- Article
The Preparation of ZnFe2O4 from Coal Gangue for Use as a Photocatalytic Reagent in the Purification of Dye Wastewater via the PMS Reaction
- Mingxian Zhang,
- Jinsong Du and
- Xuemei Zheng
- + 1 author
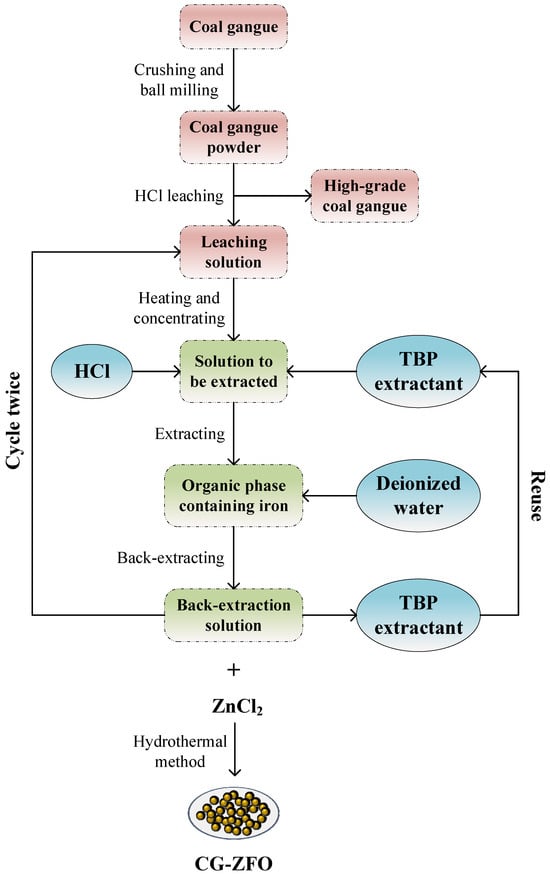
The widespread application of Rhodamine B (RhB) poses a serious threat to the aquatic environment. ZnFe2O4, as a catalyst material, can effectively activate persulfate (PMS) and respond to visible light, thus effectively degrading RhB with the joint assistance of sunlight and PMS. This study recovered Fe2O3 from high-iron coal gangue through an activating–acid leaching–extracting–back-extracting process and synthesized ZnFe2O4 catalysts (CG-ZFO) using coal gangue back-extraction liquid as the Fe source by a hydrothermal method and cetyltrimethylammonium bromide (CTAB)-assisted hydrothermal method. The characterization results of X-ray diffraction (XRD), scanning electron microscopy (SEM), and diffuse reflectance spectroscopy (DRS) showed that the CG-ZFO has a pure crystal phase, and the addition of CTAB can effectively improve the photoelectric performance of the catalyst. The synthesized CG-ZFO can produce a significant synergistic effect with simulated sunlight (SS) and PMS, and the constructed SS/CG-ZFO/PMS system had a good degradation effect on RhB. Based on the conclusions of free radical-quenching experiments, electron paramagnetic resonance (EPR) spectroscopy, and X-ray photoelectron spectroscopy (XPS), the main active species in the SS/CG-ZFO/PMS system was identified as 1O2, and the degradation mechanism of RhB was elucidated. CG-ZFO prepared from coal gangue holds promising potential for application in the remediation of organic dye wastewater, and this study also provides a new approach for the resource regeneration of high-iron coal gangue.
2 January 2026