Abstract
Biopharmaceuticals are medical drugs produced by means of biotechnology. In contrast with small-molecule (traditional) drugs, which are usually synthesized chemically, biopharmaceuticals are derived from biological sources, including tissues, cells, or microorganisms. Biopharmaceuticals comprise a wide extent of therapies, such as vaccines, monoclonal antibodies, cell therapies, recombinant proteins, and gene therapies, as well as biosimilars. These products are designed to become important treatment options for different diseases, including cancer, autoimmune pathological disorders, andinfectious diseases. The development of biopharmaceuticals often includes multifaceted processes, involving genetic engineering and cellular culture techniques, to guarantee efficacy and safety. Accordingly, biopharmaceuticals’ legislation is a key component for ensuring the highest quality of medical products, as well as protecting public health. As a rapidly developing area inside the pharmaceutical industry, biopharmaceuticals represent a significant advancing stage in modern medicine, offering targeted therapies that can improve patient outcomes. Accordingly, this paper seeks to provide current state-of-the-art didactic information, including better insight into various challenges related to biopharmaceuticals’ development, classification, medical use, legislation and ethics.
1. Introduction
Biopharmaceuticals are medical drugs produced using biotechnology [1]. Usually produced from living organisms, they encompass a variety of active agents such as recombinant proteins, vaccines, and monoclonal antibodies. These medications are intended to treat a number of illnesses, such as infectious diseases, autoimmune conditions, and cancer [2]. To guarantee efficacy and safety, the creation of biopharmaceuticals frequently entails intricate procedures, such as genetic engineering and cell culture techniques. Biopharmaceuticals, a quickly expanding segment of the pharmaceutical industry, offer targeted medicines that can enhance patient outcomes and constitute a substantial advancement in contemporary medicine [3]. Accordingly, this paper aims to provide current state-of-the-art information and better insight into various challenges related to biopharmaceuticals’ development, classification, medical use, legislation, and ethics. To achieve the stated goal, the key word “biopharmaceuticals” was cross-referenced with the key words crucial for this paper including “pharmacology”,”safety”,”classification”,”ethics”,”pharmaceutical industry”, and “legislation”, by using relevant databases such as the PubMed, Google Scholar, Scopus, Elsevier, Medscape, etc. To accomplish the broadest possible overview, the age span of the references used was not limited.
2. Pharmacology
Pharmacology in the context of biopharmaceuticals refers to the study of how biopharmaceutical products interact with biological systems, including their mechanisms of action, therapeutic effects, side effects, and pharmacokinetics (how the body absorbs, distributes, metabolizes, and eliminates these drugs) [4]. Understanding pharmacology is essential for the development and safe use of biopharmaceuticals. Some key aspects of biopharmaceutical pharmacology include:
- (1)
- Mechanism of Action: Biopharmaceuticals often have specific mechanisms of action that target particular pathways or cells in the body [5]. Monoclonal antibodies, for instance, can attach to particular antigens on cancer cells, marking them for immune system destruction. Understanding these mechanisms is crucial for predicting therapeutic effects and potential side effects.
- (2)
- Pharmacokinetics: This area studies how biopharmaceuticals behave in the body over time [6]. Key pharmacokinetic parameters include:
- -
- Absorption: The waythe pharmaceutical goes into the bloodstream.
- -
- Distribution: The way the pharmacological agent expands throughout the body and its tissues.
- -
- Metabolism: The ways a drug is chemically altered by the body, primarily by the liver.
- -
- Elimination: The waythe drug is eliminated from the body, primarily through urine or feces.
Biopharmaceuticals may have unique pharmacokinetic profiles compared to traditional small-molecule drugs due to their larger size and complexity.
- (3)
- Pharmacodynamics: This aspect concentrates on the effects of biopharmaceuticals on the body and how they exert their therapeutic effects [7]. It involves studying the relationship between drug concentration and effect, including the dose–response relationship and the time course of drug action.
- (4)
- Therapeutic Index: The ratio of the dose that has a therapeutic effect to the dose that has harmful consequences is known as the therapeutic index, and it serves as a gauge of a drug’s safety [8]. A higher therapeutic index indicates a safer pharmaceutical. Understanding this index is crucial for determining appropriate dosing regimens for biopharmaceuticals.
- (5)
- Adverse Effects and Toxicity: Biopharmaceuticals can have unique side effects due to their mechanisms of action and interactions related to the immune system [9]. Monitoring and understanding these unwanted effects are essential for ensuring patient safety and optimizing treatment protocols.
- (6)
- Drug Interactions: Biopharmaceuticals may interact with other medications, leading to altered effects or increased toxicity [10]. Understanding potential drug–drug interactions is vital for clinicians when prescribing biopharmaceuticals, especially for patients on multiple medications.
- (7)
- Personalized Medicine: Developments in pharmacogenomics—the study of how genes influence an individual’s reaction to medications—are becoming more and more critical in the biopharmaceutical industry [11]. By customizing therapies according to each patient’s unique genetic profile, specific side effects can be minimized and effectiveness increased.
- (8)
- Regulatory Considerations: The pharmacological evaluation of biopharmaceuticals is a critical component of the regulatory approval process [12]. Before novel biopharmaceutical drugs canbe put on the market, regulatory bodies need thorough pharmacological data to evaluate their safety and effectiveness.
We may say that pharmacology plays a vital role in the occurrence, authorization, and clinical use of biopharmaceuticals. Researchers and medical personnel can enhance patient outcomes, optimize treatment plans, and guarantee the safe use of these cutting-edge medicines by having a solid understanding of pharmacological principles.
3. Safety
Safety in the context of biopharmaceuticals refers to the assessment and management of risks associated with the use of biopharmaceutical products, including their development, manufacturing, and clinical application [13]. Ensuring the safety of biopharmaceuticals is critical for protecting patient health and maintaining public trust in these therapies. The key aspects related to the safety of biopharmaceuticals include (Figure 1):
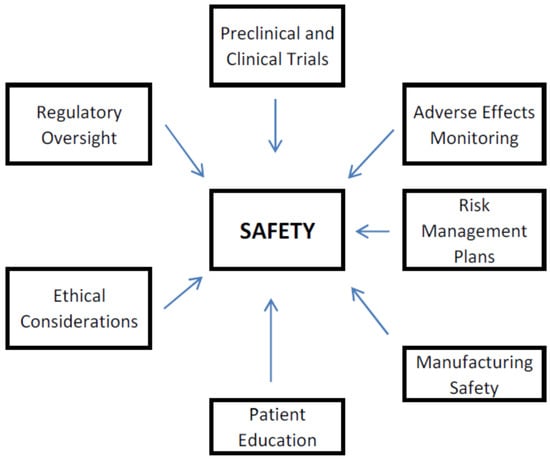
Figure 1.
Factors Influencing Safety of Biopharmaceuticals.
- (1)
- Preclinical and Clinical Trials: Prior to a biopharmaceutical being approved for public exploitation, it undergoes rigorous testing in preclinical studies (often using animal models) and multiple phases of clinical trials involving human participants [14]. These trials are intended to estimate the safety, efficacy, and optimal dosing of the product. Safety assessments include monitoring for adverse effects, determining the maximum tolerated dose, and identifying any potential toxicity, including unacceptable ones.
- (2)
- Adverse Effects Monitoring: Once a biopharmaceutical is on the market, ongoing monitoring for adverse effects is essential [15]. This includes collecting data on any side effects reported by patients and healthcare providers. Regulatory agencies, such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA), obligate manufacturers to report adverse events and may mandate post-marketing surveillance studies to assess safety further. Moreover, to identify any safety signals and take appropriate action, pharmacovigilance involves gathering and analyzing data on adverse occurrences, such as label changes, warnings, or even product recalls if significant safety issues arise [16].
- (3)
- Risk Management Plans: Biopharmaceutical companies are often required to develop risk management plans that summarize strategies for detecting, assessing, and justifying risks associated with their products [17]. These plans may include risk communication strategies, safety monitoring protocols, and plans for managing specific safety concerns.
- (4)
- Manufacturing Safety: The production of biopharmaceuticals must adhere to strict Good Manufacturing Practices (GMP) to guarantee end-product quality and safety [18]. This includes rigorous quality control measures, contamination prevention, and validation of manufacturing processes to minimize risks associated with the production of biopharmaceuticals.
- (5)
- Patient Education: Educating patients about the potential benefits and risks of biopharmaceuticals is vital for informed decision-making [19]. They should be made aware of possible side effects, how to recognize them, and when to seek medical attention. Clear communication from healthcare providers can enhance patient safety.
- (6)
- Ethical Considerations: Ethical considerations play a role in ensuring safety, particularly in clinical trials. Informed consent processes must adequately inform participants about potential risks, and researchers must prioritize participant safety throughout the study [20].
- (7)
- Regulatory Oversight: To guarantee the safety of biopharmaceuticals, regulatory bodies are essential [21]. They review clinical trial data, evaluate manufacturing processes, and monitor post-marketing safety data to ensure that biopharmaceuticals meet safety standards before and after they reach the market.
Ensuring the safety of biopharmaceuticals involves a comprehensive approach that includes rigorous testing, ongoing monitoring, risk management, and regulatory oversight. By prioritizing safety, the biopharmaceutical industry can help ensure that these innovative therapies provide effective treatment while minimizing risks to patients.
4. Classification
Biopharmaceutical drugs, often referred to simply as biopharmaceuticals, are therapeutic products that are produced using biological processes and living organisms. Unlike small-molecule traditional drugs, which are typically chemically synthesized, biopharmaceuticals result from biological sources, such as cells, tissues, or microorganisms [22]. They comprise an extensive range of products, each with distinctive characteristics and applications. Here are some key categories and examples of biopharmaceutical drugs (Table 1):

Table 1.
Classification of Biopharmaceuticals.
- (1)
- Monoclonal Antibodies (mAbs)
Monoclonal antibodies are engineered proteins designed to target specific antigens on cells [23]. They are frequently used to treat a wide range of illnesses, such as infectious diseases, autoimmune conditions, and malignancies. Examples include rituximab, used to treat certain types of lymphoma and autoimmune diseases, and trastuzumab, used for HER2-positive breast cancer.
- (2)
- Vaccines
By boosting the immune system, biopharmaceutical vaccines aim to prevent infectious illnesses [24]. They can be made from live attenuated organisms, inactivated organisms, or recombinant proteins. Examples include hepatitis B vaccine, a recombinant vaccine that protects against hepatitis B virus, and mRNA vaccines, such as COVID-19 vaccines, which use messenger RNA to instruct cells to create a viral protein that prompts an immune response.
- (3)
- Recombinant Proteins
These are proteins produced through recombinant DNA technology [25]. They can serve various therapeutic purposes, including hormone replacement and enzyme replacement therapies. Examples include insulin, used to manage diabetes, anderythropoietin (EPO), which stimulates red blood cell production and is used to treat anemia.
- (4)
- Biosimilars
Biosimilars are biologic medications that closely resemble reference biopharmaceuticals that have already received approval [26]. They are designed to have no clinically significant differences in purity, safety, and potency. An example includes Zarxio, a biosimilar to filgrastim, used to stimulate white blood cell production.
- (5)
- Gene Therapies
To treat or prevent disease, they entail adding, removing, or changing genetic material within a patient’s cells [27]. Examples includeLuxturna, a gene therapy for a rare form of inherited blindness, andZolgensma, a gene therapy for spinal muscular atrophy.
- (6)
- Cell Therapies
These involve the use of living cells to treat diseases [28]. This category includes stem cell therapies and CAR T-cell therapies. Examples includeKymriah, a CAR T-cell therapy for specific types of blood cancers, and Yescarta, another CAR T-cell therapy used for large B-cell lymphoma.
- (7)
- Fusion Proteins
These proteins are made by combining two or more genes that were previously coded for different proteins [29]. Their therapeutic qualities may be improved. An example is etanercept, a fusion protein used to treat autoimmune diseases like rheumatoid arthritis.
Some of the key groups, in accordance with their relevance, are described in more detail, as follows:
(I) Monoclonal antibodies (mAbs) are a class of biopharmaceuticals that are engineered to target specific antigens, typically proteins found on the surface of cells. They are produced by creating identical copies, or clones, of a single type of immune cell, which allows for the generation of antibodies that are uniform in structure and function [30].
There are numerous uses for monoclonal antibodies in medicine [31], including:
- ①
- Cancer Treatment: Many mAbs are designed to target cancer cells specifically, helping to inhibit tumor growth or mark cancer cells for destruction by the immune system.
- ②
- Autoimmune Diseases: In diseases including multiple sclerosis and rheumatoid arthritis, some monoclonal antibodies are utilized to alter the immune response.
- ③
- Infectious Diseases: mAbs can be used to neutralize pathogens, such as viruses, and are being explored for use in treating diseases like COVID-19.
- ④
- Diagnostic Tools: They are also employed in various diagnostic tests, helping to detect specific biomarkers associated with diseases.
The development of monoclonal antibodies involves sophisticated techniques, including hybridoma technology and recombinant DNA technology, ensuring that these therapies are both safe and effective for patients. As research continues, the potential for new mAb therapies is expanding, making them a vital component of modern biopharmaceuticals.
(II) Biopharmaceutical vaccines are a type of vaccine developed using biotechnological methods, often involving the use of living organisms or their components to elicit an immune response [32]. By stimulating the body’s immune system to identify and fight off pathogens like bacteria and viruses, these vaccinations aim to prevent infectious diseases.
There are several types of biopharmaceutical vaccines [33], including:
- ①
- Recombinant Vaccines: These vaccines are created by inserting genes that code for specific antigens from a pathogen into a harmless organism, such as yeast or bacteria. The organism then produces the antigen, which can be purified and used to promote an immune response. An illustration is the hepatitis B vaccine.
- ②
- Subunit Vaccines: These comprise only specific parts of the pathogen (such as proteins or sugars) as opposed to the whole pathogen. This approach reduces the risk of adverse reactions while still providing immunity. The human papillomavirus (HPV) vaccine is an exemplar.
- ③
- mRNA Vaccines: A newer technology that uses messenger RNA to teach cells to make a protein that is part of the pathogen, prompting an immune reaction. Two well-known mRNA vaccines are the COVID-19 vaccines created by Moderna and Pfizer–BioNTech.
- ④
- Viral Vector Vaccines: These trigger an immune response by introducing the pathogen’s genetic material into the body through a harmless virus. The Johnson & Johnson COVID-19 vaccine is an exemplar of this type.
Biopharmaceutical vaccines have revolutionized public health by providing effective means to prevent diseases that can lead to significant morbidity and mortality [34]. They undergo a rigorous safety and effectiveness examination before being made available to the general population. Ongoing research continues to enhance vaccine technology, aiming to improve the efficacy and broaden the range of preventable diseases.
(III) Recombinant proteins are a significant category of biopharmaceuticals produced through recombinant DNA technology [35]. In this procedure, a gene encoding a particular protein is inserted into a host organism, such as yeast, bacteria, or mammalian cells, which then expresses the protein. The resulting recombinant proteins can be utilized for various therapeutic and diagnostic objectives [36].
Key applications of recombinant proteins in biopharmaceuticals include:
- ①
- Therapeutic Proteins: Many recombinant proteins are utilized as therapeutic agents to treat various medical conditions. Examples include:
- -
- Insulin: Recombinant human insulin is widely used to manage diabetes.
- -
- Growth Hormones: Children and adults with growth disorders can be treated with recombinant human growth hormone.
- -
- Erythropoietin (EPO): This protein stimulates red blood cell production and is utilized to treat anemia, predominantly in patients having chronic kidney disease.
- ②
- Monoclonal Antibodies: While monoclonal antibodies themselves are a distinct class, they are often produced as recombinant proteins. These antibodies are intended to treat different diseases, including cancers and autoimmune disorders.
- ③
- Vaccines: Some vaccines utilize recombinant proteins as antigens to stimulate an immune response. Hence, the hepatitis B vaccine contains a recombinant form of the hepatitis B surface antigen.
- ④
- Enzymes: Recombinant proteins can also serve as enzymes for therapeutic purposes, such as in enzyme replacement therapy for conditions like Gaucher disease.
- ⑤
- Diagnostic Tools: Recombinant proteins are used in various diagnostic assays and tests, helping to detect specific diseases or conditions.
The production of recombinant proteins involves several steps, including gene cloning, expression, purification, and formulation [37]. This technology allows for the large-scale production of proteins that are identical to their naturally occurring counterparts, ensuring consistency and safety in therapeutic applications.
As research advances, the potential for new recombinant proteins continues to grow, contributing to the development of innovative treatments and improving patient care [38].
(IV) Biosimilars are biologic medicinal products highly similar to previously approved reference biopharmaceuticals. They are developed to have no clinically significant differences in safety, purity, and potency [39]. Put otherwise, a company creating a biosimilar must demonstrate that the purity, chemical identity, and bioactivity of its product are very comparable to those of the reference product. In terms of safety, purity, and potency, assessed by human pharmacokinetic and pharmacodynamic studies as well as additional clinical studies if necessary, the manufacturer must also demonstrate that there are no clinically significant differences between its product and the reference product. Biosimilars have become more common in the treatment of pathological conditions like cancer, autoimmune diseases (e.g., rheumatoid arthritis, Crohn’s disease), and chronic kidney disorder (for anemia management) [40,41,42]. Several biosimilars are now being approved for multiple indications (e.g., oncology, autoimmune diseases), making them more flexible treatment alternatives and allowing for broader market dissemination. Only when a healthcare professional prescribes explicitly a biosimilar medicine by name can it be dispensed in place of another biological product [43]. Still, in the US, interchangeable biosimilars can sometimes be substituted at the pharmacy level in certain states, and the EU has varying rules per country; for example, France allows limited substitution now [43,44]. In 2022, an additional statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU was published by the European Medicines Agency, therefore providing a harmonized position on this matter [45]. These drugs have the potential to reduce drug costs, thus offering lower-cost treatment options for patients.
5. Ethical Challenges
Biopharmaceutical ethics encompasses the moral principles and considerations that guide the research, development, production, and distribution of biopharmaceutical products [46]. Given the significant impact these products have on public health, patient safety, and the broader society, ethical considerations are paramount. Key areas of focus in biopharmaceutical ethics include:
- (1)
- Clinical Trials: Ethical conduct in clinical trials is crucial. This includes obtaining informed consent from participants, ensuring their safety, and maintaining transparency about the risks and benefits of participation [47]. Researchers must also consider the equitable selection of participants to avoid exploitation of vulnerable populations.
- (2)
- Access and Equity: There are ethical concerns regarding access to biopharmaceuticals, particularly in low-income or underserved populations [48]. Important issues of affordability, availability, and distribution must be addressed to ensure that all individuals have access to necessary treatments, regardless of socioeconomic status. The gene therapy pricing would be an excellent example.
- (3)
- Intellectual Property: The balance between protecting intellectual property rights and ensuring public access to essential medications is a significant ethical dilemma [49]. While patents incentivize innovation, they can also lead to high drug prices, limiting access for patients who need them.
- (4)
- Transparency and Honesty: Biopharmaceutical companies have an ethical obligation to provide accurate information about their products, including potential side effects, efficacy, and any conflicts of interest [50]. Transparency in marketing and communication is essential to maintain public trust.
- (5)
- Research Integrity: Ethical research practices are vital to ensure the validity and reliability of scientific findings. This includes avoiding fabrication or falsification of data, as well as addressing any potential biases in research design and reporting.
- (6)
- Post-Market Surveillance: After a biopharmaceutical product is granted and on the market, ongoing monitoring for safety and efficacy is essential [51]. Ethical considerations include the responsibility of companies to report adverse effects and the obligation of regulatory bodies to act on this information to protect public health.
- (7)
- Environmental Impact: The production and disposal of biopharmaceuticals can have environmental implications [52]. Ethical considerations include minimizing waste, reducing carbon footprints, and ensuring sustainable practices in manufacturing processes.
- (8)
- Global Health Considerations: In a globalized world, ethical issues extend beyond national borders. Biopharmaceutical companies must consider the implications of their actions on global health, including the distribution of vaccines and treatments during pandemics or health crises [53].
Addressing these ethical considerations requires collaboration among stakeholders, including researchers, healthcare professionals, policymakers, and the public. Ongoing dialogue and ethical frameworks are essential to navigate the complexities of biopharmaceutical development and ensure that advancements in medicine benefit society as a whole.
6. Biopharmaceutical Industry
The biopharmaceutical industry is a sector of the pharmaceutical industry that centers on the development, production, and commercialization of biopharmaceutical products [54]. A vast array of treatments, including monoclonal antibodies, vaccines, recombinant proteins, and gene therapies, is included in these items, which are usually sourced from biological sources [55]. In order to improve patient care and advance medical science, the industry is indeed essential, as the concept of biopharmaceuticals is based on modern market needs, which are strongly linked to population morbidity statistics and the imperfections of modern pharmacotherapy. The need to search for new and effective drugs, along with the confirmed advantages and disadvantages of biopharmaceuticals, certainly provides new opportunities for further improvements.
Some key aspects of the biopharmaceutical industry include (Table 2):

Table 2.
Key aspects of the biopharmaceutical industry.
- (1)
- Research and Development (R&D): The biopharmaceutical industry is heavily invested in R&D to discover and develop new therapies [56]. This process often involves significant investment in basic research, preclinical studies, and clinical trials to ensure the safety and efficacy of new products. The R&D phase can take many years and requires collaboration among scientists, clinicians, and regulatory bodies.
- (2)
- Regulatory Environment: Biopharmaceuticals are subject to rigorous regulatory oversight to guarantee their safety, efficacy, and quality [57]. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), evaluate clinical trial data and grant approvals for new products. Compliance with Good Manufacturing Practices (GMP) is also essential in the production of biopharmaceuticals.
- (3)
- Manufacturing: Cell culture, fermentation, purification, and formulation are some of the intricate procedures involved in the development of biopharmaceuticals [58]. Manufacturing facilities must follow strict quality control guidelines to guarantee the efficacy and safety of their products. Advances in bioprocessing technologies are continually improving efficiency and scalability in production.
- (4)
- Market Dynamics: The biopharmaceutical industry is characterized by rapid growth and innovation. The rising incidence of chronic illnesses, developments in biotechnology, and the increasing need for individualized medicine are the leading causes of this expansion. The market is also influenced by patent expirations, competition from biosimilars, and pricing pressures [59].
- (5)
- Collaboration and Partnerships: The biopharmaceutical sector frequently depends on partnerships between businesses, educational institutions, and research groups [60]. Partnerships can facilitate access to new technologies, share risks in R&D, and enhance the development of innovative therapies.
- (6)
- Ethical Considerations: As discussed earlier, ethical issues play a significant role in the biopharmaceutical industry. Companies must navigate challenges related to clinical trial ethics, access to medications, intellectual property rights, and transparency in communication [61].
- (7)
- Global Impact: The biopharmaceutical industry has a global reach, with companies operating in various countries and regions [62]. This international presence allows for the sharing of knowledge and resources but also presents challenges related to regulatory differences, market access, and public health considerations.
- (8)
- Future Trends: The industry is evolving with trends such as the rise in gene and cell therapies, advancements in precision medicine, and the incorporation of artificial intelligence with data analytics in drug development. Additionally, the COVID-19 pandemic has accelerated innovation and collaboration within the industry, highlighting the importance of rapid response capabilities in public health emergencies [63].
Overall, the biopharmaceutical industry is a dynamic and essential component of modern healthcare, driving advancements in treatment options and improving patient outcomes.
7. Legislation
Biopharmaceuticals legislation encompasses the laws, regulations, and guidelines that regulate the research, development, manufacturing, approval, and marketing of biopharmaceutical products [64]. This legislation is critical for ensuring the safety, efficacy, and quality of biopharmaceuticals, as well as protecting public health.
These are some crucial aspects of biopharmaceuticals legislation (Figure 2):
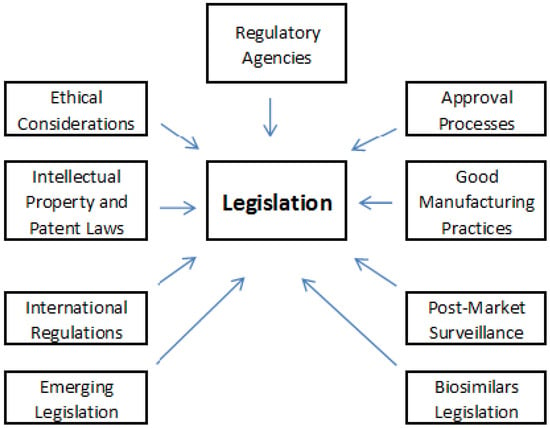
Figure 2.
Key aspects of biopharmaceuticals’ legislation.
- (1)
- Regulatory Agencies
Different countries have regulatory agencies responsible for overseeing biopharmaceuticals [65]. Key agencies include:
- -
- U.S. Food and Drug Administration (FDA): In the United States, the FDA regulates the approval and monitoring of biopharmaceuticals, comprising biologics and biosimilars.
- -
- European Medicines Agency (EMA): In the European Union, the EMA is in charge of supervision, the scientific evaluation, as well as safety monitoring of medicines, including biopharmaceuticals.
- -
- Other National Agencies: Many states have their individual regulatory bodies, such as Health Canada, the Medicines and Healthcare Products Regulatory Agency (MHRA) in the UK, and the Therapeutic Goods Administration (TGA) in Australia.
- (2)
- Approval Processes
Legislation outlines the processes for the approval of biopharmaceuticals [66], which typically include:
- -
- Preclinical Studies: Before human trials, biopharmaceuticals must undergo laboratory and animal testing to assess safety and efficacy.
- -
- Clinical Trials: Biopharmaceuticals must go through multiple phases of clinical trials involving human participants to examine their optimal dosing, safety, and efficacy.
- -
- New Drug Applications (NDAs) or Biologics License Applications (BLAs): After successful clinical trials, companies submit applications to regulatory agencies for approval to market their products.
- (3)
- Good Manufacturing Practices (GMP)
Legislation mandates adherence to Good Manufacturing Practices (GMP) to ensure that biopharmaceuticals are produced consistently and meet quality norms. GMP regulations encompass all aspects of production, including facility design, equipment, personnel training, and quality control [67].
- (4)
- Post-Market Surveillance
Once biopharmaceuticals are on the market, legislation requires ongoing monitoring for safety and efficacy. This includes:
- -
- Pharmacovigilance: The collection and analysis of data on adverse effects and other safety concerns.
- -
- Risk Management Plans: Companies may be required to develop plans to identify, assess, and mitigate risks associated with their products.
- (5)
- Biosimilars Legislation
With the increasing use of biosimilars (biological products highly similar to already granted reference products), specific legislation has been developed to govern their approval and marketing. In the U.S., the Biologics Control Act and the Biologics Price Competition and Innovation Act (BPCIA) outline the regulatory framework for biosimilars [68].
- (6)
- Intellectual Property and Patent Laws
Legislation related to intellectual property rights plays a significant part in the biopharmaceutical industry [69]. Patent laws protect the innovations of biopharmaceutical companies, allowing them to recoup their investments in research and development. However, these laws also raise ethical and accessibility concerns regarding the affordability of medications.
- (7)
- Ethical Considerations
Legislation often includes ethical guidelines for conducting clinical trials, including informed consent, the protection of vulnerable populations, and the ethical treatment of research subjects. Regulatory agencies may require compliance with ethical standards set by organizations such as the Declaration of Helsinki.
- (8)
- International Regulations
As biopharmaceuticals are developed and marketed globally, international regulations and guidelines, such as those from the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) and the World Health Organization (WHO), play a role in harmonizing standards across countries [70,71].
- (9)
- Emerging Legislation
As the biopharmaceutical landscape evolves, new legislation may emerge to address advancements in technology, such as gene therapies, cell therapies, and personalized medicine. Regulatory agencies are continually adapting their frameworks to keep pace with innovation while ensuring patient safety.
In summary, biopharmaceuticals legislation is a complex and dynamic field that has a critical role in ensuring the safety, efficacy, and quality of biopharmaceutical products. It involves collaboration among regulatory agencies, industry stakeholders, and the public to create a framework that supports innovation while protecting public health.
8. Key Considerations
Biopharmaceuticals are often more complex than traditional drugs, which can make their development and manufacturing more challenging. To guarantee their quality, safety, and efficacy, biopharmaceuticals must adhere to strict ethical and regulatory standards. The development and production of biopharmaceuticals can be expensive, leading to higher costs for patients and healthcare systems. Still, biopharmaceutical drugs represent a diverse and rapidly evolving field within medicine, offering innovative treatment options for a broad range of diseases. Their development is indeed driven by the latest advances in biotechnology and a deeper understanding of biological processes. Still, some real-world failures (e.g., immunogenicity leading to treatment discontinuations) and forward-looking debates (e.g., regulatory hurdles for CRISPR-based gene therapies) need new prospects.
Funding
The Fogarty International Center at the US National Institutes of Health (NIH) and the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia supported this article through grants 2R25 TW008171-06A1 and 200110, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Rasmussen, A.S.B.; Hammou, A.; Poulsen, T.F.; Laursen, M.C.; Hansen, S.F. Definition, categorization, and environmental risk assessment of biopharmaceuticals. Sci. Total Environ. 2021, 789, 147884. [Google Scholar] [CrossRef]
- Kesik-Brocka, M. Progress in biopharmaceutical development. Biotechnol. Appl. Biochem. 2018, 65, 306–322. [Google Scholar] [CrossRef]
- Moorkens, E.; Meuwissen, N.; Huys, I.; Declerck, P.; Vulto, A.G.; Simoens, S. The Market of Biopharmaceutical Medicines: A Snapshot of a Diverse Industrial Landscape. Front. Pharmacol. 2017, 8, 314. [Google Scholar] [CrossRef]
- Samanen, J. Similarities and differences in the discovery and use of biopharmaceuticals and small-molecule chemotherapeutics. Introd. Biol. Small Mol. Drug Res. Dev. 2013, 161–203. [Google Scholar] [CrossRef]
- Halpern, W.; Hutto, D. Biopharmaceuticals. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Bolon, B., Haschek, W.M., Ochoa, R., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 751–782. ISBN 978-0-12-415759-0. [Google Scholar] [CrossRef]
- Tang, L.; Persky, A.M.; Hochhaus, G.; Meibohm, B. Pharmacokinetic aspects of biotechnology products. J. Pharm. Sci. 2004, 93, 2184–2204. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Le Heuzey, J.Y.; Mabo, P. Narrow therapeutic index drugs: A clinical pharmacological consideration to flecainide. Eur. J. Clin. Pharmacol. 2015, 71, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Tovey, M.G.; Lallemand, C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Ther. Adv. Drug Saf. 2011, 2, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Bittner, B.; Richter, W.; Schmidt, J. Subcutaneous Administration of Biotherapeutics: An Overview of Current Challenges and Opportunities. BioDrugs 2018, 32, 425–440. [Google Scholar] [CrossRef]
- Harper, A.R.; Topol, E.J. Pharmacogenomics in clinical practice and drug development. Nat. Biotechnol. 2012, 30, 1117–1124. [Google Scholar] [CrossRef]
- Amouzadeh, H.R.; Engwall, M.J.; Vargas, H.M. Safety Pharmacology Evaluation of Biopharmaceuticals. Handb. Exp. Pharmacol. 2015, 229, 385–404. [Google Scholar] [CrossRef]
- Ahmed, T.; Kollipara, S.; Boddu, R.; Bhattiprolu, A.K. Biopharmaceutics Risk Assessment-Connecting Critical Bioavailability Attributes with In Vitro, In Vivo Properties and Physiologically Based Biopharmaceutics Modeling to Enable Generic Regulatory Submissions. AAPS J. 2023, 25, 77. [Google Scholar] [CrossRef]
- Kepplinger, E.E. FDA’s Expedited Approval Mechanisms for New Drug Products. Biotechnol. Law. Rep. 2015, 34, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, N.; Edwards, I.R.; Felix, T.; Graze, P.R.; Litten, J.B.; Strober, B.E.; Warnock, D.G. Pharmacovigilance and biosimilars: Considerations, needs and challenges. Expert. Opin. Biol. Ther. 2013, 13, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Pharmacovigilance: Importance, concepts, and processes. Am. J. Health Syst. Pharm. 2017, 74, 606–612. [Google Scholar] [CrossRef]
- Milá Cáceres, L. Quality Risk Management Application Review in Pharmaceutical and Biopharmaceutical Industries. Bioprocess. J. 2010, 9, 26–37. [Google Scholar] [CrossRef]
- Hock, S.C.; Kian, S.M.; Wah, C.L. Global challenges in the manufacture, regulation and international harmonization of GMP and quality standards for biopharmaceuticals. Generics Biosimilars Initiat. J. 2020, 9, 52–64. [Google Scholar] [CrossRef]
- Wise, J.; Möller, A.; Christie, D.; Kalra, D.; Brodsky, E.; Georgieva, E.; Jones, G.; Smith, I.; Greiffenberg, L.; McCarthy, M.; et al. The positive impacts of Real-World Data on the challenges facing the evolution of biopharma. Drug Discov. Today 2018, 23, 788–801. [Google Scholar] [CrossRef]
- Radenković, M. Importance of decisional capacity tools in obtaining informed consent in clinical settings. Bioethics 2023, 37, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Taraban, M.B.; Wang, W.; Briggs, K.T. Improving Biopharmaceutical Safety through Verification-Based Quality Control. Trends Biotechnol. 2017, 35, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Poduval, P.; Parsekar, S.; Meena, S.N. Small molecules vs biologics. In New Horizons in Natural Compound Research; Meena, S.N., Nandre, V., Kodam, K., Meena, R.S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 179–199. [Google Scholar] [CrossRef]
- Stone CAJr Spiller, B.W.; Smith, S.A. Engineering therapeutic monoclonal antibodies. J. Allergy Clin. Immunol. 2024, 153, 539–548. [Google Scholar] [CrossRef]
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines 2021, 9, 1490. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of Recombinant DNA Technology to Improve Life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Blandizzi, C.; Meroni, P.L.; Lapadula, G. Comparing Originator Biologics and Biosimilars: A Review of the Relevant Issues. Clin. Ther. 2017, 39, 1026–1039. [Google Scholar] [CrossRef]
- Dunbar, C.E.; High, K.A.; Joung, J.K.; Kohn, D.B.; Ozawa, K.; Sadelain, M. Gene therapy comes of age. Science 2018, 359, eaan4672. [Google Scholar] [CrossRef] [PubMed]
- El-Kadiry, A.E.; Rafei, M.; Shammaa, R. Cell Therapy: Types, Regulation, and Clinical Benefits. Front. Med. 2021, 8, 756029. [Google Scholar] [CrossRef] [PubMed]
- Mertens, F.; Johansson, B.; Fioretos, T.; Mitelman, F. The emerging complexity of gene fusions in cancer. Nat. Rev. Cancer 2015, 15, 371–381. [Google Scholar] [CrossRef]
- Yokoyama, W.M.; Christensen, M.; Santos, G.D.; Miller, D.; Ho, J.; Wu, T.; Dziegelewski, M.; Neethling, F.A. Production of monoclonal antibodies. Curr. Protoc. Immunol. 2013, 102, 2–5. [Google Scholar] [CrossRef]
- Rawat, S.; Hussain, M.S.; Mohapatra, C.; Kaur, G. An overview of monoclonal antibodies and their therapeutic applications. Nat. Volatiles Essent. Oils 2021, 8, 4121–4130. [Google Scholar]
- Butler, M.; Meneses-Acosta, A. Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl. Microbiol. Biotechnol. 2012, 96, 885–894. [Google Scholar] [CrossRef]
- Josefsberg, J.O.; Buckland, B. Vaccine process technology. Biotechnol. Bioeng. 2012, 109, 1443–1460. [Google Scholar] [CrossRef]
- Haider, R. Pharmaceutical and Biopharmaceuticals Industries: Revolutionizing Healthcare. Asian J. Nat. Sci. 2023, 2, 83–89. [Google Scholar] [CrossRef]
- Parasuraman, S.; Kumar, L.N.; Thanapakiam, G.; Sayem, A.S.; Chuah, J.J.; Venkateskumar, K. Biopharmaceutical Production by Recombinant DNA Technology: Future Perspectives. In Microbial Products for Health and Nutrition; Kothari, V., Ray, S., Kumar, P., Eds.; Springer Nature: Singapore, 2024; pp. 285–303. ISBN 978-981-97-4234-9. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Therapeutic proteins. Methods Mol. Biol. 2012, 899, 1–26. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development. Front. Bioeng. Biotechnol. 2019, 7, 420. [Google Scholar] [CrossRef]
- Lagassé, H.A.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Sauna, Z.E.; Kimchi-Sarfaty, C. Recent advances in (therapeutic protein) drug development. F1000Res 2017, 6, 113. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.S.; Olech, E.; McClellan, J.E.; Kirchhoff, C.F. Development of biosimilars. Semin. Arthritis Rheum. 2016, 45, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Liu, M.; Wang, H.; Fan, J. Biologics and Biosimilars: Potential Therapeutics for Autoimmune Renal Diseases. In Biologics and Biosimilars; Feng, X., Xie, H.-G., Malhotra, A., Yang, C.F., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 301–328. ISBN 9781032262024. [Google Scholar] [CrossRef]
- Malakar, S.; Gontor, E.N.; Dugbaye, M.Y.; Shah, K.; Sinha, S.; Sutaoney, P.; Chauhan, N.S. Cancer treatment with biosimilar drugs: A review. Cancer Innov. 2024, 3, e115. [Google Scholar] [CrossRef]
- Akram, M.S.; Pery, N.; Butler, L.; Shafiq, M.I.; Batool, N.; Rehman, M.F.U.; Grahame-Dunn, L.G.; Yetisen, A.K. Challenges for biosimilars: Focus on rheumatoid arthritis. Crit. Rev. Biotechnol. 2021, 41, 121–153. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, J.; Barry, S.P.; Bermingham, M.; Morris, J.M.; Griffin, B.T. Regulation of biosimilar medicines and current perspectives on interchangeability and policy. Eur. J. Clin. Pharmacol. 2019, 75, 1–11. [Google Scholar] [CrossRef]
- Afzali, A.; Furtner, D.; Melsheimer, R.; Molloy, P.J. The Automatic Substitution of Biosimilars: Definitions of Interchangeability are not Interchangeable. Adv. Ther. 2021, 38, 2077–2093. [Google Scholar] [CrossRef]
- European Medicines Agency. Statement on the Scientific Rationale Supporting Interchangeability of Biosimilar Medicines in the EU-EMA/627319/2022. Last Updated on 21 April 2023. Available online: https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf (accessed on 23 October 2025).
- Van Campen, L.E.; Poplazarova, T.; Therasse, D.G.; Turik, M. Biopharmaceutical Bioethics Working Group. Considerations for applying bioethics norms to a biopharmaceutical industry setting. BMC Med. Ethics 2021, 22, 31. [Google Scholar] [CrossRef]
- Radenković, M. Informed Consent. The Current Standing. In Heading Towards Humans Again: Bioethics in the New Age of Science; Radenkovic, M., Ed.; Trivent Pub: Budapest, Hungary, 2022; pp. 41–60. ISBN 978-6156405050. [Google Scholar] [CrossRef]
- Lalor, F.; Fitzpatrick, J.; Sage, C.; Byrne, E. Sustainability in the biopharmaceutical industry: Seeking a holistic perspective. Biotechnol. Adv. 2019, 37, 698–707. [Google Scholar] [CrossRef]
- Nidhi, A. The Crossroad between intellectual property and clinical trials: Balancing incentives for innovation with access to healthcare. J. Intellect. Prop. Rights (JIPR) 2023, 28, 284–292. [Google Scholar] [CrossRef]
- Wolitz, R.E. A corporate duty to rescue: Biopharmaceutical companies and access to medications. Indiana Law J. 2019, 94, 1163. [Google Scholar]
- Brennan, F.R.; Baumann, A.; Blaich, G.; de Haan, L.; Fagg, R.; Kiessling, A.; Kronenberg, S.; Locher, M.; Milton, M.; Tibbitts, J.; et al. Nonclinical safety testing of biopharmaceuticals--Addressing current challenges of these novel and emerging therapies. Regul. Toxicol. Pharmacol. 2015, 73, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.V.; McLaughlin, J.M.; Cue, B.W.; Dunn, P.J. Environmental considerations in biologics manufacturing. Green. Chem. 2010, 12, 755–766. [Google Scholar] [CrossRef]
- née Lybecker, K.M.A. Health and biopharmaceutical innovation. In Handbook of Innovation and Intellectual Property Rights; Edward Elgar Publishing: Cheltenham/Camberley, UK, 2024; pp. 396–416. ISBN 9781800880610. [Google Scholar] [CrossRef]
- Tulum, Ö.N. Innovation and Financialization in the U.S. Biopharmaceutical Industry. Faculty of Economics, University of Ljubljana. COBISS.SI-ID–24642790, 2018, 241. Available online: http://www.cek.ef.uni-lj.si/doktor/tulum.pdf (accessed on 23 October 2025).
- Mahler, S. Safety of biologics therapy: Monoclonal antibodies, cytokines, fusion proteins, hormones, enzymes, coagulation proteins, vaccines, botulinum toxins. MAbs 2017, 9, 885–888. [Google Scholar] [CrossRef]
- Carter, P.H.; Berndt, E.R.; DiMasi, J.A.; Trusheim, M. Investigating investment in biopharmaceutical R&D. Nat. Rev. Drug Discov. 2016, 15, 673–674. [Google Scholar] [CrossRef]
- Christiansen, G.D. Quality control and quality assurance issues in biopharmaceutical processing. In Biotechnology; Avis, K.E., Wagner, C.M., Wu, V.L., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 33–71. ISBN 9781003055297. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Shrivastava, A. Scale up of biopharmaceuticals production. In Nanoscale Fabrication, Optimization, Scale-Up and Biological Aspects of Pharmaceutical Nanotechnology; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 133–172. ISBN 9780128136294. [Google Scholar]
- Vandenplas, Y.; Simoens, S.; Van Wilder, P.; Vulto, A.G.; Huys, I. Off-Patent Biological and Biosimilar Medicines in Belgium: A Market Landscape Analysis. Front. Pharmacol. 2021, 12, 644187. [Google Scholar] [CrossRef]
- Powell, W.W. Learning from collaboration: Knowledge and networks in the biotechnology and pharmaceutical industries. Calif. Manag. Rev. 1998, 40, 228–240. [Google Scholar] [CrossRef]
- Gøtzsche, P.C. Why we need easy access to all data from all clinical trials and how to accomplish it. Trials 2011, 12, 249. [Google Scholar] [CrossRef]
- Downs, J.B.; Velamuri, V. Business model innovation opportunities for the biopharmaceutical industry: A systematic review. J. Commer. Biotechnol. 2016, 22, 19–63. [Google Scholar] [CrossRef]
- Geurts, A.; Geerdink, T.; Sprenkeling, M. Accelerated innovation in crises: The role of collaboration in the development of alternative ventilators during the COVID-19 pandemic. Technol. Soc. 2022, 68, 101923. [Google Scholar] [CrossRef]
- Fisher, A.C.; Kamga, M.H.; Agarabi, C.; Brorson, K.; Lee, S.L.; Yoon, S. The Current Scientific and Regulatory Landscape in Advancing Integrated Continuous Biopharmaceutical Manufacturing. Trends Biotechnol. 2019, 37, 253–267. [Google Scholar] [CrossRef]
- Yu, L.X.; Woodcock, J. FDA pharmaceutical quality oversight. Int. J. Pharm. 2015, 491, 2–7. [Google Scholar] [CrossRef]
- Wang, J.; Chow, S.C. On the regulatory approval pathway of biosimilar products. Pharmaceuticals 2012, 5, 353–368. [Google Scholar] [CrossRef]
- Griffiths, E. Quality standards for biopharmaceuticals: The importance of good manufacturing practice. GaBI J. 2020, 9, 97–99. [Google Scholar] [CrossRef]
- Darrow, J.J. Biosimilar approvals and the BPCIA: Too soon to give up. Health Aff. Forefr. 2019. [Google Scholar] [CrossRef]
- Toma, A.; Secundo, G.; Passiante, G. Open innovation and intellectual property strategies: Empirical evidence from a bio-pharmaceutical case study. Bus. Process Manag. J. 2018, 24, 501–516. [Google Scholar] [CrossRef]
- Ojha, A.; Bhargava, S. International council for harmonisation (ICH) guidelines. In Regulatory Affairs in the Pharmaceutical Industry; Javed, A., Sanjula, B., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 47–74. ISBN 978-0-12-822211-9. [Google Scholar] [CrossRef]
- Walsh, G. Biopharmaceutical benchmarks. Nat. Biotechnol. 2000, 18, 831–833. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Published by MDPI on behalf of the Österreichische Pharmazeutische Gesellschaft. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).