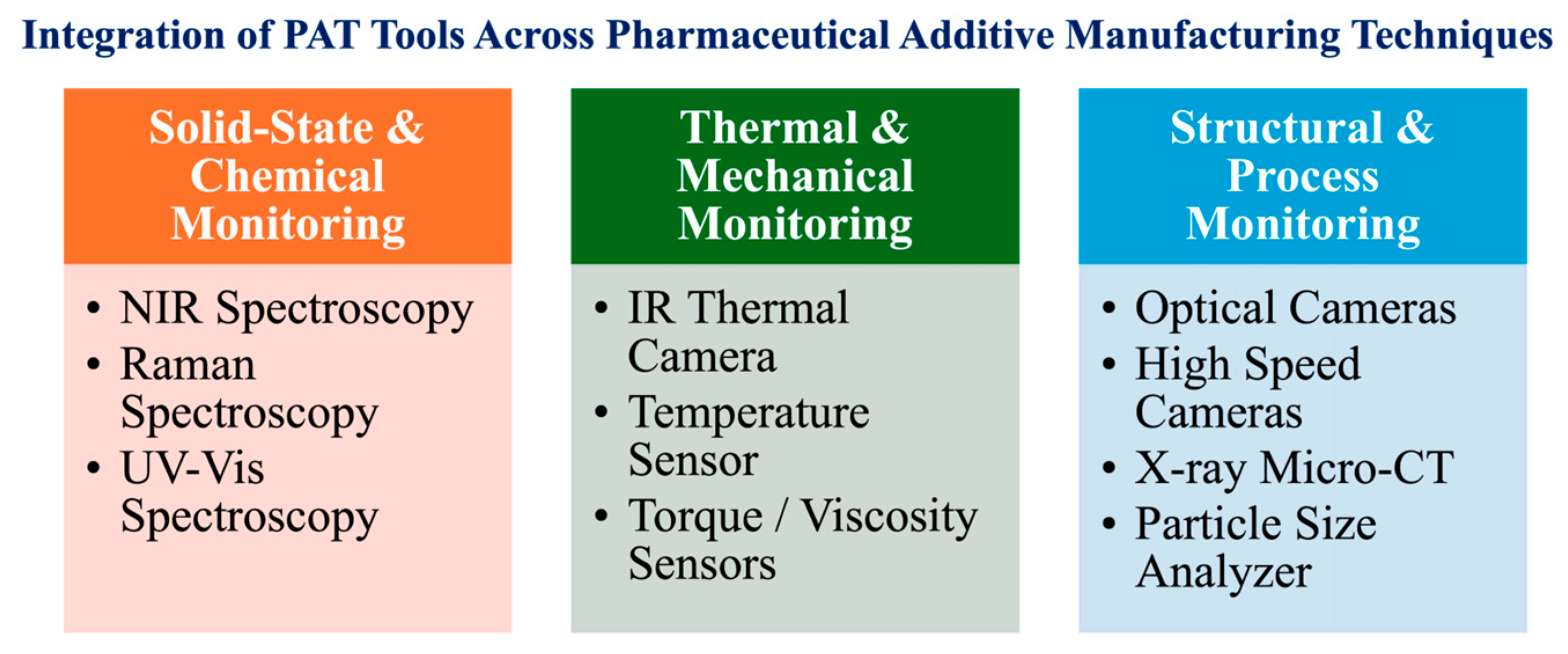
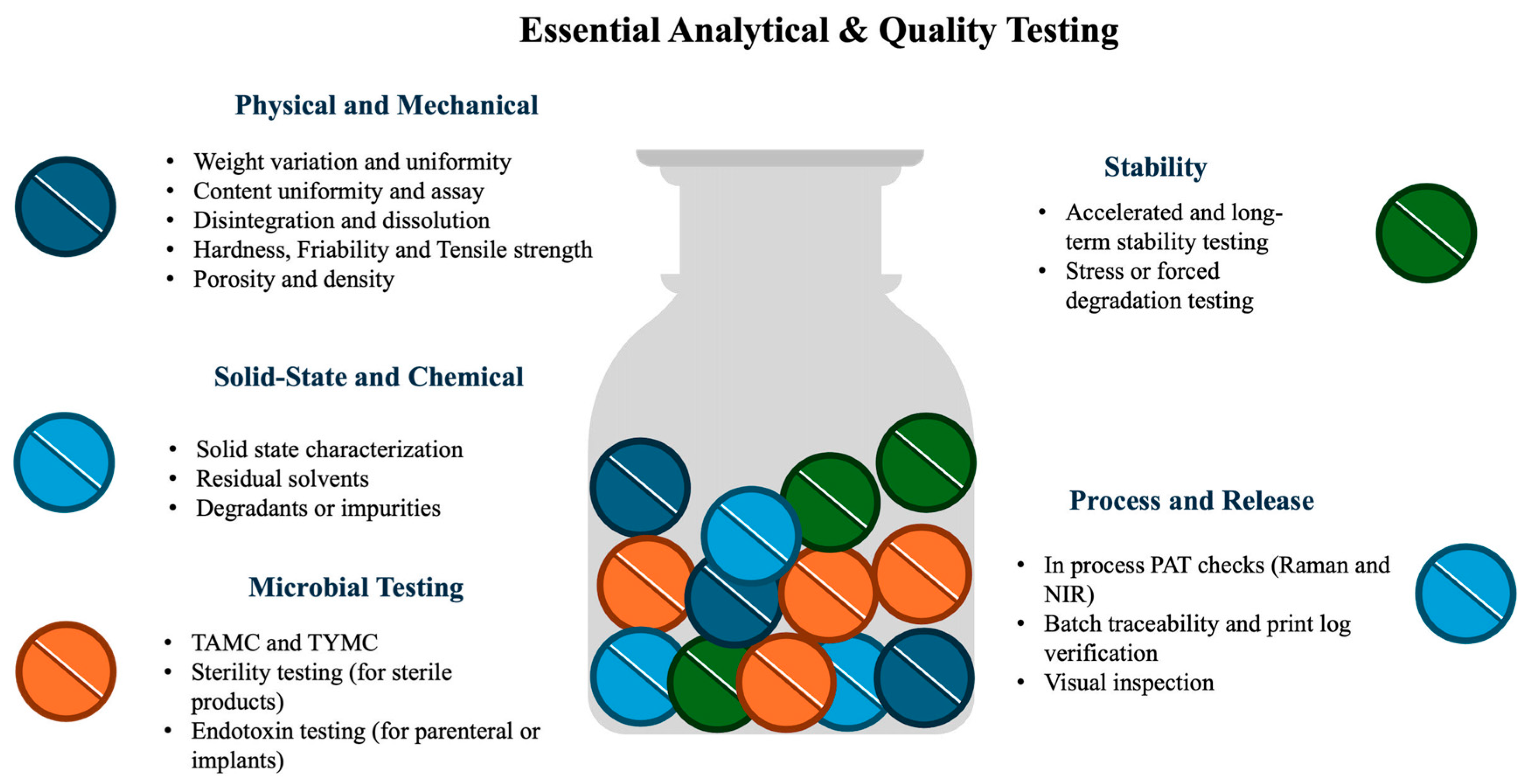
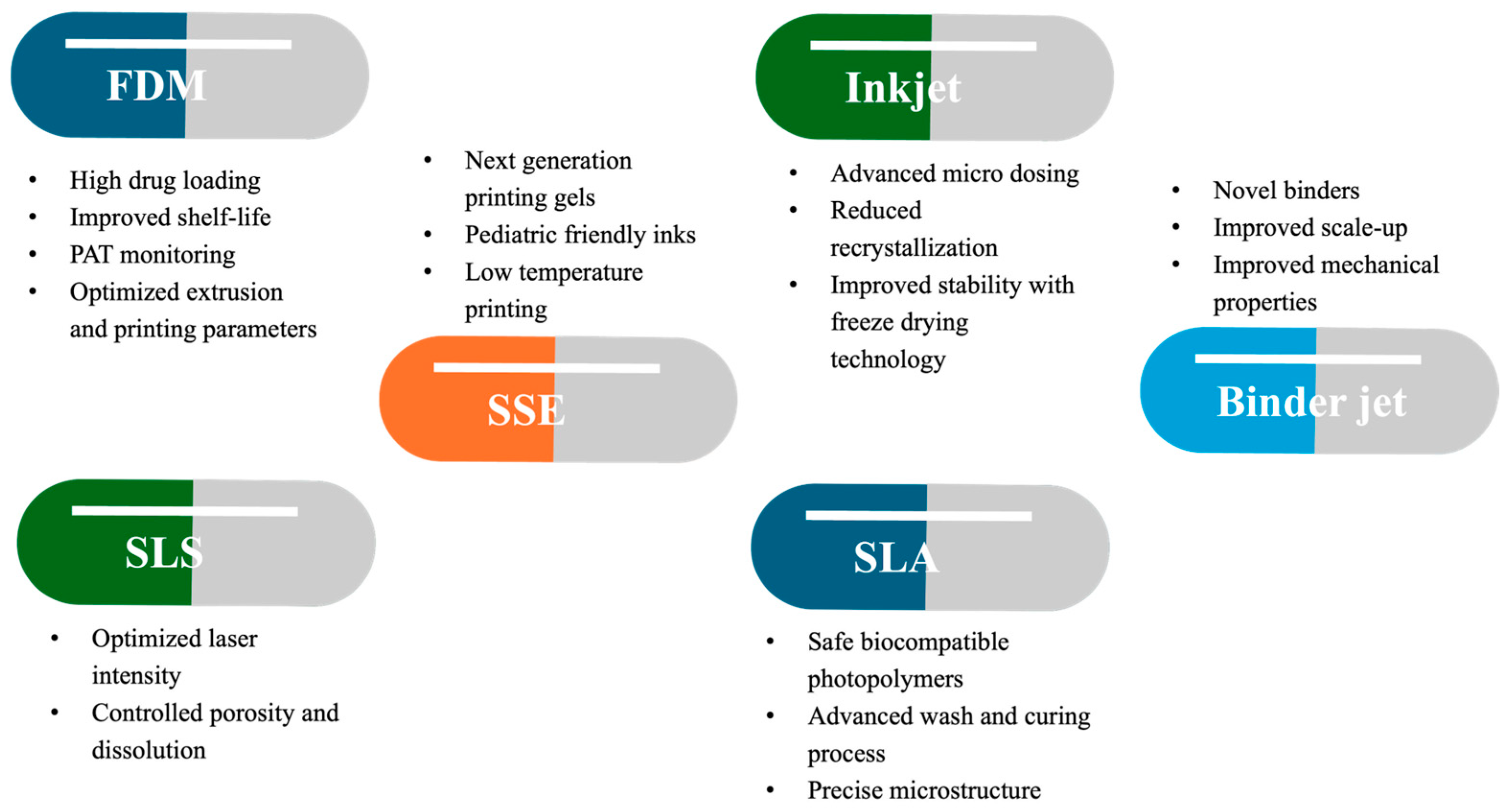
| Timeframe | Stage | Key Findings | Milestones |
|---|---|---|---|
| 2000–2014 | Early concept and prototyping |
|
|
| 2015–2020 | Innovation phase |
|
|
| 2020–2025 | Specialized applications |
|
|
| Next 15 years | Future outlook |
|
|
| 3D Printing Platform | Mechanism | Materials | Advantages | Limitations | Common Applications |
|---|---|---|---|---|---|
| FDM | Drug-loaded polymeric filament is melted and extruded layer by layer | Polymers such as HPMC, HPC, Eudragit, PEO, PVA, PVP |
|
|
|
| SSE | Semi-solid material is extruded through a nozzle and deposited on the build platform layer by layer | HPMC, Poloxamers, Lipids, Starch paste |
|
|
|
| Inkjet | The fine droplets of formulation solution sprayed onto the build platform layer by layer | Formulation materials dissolved in aqueous/organic solvents |
|
|
|
| Binder jet | The fine droplets of binder solution are sprayed on top of successive powder layers | Binder solution sprayed onto common powder excipients such as microcrystalline cellulose and starch |
|
|
|
| SLS | The powder particles are fused with the application of a laser in the shape of a 3D design | Polymers such as PVP, Eudragit, and PVA |
|
|
|
| SLA | The drug solution, consisting of photopolymer resin, is cured in the shape of a 3D design with the application of UV light | Methacrylate, PEG diacrylate resins |
|
|
|
| Platform | Key Process Parameters | Typical Operating Conditions | Solid State of Drug |
| FDM |
|
|
|
| SSE |
|
|
|
| Inkjet |
|
|
|
| Binder jet |
|
|
|
| SLS |
|
|
|
| SLA |
|
|
|
| 3D Printing Technique | Commonly Used Materials | Strengths | Limitations |
|---|---|---|---|
| FDM | PVA, HPC, PEO, PLA, PEG-based polymers, PVP, HPMC | Suitable for solubility enhancement and for developing controlled release formulations |
|
| SSE | Glycerin-based gels, starch gel, poloxamer gel, Carbopol, cellulose-based excipients | Can be processed at ambient or low temperatures |
|
| Inkjet | HPMC and PVP-based solvent solutions | Suitable for nano to micro range dosing |
|
| Binder jet | Lactose, mannitol, cellulose-based materials, PVP | Can be processed at ambient or low temperatures, suitable for thermally sensitive drugs |
|
| SLS | Lactose, mannitol, PVP, PVA, Eudragit | Suitable for complex geometries and partial drug amorphization |
|
| SLA | Polyethylene glycol diacrylate, polycaprolactone diacrylate | High resolution and ideal for developing implants |
|
| 3D Printing Technique | Primary Degradation Risk | Root Cause | Impact on Drug Product | Risk Mitigation Strategy |
|---|---|---|---|---|
| FDM | Thermal degradation |
|
|
|
| SSE | Hydrolysis and microbial degradation |
|
|
|
| Inkjet | Crystallization and chemical degradation |
|
|
|
| Binder jet | Hydrolysis and binder instability |
|
|
|
| SLS | Thermal and oxidative degradation |
|
|
|
| SLA | Unreacted monomers or photodegradation |
|
|
|
| 3D Printing Technique | Stability Challenges | Implications |
|---|---|---|
| FDM |
|
|
| SSE |
|
|
| Inkjet |
|
|
| Binder jet |
|
|
| SLS |
|
|
| SLA |
|
|
| Parameter | Additive Manufacturing | Traditional Manufacturing |
|---|---|---|
| Production speed | Slow (few minutes/unit) | Fast (hundreds/minute) |
| Batch size | Small scale | Large scale |
| Scalability | Limited | Depends on equipment capacity |
| Cost efficiency | High cost, minimal to no waste | Low cost |
| Quality consistency | Vary between printers | Highly consistent |
| Flexibility | Flexible to adjust dose and geometry | No flexibility |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidiyala, N.; Sunkishala, P.; Mandati, P.; Parupathi, P.; Nyavanandi, D. Integrating 3D Printing and Additive Manufacturing into Personalized Medicine for Pharmaceuticals: Opportunities, Limitations, and Future Perspectives. Sci. Pharm. 2025, 93, 61. https://doi.org/10.3390/scipharm93040061
Vidiyala N, Sunkishala P, Mandati P, Parupathi P, Nyavanandi D. Integrating 3D Printing and Additive Manufacturing into Personalized Medicine for Pharmaceuticals: Opportunities, Limitations, and Future Perspectives. Scientia Pharmaceutica. 2025; 93(4):61. https://doi.org/10.3390/scipharm93040061
Chicago/Turabian StyleVidiyala, Nithin, Pavani Sunkishala, Preethi Mandati, Prashanth Parupathi, and Dinesh Nyavanandi. 2025. "Integrating 3D Printing and Additive Manufacturing into Personalized Medicine for Pharmaceuticals: Opportunities, Limitations, and Future Perspectives" Scientia Pharmaceutica 93, no. 4: 61. https://doi.org/10.3390/scipharm93040061
APA StyleVidiyala, N., Sunkishala, P., Mandati, P., Parupathi, P., & Nyavanandi, D. (2025). Integrating 3D Printing and Additive Manufacturing into Personalized Medicine for Pharmaceuticals: Opportunities, Limitations, and Future Perspectives. Scientia Pharmaceutica, 93(4), 61. https://doi.org/10.3390/scipharm93040061







