Abstract
Increased needs arising from efficient utilization of renewable energy sources and the emerging use of portable electronic devices have introduced new requirements and challenges, such as fast charging and discharging, high-speed energy delivery, longer lifetime, and recyclability. To meet these demands, the innovative use of supercapacitors is essential, as they can complement the batteries currently in use. One of the major disadvantages of supercapacitors is that their energy storage capacity (5–20 Wh/kg) is currently insufficient, compared to the capacity of batteries (~1000 Wh/kg). Supercapacitors have higher specific power (10 kW/kg) but lower specific energy density, which is another significant disadvantage compared to batteries. This has prompted researchers around the world to find innovative solutions to enhance the energy density of these materials. Carbon-based nanomaterials are one of the most widely used electrode materials for supercapacitors; therefore, the development of carbon-based nanomaterials plays crucial role in evolution of supercapacitors, due to their high electrical conductivity, large specific surface area, and excellent mechanical strength compared to conventional electrode materials graphite, copper, platinum, etc. Significant results have been reported in the scientific literature on novel carbon-based nanostructured materials such as carbon nanotubes, vertically aligned carbon nanotubes, graphene, activated carbon, or carbon nanoballs, which have a hierarchical pore structure, as well as hybrid systems combining these materials and the introduction of alternative electrolytes. This manuscript reviews briefly the background and fundamental characteristics of supercapacitors, classifying them. It also mentions the general electrochemical measurement methods used to evaluate the energy storage properties of supercapacitors, with emphasis on their specific characteristics and limitations. The integral components of supercapacitors, especially electrode materials, are considered to have considerable impact on the performance of supercapacitor devices (e.g., long life cycle, storage capacity, and high power density).
1. Introduction
As technology and economy evolve, the global demand for energy keeps growing. Efficient energy storage and conversion are the foundation of today’s society, driven by the mutual promotion of environmental awareness and economic development. Supplies of fossil fuel are limited, and their use causes significant environmental pollution [1,2,3,4]. Therefore, considerable attention has been given to the applicability and integration of renewable solar and wind energy sources into everyday life. Energy utilization and transformation are essential for renewable energy sources, given their global prevalence. Due to low energy density, batteries are not suitable in the long term for reducing the imbalance between renewable energy production and efficient energy storage [5]. The aim of research and development is to lower and step-by-step exclude environmental and economic disadvantages associated with the use of traditional energy sources. The energy generated by renewable energy sources is often unstable, and its direct application faces various obstacles, including solar panels, can only convert sunlight into electricity when sunlight is available. The mechanism of energy storage is not well developed, particularly for seasonal energy (wind and tidal) facing the same challenges. Certain renewable energies are capable of generating electricity continuously; however, these power plants are site-limited due to their requirements. Renewable energy sources are not aligned with peak electricity consumption periods, leading to major energy losses in our daily lives [5]. Thus, energy storage devices are essential for the efficient use of these energies. The technology of energy storage is crucial in managing the variable nature of renewable energies and to satisfy the energy demands of emerging electronic devices [6]. Battery-, fuel cell-, and electrochemical capacitor (also known as supercapacitor (SC))-based electrochemical energy has a key role in meeting today’s energy demands efficiently. While these devices have similar electrochemical properties, the mechanisms of energy storage and conversion differ [7,8,9]. Nowadays, SCs have attracted a great deal of interest owing to their unique characteristics, such as high specific capacity, fast charging and discharging capability, and long lifetime [10]. SCs are capable of delivering high specific power (10,000 W/kg) and short-duration (between seconds and minutes) high current pulses [11]. Their outstanding lifetime, which often extends beyond thousands or even millions of charge and discharge cycles, sets them apart from traditional batteries (Table 1). Such exceptional robustness results due to the electrostatic character of energy storage in supercapacitors, which minimizes quality degradation through multiple cycles [12]. SCs can operate by standing alone or integrated with other storage systems such as batteries and can be used in a variety of applications including industrial power management, solar energy production, hybrid electric vehicles, and consumer electronics [13,14]. In addition, their recyclability and longer lifetime meet the demands of environmental sustainability.
Despite many advantages of SCs, the principal weakness of SCs is their relatively low energy density (5–20 Wh/kg), which is approximately 20–40 times lower than the conventional rechargeable Li-ion batteries (100–265 Wh/kg) [14,15].

Table 1.
Comparison of properties of commercial supercapacitors and lithium-ion batteries.
Table 1.
Comparison of properties of commercial supercapacitors and lithium-ion batteries.
| Parameters | Electric Double-Layer Supercapacitor | Lithium-Ion Battery | Refs. |
|---|---|---|---|
| Charge capacity | 1–5000 F/3.04 Wh | 1000–100,000 F/12.1 Wh | [16,17] |
| Energy density [Wh/kg] | 1–20 | 20–300 | [14,15,16,17] |
| Power density [W/kg] | 2000–10,000 | 50–200 | [11,16] |
| Maximum power [W] | 7020 | 18 | [16] |
| Charge time [s] | 1–60 | 3600–18,000 | [16] |
| Self-discharge | 5–60% per two weeks | <4% per month | [18,19] |
| Nominal voltage [V] | 2.7–3.0 | 3.6 | [16,17] |
| Cycle life (charge–discharge cycles) | 500,000–1,000,000 | 250–1000 | [20,21] |
| Temperature range [°C] | −40 to 70 | −20 to 60 | [22,23] |
| Price per kWh [US-$] | 5000–10,000 | 100–1000 | [16,24] |
Hence, extensive research has aimed to improve the energy storage capacity of SCs while maintaining their high power output capability. The challenge is to design versatile and improved energy storage systems for a wide range of applications. To achieve this aim, researchers focus on improving energy density, with particular emphasis on electrode material and structure innovation [25,26,27]. Recent studies have focused on a particular group of materials, offering an overview of recent developments in the state of the art, the most characteristic physicochemical properties, supercapacitive performance, and methods of fabrication of such materials [28,29,30].
2. Supercapacitors—A Brief History
The concept of electrical charge storage on surfaces can be traced back to the days of ancient Greece, where the concept was inspired by the friction properties of amber [31]. The molecular understanding of electricity can be traced back to the 19th century, to the early studies on electrons by Michael Faraday and later by J. J. Thomson and Millikan [32]. The Leyden jar, invented by Pieter van Musschenbroek, marked a crucial step forward, introducing the principles of charge separation and storage, which was originally known as a “condenser” and later as a “capacitor” [33].
Helmholtz introduced the concept of electric double layer (EDL) in the 19th century (Figure 1). The Helmholtz model is based on the idea of particles with opposite charges forming layers at the interface between the electrode and the electrolyte, which are separated by atomic distances, just like in the case of regular capacitors. This was later improved by Gouy and Chapman (Figure 1), who introduced the concept of a diffuse layer, in which the ions surrounding the charged surface are not strongly bound to it but instead create a diffuse interface. The kinetic energy of the ions in the electrolyte partially affects the thickness of this diffuse interface [34,35].
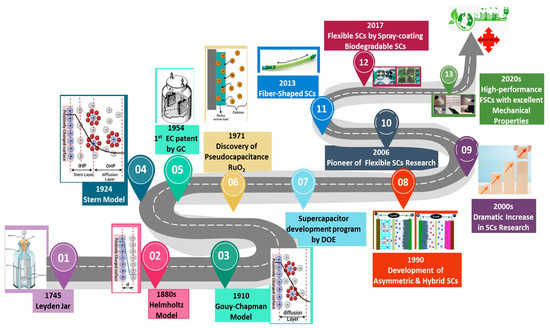
Figure 1.
Development timeline of supercapacitors [35].
Based on the earlier concepts, Stern presented a model in which the particles were divided into inner region (compact or Stern layer) and diffuse layer (Figure 1). Based on this theory, the size of ions is limited, restricting their access to the surface. The Stern layer is formed by ions adsorbed onto the surface, which form two planes: one consisting of specifically adsorbed ions (the inner Helmholtz plane, or IHP) and the other consisting of non-specifically adsorbed counterions (the outer Helmholtz plane, or OHP). This diffusion layer is defined by the Gouy–Chapman model where the diffusion layer is formed by the kinetic energy of the counterions and depends on the thickness of the layer [32,36,37]. The first patent for storing charges in EDL was issued in 1957 [38]. The same patent detailed the formation of the double layer of charges at the interface of the electrolyte and the solid material. During the 1960s, Standard Oil Company (Cleveland, OH, USA) committed itself to the development of this technology and made considerable progress; however, the technology’s subsequent development was halted due to a lack of sales. The Nippon Electric Company (NEC) in Tokyo, Japan, eventually acquired the technology in the 1970s [39]. The company began manufacturing low-power devices under the brand name “Supercapacitor” for memory storage [40]. Subsequently, this technology was adopted by other manufacturers, who launched their own branded versions, such as the “Panasonic Gold Capacitor” developed by the Matsushita Electric Industrial Company (Kadoma, Japan) in 1978 and “Dynacap” by ELNA Co. Ltd. (Yokohama, Japan) in 1987 [41,42]. The extensive fundamental work on ruthenium(IV) oxide (RuO2) in 1971 created a new class of electrochemical capacitors called pseudocapacitors. The newly discovered pseudocapacitance has enabled these devices to store greater amounts of charge [43]. In 1982, the Pinnacle Research Institute (Fort Lauderdale, FL, USA) developed the very first high-performance double-layer capacitor, which was marketed under the brand name “Ultracapacitor”. The US Department of Energy later pursued the development of hybrid electric vehicles using this technology, and in 1992, Maxwell Laboratories Inc. (San Diego, CA, USA) took over the development and began manufacturing a wide range of supercapacitors, including asymmetric supercapacitors, electric double-layer capacitors (EDLCs) and pseudocapacitors [44,45,46]. Nowadays, an increasing number of companies are involved in the manufacture of supercapacitors (Table 2), including Nippon Chemi-con Corporation (Tokyo, Japan), KEMET Electronics Corporation, a subsidiary of Yageo Corporation (Fort Lauderdale, FL, USA), CAP-XX Ltd. (Sydney, Australia), Ness Capacitor Co. Ltd. (Yongin-si, Republic of Korea), etc.

Table 2.
Commercially available SCs (original data from ref. [47] (Open Access) and updated based on available information on the website of each company on 28 October 2025).
The research in this field is constantly expanding (Figure 2) as scientists focus on advancing knowledge, bringing together the latest findings, and improving current understanding [40,48,49,50,51,52,53,54] towards a more sustainable future, evidenced by real-world applications such as supercapacitor-assisted buses for public transportation in urban areas of South Korea and China [55,56,57,58,59,60].
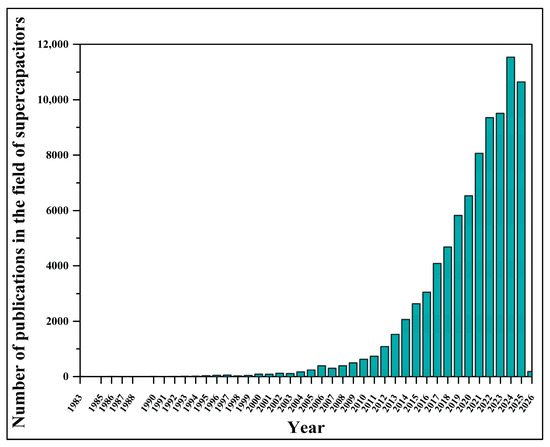
Figure 2.
Timeline of publications in the field of supercapacitors from the beginning to the present day, based on Science Direct (https://www.sciencedirect.com, accessed on 24 August 2025).
3. Principles of Supercapacitor Technology
This chapter reviews supercapacitors in more detail, discussing their classification and specifications. The operating principles of supercapacitors are basically like electrostatic capacitors.
3.1. Characteristics and Principles of Supercapacitors
SCs integrate the strengths of batteries and conventional capacitors using the principles of electrostatics to regulate the efficient storage and release of energy [61]. Supercapacitors provide greater power density than batteries and greater energy density than conventional capacitors [62]. Supercapacitors (Figure 3) consist of an electrolyte, a separator, and two electrodes (anode and cathode). The electrolyte and electrodes generate an electric field which stores energy. Typically, the anode and cathode are porous materials separated by ion-permeable membranes [63]. In hybrid and asymmetric cells, the electrodes may differ from each other, whereas they are identical in symmetric cells. The main operating principle of supercapacitors is as follows [64]: when SC connects to the power supply during the charging process, the electrons are forced to migrate from the positive electrode to the negative electrode through this external circuit. This migration creates a potential difference across the SC; consequently, anions move toward the positive electrode and cations move toward the negative electrode in the electrolyte. To compensate for the imbalance of the external charge, electrostatic double layers are formed, in which energy is stored at the interfaces between the electrolyte and the electrodes. During the charging process, the voltage usually linearly rises across the supercapacitor, until it reaches its maximum nominal value. During discharge, the reverse of the process described above occurs when a device is connected to the SC: electrons migrate to the positive electrode through the external circuit, thereby transferring the energy stored in the SC to the connected device. Several multicharged ions can indeed dissociate, but in most cases, neutral molecules are dissociated in the electrolyte. The electrodes are essentially neutral, since the anions and cations migrate from the negative and positive electrodes, respectively. The voltage of the supercapacitor drops during discharge, typically in a linear sequence, until the energy is used up.
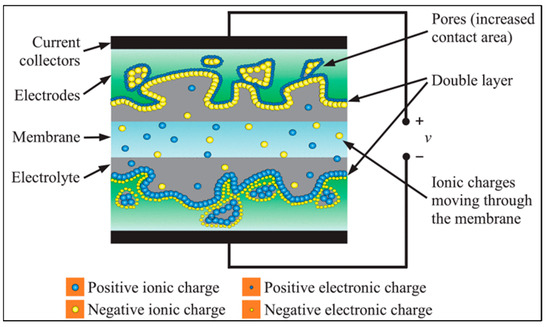
Figure 3.
Scheme of a supercapacitor cell [65].
The comparison of different energy storage devices can be visualized with the Ragone diagram [56], which enables comparison by plotting power density (W/kg) on the y-axis and energy density (Wh/kg) on the x-axis (Figure 4).
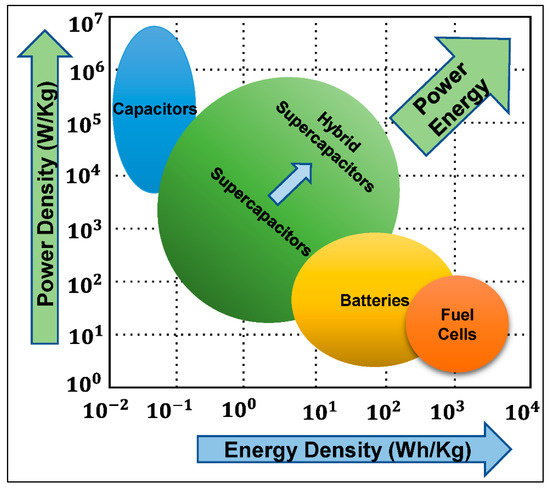
Figure 4.
Ragone diagram: visual comparison of the power–energy density distribution of energy storage devices [66].
As can be seen, traditional capacitors have the lowest capacity to store energy, yet they can discharge extremely fast, providing the highest power density. Momentary power output of batteries is limited, yet their ability to store considerable quantities of energy in a small volume is remarkable. Fuel cells can provide more energy density but require a series of chemical reactions to release energy. Supercapacitors fill the gap by bridging the divide between traditional capacitors, while offering an exceptional power density. Different subcategories of SCs exhibit further diversity in the energy–power space, as shown in Figure 4. Other relevant parameters must also be considered to achieve a complete understanding of advantages and limitations of these energy storage technologies besides the Ragone plot, including cycle lifetime and degradation, costs and resource extraction, toxicity, safety, etc. [35,54,67,68,69,70,71,72]. SCs offer considerable advantages in terms of lifetime, as the charge-storage mechanism allows them to operate between 100,000 and 1 million cycles. Charges are stored physically on the surface of the electrodes and do not involve irreversible chemical reactions, which is why SCs can greatly outperform batteries in terms of cycle life [73]. The lifespan of batteries is finite as swelling occurs in the active material during charge and discharge periods as a result of characteristic redox reactions. The volume of most SC electrodes does not change as their electrostatic storage is largely reversible. In addition, the mechanism of charge storage generates less heat than conventional rechargeable batteries, enabling safer and more robust operation [67].
3.2. Classification of Supercapacitors
Supercapacitors are classified by their manufacturing methods and structural design. They can have different shapes, like cylindrical, flat, or rectangular casings [74]. SCs can be classified into three major groups based on their energy storage mechanism: electric double-layer capacitors (EDLCs), pseudocapacitors (PCs), and hybrid capacitors. EDLCs and PCs differ from each other in terms of energy storage mechanism. In EDLCs, the double layer contains anions and cations which are accumulated at the interface between the electrodes and the electrolyte and electrostatically stored. The active electrode materials used in EDLCs are nanoporous materials with high specific surface area of more than 1000 m2/g. Such nanoporous materials are mainly carbon-based, as they are cheap, readily available, and easy to manufacture [75]. Carbon-based materials the most frequently used in EDLCs are carbon nanotubes (CNTs), activated carbon (AC), graphene, carbon aerogels, carbon foams, etc. [48,76,77]. EDLCs can store large number of charges, due to the high surface area of the electrode, resulting in higher capacitance values. The surface density depends upon the applied voltage; hence, the electrode capacitance changes with the electrode potential. The sole electrochemical reaction involves adsorption and desorption of ions on the electrode which makes this mechanism inherently fast energy storage [78]. The charge storage of PCs is based on faradaic process that involves the transfer of charge carriers electrostatically [79] by rapid redox reactions on or near the electrode surface when voltage is applied. The electrochemical behavior of PCs is similar to EDLCs, involves electron transfer, and changes the oxidation state of the electrode, which results in significantly higher specific capacitance than EDLCs. Three major types of pseudocapacitance can be classified as follows [80]: (i) redox pseudocapacitance, typically involving the electrochemical adsorption of ions near the electrode surface with faradaic charge transfer and occurring mainly in aqueous electrolytes, for example manganese(IV) dioxide (MnO2) and RuO2 [81]; (ii) intercalation pseudocapacitance, which happens by the intercalation of ions into the layers of redox-active materials together with faradaic charge transfer without the occurrence of a crystalline phase transition, similar to the intercalation of ions in the electrode of Li-ion batteries for which usually involves a phase transition [80] (including niobium pentoxide (Nb2O5) [82]; and (iii) adsorption pseudocapacitance, determined by single-layer adsorption, like platina electrode [83]. The rate of reaction, determined by surface coverage, intercalation, and surface redox, is nearly linearly dependent on potential (V) for all types [84,85]. The intercalation pseudocapacitance is one of the most promising types, which stores charge in the electrode material through rapid intercalation and deintercalation of ions, thereby overcoming the gap between conventional batteries and supercapacitors [86,87]. Conducting polymers and transition metal oxides can also be applied as electrode materials, combining the pseudocapacitive and electrostatic charge-storage mechanisms and enhancing energy density and the performance of SCs [54,88,89,90,91,92]. Conductive polymer-based SCs provide high capacitance and low internal resistance at lower production cost than carbon-based EDLCs [93]. One of their disadvantages is that they maintain a lower power density and shorter life cycle because of their poor mechanical stability as the electrodes swelling and shrinking during the redox reactions [40,84,94]. Hybrid capacitors integrate the energy storage capacity of PCs or battery-like electrodes and the energy delivery capacity of EDLC electrodes in a single cell. Hybrid capacitors combine both polarizable (e.g., carbon-based) and non-polarizable (e.g., metal or conductive polymer) electrodes. They can maintain high energy storage capacity through a combination of Faradaic and non-Faradaic processes derived from each electrode [95,96,97,98]. The result of combining both electrode types leads to the suppression of the limiting qualities of each electrode, thereby enabling the use of higher working potentials and exhibiting a higher specific capacity (generally two to three times higher) compared to EDLCs and pseudocapacitors. This enables the development of low-cost SCs with high electrical conductivity, high mechanical robustness, and high chemical resistance. There are three additional classes of hybrid capacitors: asymmetric and battery type and composite hybrid SCs [96,98,99].
3.3. Characteristics of Supercapacitors
The three fundamental parameters required for effective characterization of the energy storage capacity and performance of SCs: total capacity (CT), equivalent series resistance (RES), and operating voltage (VO). Additional factors are also crucial in scientific research for the development of new electrode materials and innovative cell designs, including energy and power density, stable lifetime, operating voltage range, etc. To characterize the supercapacitive behavior of SCs, typically three techniques are used as follows: cyclic voltammetry, galvanostatic charge–discharge, and electrochemical impedance spectroscopy. The primary purpose of these techniques is to evaluate the electrochemical characteristics of energy storage systems from different perspectives. In general, parameters are measured as follows: current, time, and voltage. The other parameters can be calculated using these data [54].
Cyclic voltammetry (CV) can analyze the electrochemical behavior of electrode materials. If the used voltage is greater than the estimated voltage, the current can be checked with the Nernst equation [100]. During CV analysis, electrochemical behavior occurs during both scans (forward and backward), which can be distinguished from pseudocapacitive behavior based on the observed peaks and the general profile of the EDLC behavior of the electrode material [101]. The CV technique examines the electrochemical behavior of the electrode material in a three-electrode configuration with reference and counter electrodes. The main limitation of CV is that it overlooks thermodynamic aspects and only considers the kinetic properties of the material [102,103].
The galvanostatic charge and discharge (GCD) technique analyzes the changes in potential over time. During the measurement, the electrode is charged and discharged at a specific point in time according to the properties of the material type (electrical activity, specific surface area, etc.) [104,105]. In particular, energy balance, power density, specific capacity, and stability are calculated from data on the potential window and charge and discharge times. The general shape of the charge–discharge diagram (Figure 5) shows the behavior differences of EDLC and pseudocapacitance based on the shape of the shoulders induced by Faraday currents [106,107].
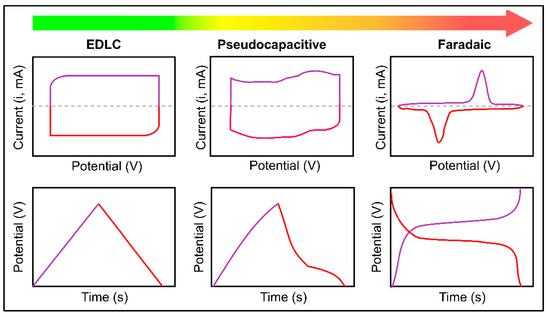
Figure 5.
Schematic illustration of cyclic voltammograms and corresponding charge–discharge curves of EDLC, pseudocapacitor, and Faradaic materials [107] (Open Access).
Electrochemical impedance spectroscopy (EIS) is used to validate the parameters relevant for SCs. The data gained from EIS analysis is processed using appropriate software, such as ZView® (Scribner Associates, Southern Pines, NC, USA), to determine the equivalent circuit required for analysis. The equivalent circuit features illustrate the overall properties of SCs, including equivalent series resistance, non-ideal behavior, and charge transfer capacitance [108].
Due to the different mechanisms of these measuring techniques, the results may seem contradictory; hence, in addition to the results, it is vital to indicate which parameters were used [54,109,110,111,112,113].
4. Carbon-Based Electrode Materials for Supercapacitors
The functional characteristics of SCs depend on the choice of materials used as electrolyte, electrode, separator, and collector. Nearly all characteristics of the structure and charge-storage mechanism of supercapacitors rely on these materials. Numerous studies have been conducted to enhance energy density, power density, lifetime, and voltage properties.
The charging and storage capabilities of SCs are strongly dependent on the used electrode material; therefore, the development of SCs focuses on novel, enhanced-performance electrode materials. Ideally, electrodes should have excellent electrical conductivity, adequate electrochemical and thermal resistance, a high surface area for electrochemical activity, and good wettability by the electrolyte. Recyclability and cost-effectiveness are also important considerations [28,37,114,115]. The electrochemical performance of electrode materials depends on various parameters, including morphology, pore structure, and specific surface area. Carbon-based materials (Figure 6) have been long exploited as electrodes in energy storage, for example as electrically conductive additives, active material supports, electron transfer catalysts, intercalation supports, current collector substrates, and tools for controlling heat transfer, porosity, surface area, and capacitance [51,116,117].
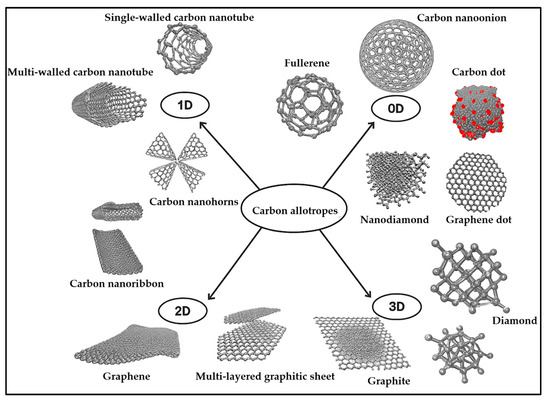
Figure 6.
Schematic illustration of carbon allotropes; reproduced ref. [117] (Open Access).
The advantages of carbon as a supercapacitor electrode material stem from its unique combination of chemical and physical properties, in particular, high conductivity, large surface area (∼1 to >2000 m2/g), high resistance to corrosion, temperature stability, controlled pore structure, fabricability and compatibility in composite materials, and comparatively modest cost [13]. Thus, in the following, carbon-based electrodes will be presented, with an emphasis on carbon nanotube-based electrodes, a comparison of the specific capacity, energy and power density, retention, and cycle life properties of these materials is shown at the end of Section 4 (Table 3).
Activated carbon (AC) is a promising electrode material in energy storage systems. ACs are synthesized using both chemical (low temperature, in the presence of potassium hydroxide (KOH)) and physical (high temperature, in atmospheres of carbon dioxide) methods [118,119,120]. Activated carbon is usually made from carbon-rich materials [121]. The two most important sources of AC production are agricultural waste or biomass rich in carbon and lignocellulose materials. The raw materials used to produce commercially available activated carbon, such as petroleum residues, lignite, coal, and peat, are not cost-effective and are available in limited quantities [122]. The production of low-cost activated carbon from agricultural by-products such as grains, corn cobs, hemp hurd sticks, almond shells, and bamboo has been reported [122,123,124,125]. AC electrodes offer optimal performance in both aqueous and organic electrolytes, as these organic materials form small pores that are not involved in the electrochemical mechanism [126,127].
Carbon nano-onions (CNOs) are carbon skins with a spherical morphology, discovered by accident in transmission electron microscopy (TEM) image in 1980 by Iijima during his research on carbon black [128]. The sp2 network structure of CNOs allows rapid charge transfer and has been subsequently used as EDLC electrode material in the manufacture of SCs [129]. It has been found that, compared to other EDLC materials, there is no temperature limit to their energy storage performance, enabling their application in a broader temperature range [130,131]. CNOs have been produced by various synthesis methods, including annealing and arc discharge, that enabled industrial-scale production [132,133]. They are extensively applied in industry due to their low synthesis costs [134,135]. The energy density of CNOs can be up to 10 Wh/kg as a result of activation, and the specific capacity of CNOs is effectively increased. The activation of CNOs, on the other hand, might cause quality loss in these materials. Using CNOs as anodes and other pseudocapacitive materials as cathodes in asymmetric SCs can ultimately yield high-power, high-energy-density, and efficient energy storage solutions [129,136,137].
Graphene is a honeycomb-like single-layer structure of sp2-bonded carbon atoms. The structure of graphene consists of carbon atoms connected by covalent bonds to three neighboring carbon atoms and has unique electrical and thermal properties due to its single free electron [138]. As a 2D material, graphene has recently attracted considerable attention for use as a single-atom-thick electrode material in supercapacitor systems. Graphene-based electrode materials exhibit high specific surface area, leading to high electrical conductivity properties, chemical resistance, long cycle life, a narrow diffusion distance due to their thin structure, and great accessibility to functional groups. Based on these considerations, the extensive research and application of graphene as an integral part of developing energy storage devices is fully validated [139,140].
Carbon nanotubes (CNTs) with a 1D structure, formed by the hybridization of sp2 carbon atoms, were discovered by Iijima during the TEM characterization of fullerenes synthesized via arc discharge technique in 1991 [141]. From the perspective of physical and chemical properties, CNTs have outstanding tensile strength, good elasticity, high electrical conductivity (106–107 S/m), high thermal conductivity (~2800–6000 W/m × K), and a variety of other advanced features [142,143,144,145,146,147,148,149,150]. These excellent properties have enabled the widespread use of CNTs in many scientific fields and practical applications, including supercapacitor energy storage systems. Based on the number of wall layers, CNTs can be classified into four categories: single-walled carbon nanotubes (SWCNTs), double-walled carbon nanotubes (DWCNTs), multi-walled carbon nanotubes (MWCNTs), and a special subtype of vertically aligned carbon nanotubes (VACNTs or CNT forests) [151,152,153]. Theoretically, CNTs can have relatively high specific surface area (1100–1500 m2/g) and high conductive properties, that make them promising candidates for energy storage devices. In a new study, CNT forests were grown on copper foil using the plasma-enhanced chemical vapor deposition technique [154]. Electrochemical tests confirmed (Figure 7) that the gravimetric specific capacitance was 8 F/g and the areal specific capacitance was 3.5 mF/cm−2 at a scan rate of 5 mV/s. The electrode fabricated from these CNT forests demonstrated outstanding rate capability and retained 92% of its initial capacity after 3000 cycles.
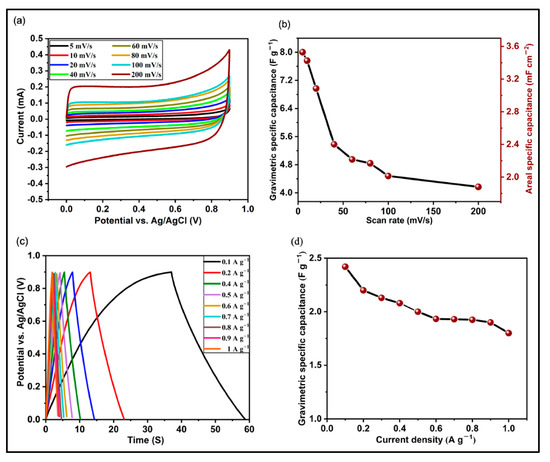
Figure 7.
(a) CV curves of VACNT-based electrodes with different scan rates, (b) specific capacitance as a function of scan rate, (c) GCD curves at different current densities, and (d) specific capacitance as a function of current density [154] (Open Access).
However, CNTs naturally aggregate easily due to Van der Waals interactions and weak dispersion capabilities, resulting a dramatic decrease in specific surface area (up to 50 m2/g), and eventually electrochemical performance. To solve these problems, CNT production methods and chemical functionalization/modification have been developed [155]. In the beginning, CNTs were manufactured using arc discharge. This method was well known and was used to synthesize carbon fibers and carbon filaments. Later, other techniques were tried to synthesize CNTs: laser ablation or chemical vapor deposition (CVD). In fact, these three are the main synthesis techniques for CNTs. Some attempts have been made to prepare carbon nanotubes using other methods, but these have not been promising alternatives. This may be due to the expensive reaction equipment, the price of used materials, or the extreme reaction environment, including high pressure or liquid nitrogen temperature [156]. Many studies have attempted to enhance the quality and the quantity of CNTs by optimizing the synthesis process. As a result, some types of CVD method were discovered as follows: microwave-enhanced, plasma-enhanced, radiofrequency-enhanced CVD, etc. [157]. Unlike conventional CNTs, vertically aligned carbon nanotubes can only be prepared efficiently using catalyst-supported chemical vapor deposition (CCVD) technology [158,159]. VACNT structures can be grown on different substrates, mainly silicon; however, there are many efforts to synthetize them on conductive metal substrates, including titanium (Ti), aluminum (Al), and stainless steel (SS) [160,161,162,163,164,165].
For integrated application in SCs, it is preferred for VACNTs to grow directly on conductive substrates, particularly metal substrates. In this way, the manufactured electrode materials can improve electrical performance by facilitating enhanced electrical contact between VACNTs and metallic current collectors and simplifying the electrode assembly process by eliminating the need for conductive binders and dopants in the electrode materials [166]. The synthesis of VACNTs includes catalytic decomposition of carbon compounds, mainly hydrocarbons, at elevated temperatures using metal catalyst nanoparticles supported on an oxide buffer layer, which are deposited on the substrate surface. Previous studies have revealed that the metal catalysts, buffer layer, and substrate have great impact on the growth of the VACNTs [167,168,169,170,171,172]. Transition metals, including iron, cobalt, and nickel, are extensively used as catalysts in the CVD method. Catalyst layers can be deposited in the form of single-component, bi-component or multi-component layer on the substrate by various thin layer deposition techniques, including physical vapor deposition, magnetron sputtering, atomic layer deposition, dip coating, thermal evaporation, pulsed laser deposition, etc. [173,174,175,176]. The properties of the synthesized VACNTs often do not meet the desired requirements, therefore, depending on the intended application and the required properties, different functionalization of VACNTs are possible: uniformly along the entire length of the nanotubes, or selectively at the CNT tips and CNT sidewalls [177,178]. Doping CNTs with heteroatoms like nitrogen, boron, and phosphorus is an effective approach to improve the specific capacitance of CNTs. Heteroatoms on the surface of CNTs can result an increase in conductivity and thus improve the specific capacitance of the structure, for example nitrogen-doped CNTs have approximately twice the specific capacity of pure CNTs [179,180,181,182].
CNTs and other carbon-based nanomaterials have been combined with transition metal oxides to form composite materials. It is generally believed that the real nanoelectrodes for supercapacitors primarily refer to electroactive materials consisting of various independent nano-arrays grown on conductive substrates. To produce supercapacitor nanoelectrodes, the most used method is to directly manufacture electroactive materials in either one-dimensional (1D) nanomatrices (nanowires, nanorods, and nanotubes) or two-dimensional (2D) nanomatrices (nanosheets and nanowalls) or three-dimensional (3D) nanoporous architectures (Figure 8) [183,184,185,186,187]. Thus, the production of VACNTs on conductive substrates is a promising area.
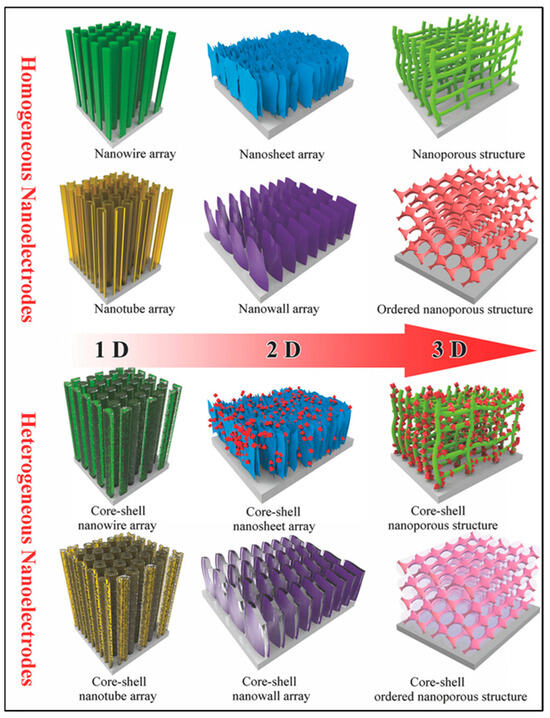
Figure 8.
Scheme of homogeneous and heterogeneous nanoelectrodes used for SCs [184].
The development of 1D, 2D, and 3D carbon-based nanostructures as electrode materials, including CNO and CNT-based arrays and graphene-based architectures, can create hierarchical porous channels due to structural interconnections. These structures also exhibit higher electrical conductivity and better structural mechanical stability, which can improve the performance metrics of supercapacitors, such as higher energy density, power density, and longer lifespan. These materials can improve the transport of ions and electrons, while the porous structure provides a large surface area for charge storage and allows for the deposition of other high-performance pseudoactive materials, such as metal oxides or conductive polymers. This can enable the development of hybrid electrodes that bridge the disadvantages of carbon-based nanostructures and pseudoactive materials and synchronize their advantageous properties [188,189,190].
For the assembly of nanocomposites based on carbon nanotubes and transition metal oxides, various methods have been used, including atomic layer deposition, which is a time- and cost-consuming technology [191]. Recently, other methods, including hydrothermal, electrochemical deposition, anodization, CVD, electrospinning, etc., techniques, have been used for the synthesis of these nanocomposites [183,192,193,194,195]. There are several types of hydrothermal processes based on different mechanisms, including solvothermal, autogenous, molten salt, and microwave-assisted [196,197,198,199]. Each of these techniques is suitable for the fabrication of nanocomposites.
MWCNT electrodes coated with nickel(II) oxide (NiO)–MnO2 are obtained by a straightforward chemical precipitation process with a defined capacity of 193.5 F/g (at a scan rate of 5 mV/s) in 6 mol/dm3 KOH electrolyte. The observed GCD curves exhibited symmetrical triangles, suggesting superior EDLC behavior and reversible charging/discharging performances [200]. Gold coatings were deposited on VACNTs using a direct current (DC) magnetron sputtering method, and the porous gold (Au) deposit was anchored to VACNTs with a large apparent surface area. The porous Au/VACNT nanoelectrodes have good surface capacitance compared to most modern gold-based electrodes (25.6 mF/cm2 at 5 mV/s) and excellent cycle stability (90% retention after 10,000 cycles) in 0.5 mol/dm3 sulfuric acid (H2SO4) aqueous solution [201]. Poly(3-methylthiophen) (P3MT) was deposited via potentiostatic pulse deposition on VACNTs grown directly on aluminum foil. VACNTs with high density (2.1011 VACNT/cm2) and anisotropic properties had a large specific surface area (340 m2/g) and served as template electrodes for the formation of the P3MT coating nanostructure. The deposited P3MT layer was up to 70% deposited inside the mat on the VACNTs, resulting in a specific capacity of 170 F/g. The calculated areal capacity was 380 mF/cm2, and the volumetric capacity was 76 F/cm2. In addition, the nanostructured electrodes showed great stability and capacity retention after 19,000 cycles. Asymmetric electrochemical capacitors were finally assembled, with a specific energy of 52 Wh/kg (14 Wh/L) and a specific power of 10 kW/kg [202]. A composite electrode with homogeneous vanadium(V) oxide (V2O5) nanoparticles were prepared via supercritical carbon dioxide (CO2) impregnation followed by heating and used as a binder-free negative electrode in aqueous asymmetric supercapacitors. The V2O5/VACNT composite electrode exhibited ideal specific capacity (284 F/g) in the potential range between −1.1 and 0 V at 2 A/g compared to saturated calomel electrode, as well as outstanding cycle stability in aqueous sodium sulfate (Na2SO4) solution. Asymmetric supercapacitors provided a high energy density of 32.3 Wh/kg at a power density of 118 W/kg and exhibited satisfactory cycle life with 76% capacity retention after 5000 cycles [203]. CNT composite nanoelectrodes decorated with Fe3O4 nanoparticles were prepared using a microwave solvothermal method. The prepared Fe3O4/CNT composite exhibited a reversible capacity of 187.1 F/g at 1 A/g, superior rate capability by maintaining 61.6% of 10 A/g (vs. 1 A/g), and 80.2% cycle stability after 1000 cycles at 1 A/g [204]. The performance and cycle stability of these composite electrodes can be significantly improved through increased conductivity [205,206].
Among the carbon-based electrode materials for SCs, porous carbon materials with a 3D structure also offer excellent electrochemical performance as cathode materials [185,186,187]. These 3D structured materials are transformed into complex polymer superstructures with characteristic flower-like morphology and structure through the controlled growth of oligomers. The resulting hierarchical porous carbon superstructures exhibited a significant surface area (2824 m2/g) providing alternative and efficient charging routes. It is noteworthy that in the hybrid system of these carbon superstructures with zinc ions, the synergistic combination of dual ion storage, ion-accessible pore structures, and endogenous zincophilic sites led to a superior performance, which included a remarkable energy density of 112.1 W/kg and 160.8 Wh/kg, exceptional cycle stability of 200,000 cycles at 20 A/g, and a high specific capacity of 262.8 mAh/g at 0.2 A/g [185].

Table 3.
Electrochemical performance characteristics of carbon-based materials for supercapacitors.
Table 3.
Electrochemical performance characteristics of carbon-based materials for supercapacitors.
| Type of Electrode | Sample Name | Capacitance (F/g) | Energy Density (Wh/kg) | Power Density (kW/kg) | Retention/Cycles (%) | Ref. |
|---|---|---|---|---|---|---|
| Graphene | rGO | 585.44 | 81.31 | 62.64 | 94.14/5000 | [207] |
| NiO@srGO/CNT | 1605.82 | N/A | N/A | 71.56/10,000 | [197] | |
| NMGO//MWCNT | 90 | 28 | 0.75 | 88/6000 | [208] | |
| 4NG | 405 | 68.1 | 558.5 | 87.7/5000 | [209] | |
| Activated Carbon | RPC | 56 | 44 | 0.564 | N/A | [210] |
| HAC-WS | 225 | 72.2 | 1.547 | 88/2500 | [211] | |
| hCNC-5.0 | 281 | 153 | 1.000 | 93/100,000 | [212] | |
| Carbon Nanotubes | CNT Am-241 | 489.6 | 68 | 9.992 | 98.5/5000 | [213] |
| VACNT | 8 | 0.20 | 0.450 | 92/3000 | [154] | |
| Fe3O4/CNT | 187.1 | N/A | N/A | 80.2/1000 | [204] | |
| V2O5/VACNT | 284 | 32.3 | 0.118 | 76/5000 | [203] | |
| P3MT/VACNT | 170 | 52 | 10 | 95/19,000 | [202] | |
| PC-CNT | 248 | 8.42 | 0.250 | 97.3/3000 | [214] | |
| SWCNT/TiO2 | 144 | 20 | 10.000 | 95/50,000 | [215] | |
| CNT-SC | 375.4 | 75.1 | N/A | 93.1/100 | [216] | |
| CNT/TiNiW-SC | 549.1 | 336.7 | N/A | 95.4/100 | ||
| CNT-NF | 250.5 | 68.19 | 27.994 | 92.42/10,000 | [217] | |
| G/CNT-SP | 500.16 | 69.46 | N/A | 87/1000 | [218] | |
| MnO2@CNT | 219 | N/A | N/A | 88/7000 | [219] | |
| Carbon Nano-onions | Co3O4/CNO | 402.35 | N/A | N/A | 76/9000 | [220] |
| PEDOT-MoO3@CNO | 428 | N/A | N/A | 76/6000 | [131] | |
| RFM-CNO-C | 160 | 5 | 0.43 | 97/3000 | [221] |
In previous research, various electrode fabrication techniques (such as screen printing, etching, pressing, lamination, deposition, coating, and direct synthesis on the current collector), electrolyte systems (such as aqueous and organic), and substrates (conductive and insulating) have been used [222,223,224,225,226,227]. The obtained properties can vary significantly, thus making it difficult to directly compare the electrochemical performance of active materials. Furthermore, few studies have addressed the use of spin coating and inkjet printing techniques in electrode fabrication, as increasing the loading of active materials generally improves the overall capacity, and there has been limited interest in extremely lightweight energy storage systems [228,229,230,231]. This suggests that the manufacturing technology itself can have a significant impact on the properties of the device for the following reasons: (i) minor changes in the manufacturing method can result in various microstructures, which affects the properties of these electrode materials, and (ii) the electrochemical performance of SCs depends on both the width of the electrodes and the interspace between the electrode pairs. The accuracy and precision of the above parameters are also influenced by the fabrication methods, which cause differences in electrochemical performance (see Table 3).
5. Conclusions and Outlook
Supercapacitors offer a promising alternative to overcome long-term energy storage problems. They are reliable and best suited for high-power transfer applications, but it is advisable to combine them with batteries. SCs can be manufactured using a variety of methods and materials. This study reviews the fundamentals of supercapacitors, their structure, properties, and the various types of carbon-based electrodes used in their construction. The high interest in carbon-based nanomaterials is also due to their high conductivity, morphological versatility, fine-tunable porous structure, controllable surface functions, natural abundance of raw materials, and low cost. VACNTs can not only provide a well-oriented “highway” for electron conduction, but also promote rapid ion transport due to the regular, small distances between CNTs and their alignment. High surface area of aligned CNTs on conductive substrates and introducing coating of transition metal oxides (NiO, V2O5, MnO2, ZnO, etc.) in order to increase the energy density are in current demand and meet the requirements to produce low cost, satisfactory cycle lifetime stability and high-energy-density supercapacitors. To achieve the full potential application of these devices, the performance and reproducibility of electrodes must be enhanced by further designing and developing these nanostructures. Therefore, studies focusing on the development of nanoscale materials that improve the capacitive performance of supercapacitors while maintaining high cycle life and dynamic reversibility are of particular importance. The hybridization of carbon-based nanostructures and metal oxides to form composite materials is recommended and is expected to yield significant success and coordinated efforts. Another important aspect is the possibility of integrating batteries and supercapacitors, as the strengths of one compensate for the weaknesses of the other. Nevertheless, future studies should focus on the following research directions: (i) developing new materials and designs that can significantly increase the energy density of supercapacitors, (ii) developing hybrid systems that combine the advantages of supercapacitors and batteries, thereby providing greater energy density and longer life cycles, (iii) developing environmentally friendly and sustainable materials, and (iv) novel materials and designs need to be developed to improve the reliability and lifespan (charge/discharge cycle) of SCs. Key challenges include bridging the gap between laboratory performance and commercial viability, and transitioning to more environmentally friendly processing methods. In the future, integrating advances in electrodes and engineering design will be essential to harnessing the full potential of SCs in modern energy systems.
Author Contributions
Conceptualization, methodology, format analysis, data curation, writing—original draft preparation, L.N.; conceptualization, supervision, K.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The research work L. Nánai (EKÖP-24-4-II-18) is supported by the University Research Scholarship Program of the Ministry for Culture and Innovation from the source of the National Research, Development and Innovation Fund.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| 1D | One-Dimensional |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| AC | Activated Carbon |
| Al | Aluminum |
| Au | Gold |
| CCVD | Catalyst-Supported Chemical Vapor Deposition |
| CNO | Carbon Nano-onion |
| CNT | Carbon Nanotube |
| CO2 | Carbon Dioxide |
| CT | Total Capacity |
| CV | Cyclic Voltammetry |
| CVD | Chemical Vapor Deposition |
| DC | Direct Current |
| DWCNT | Double-Walled Carbon Nanotube |
| EDL | Electric Double Layer |
| EDLC | Electric Double-Layer Capacitor |
| EIS | Electrochemical Impedance Spectroscopy |
| GCD | Galvanostatic Charge–Discharge |
| GO | Graphene Oxide |
| HAC | Hierarchical Activated Carbon |
| hCNC | Hierarchical Carbon Nanocages |
| H2SO4 | Sulfuric Acid |
| IHP | Inner Helmholtz Plane |
| KOH | Potassium Hydroxide |
| MnO2 | Manganese(IV) Dioxide |
| MWCNT | Multi-Walled Carbon Nanotube |
| Na2SO4 | Sodium Sulfate |
| Nb2O5 | Niobium Pentoxide |
| NEC | Nippon Electric Company |
| NG | Nitrogen Doped Graphene |
| NiO | Nickel(II) Oxide |
| OHP | Outer Helmholtz Plane |
| P3MT | Poly(3-Methylthiophene) |
| PC | Pseudocapacitor |
| RES | Equivalent Series Resistance |
| rGO | Reduced Graphene Oxide |
| RPC | Rose Petal-Derived Porous Carbons |
| RuO2 | Ruthenium(IV) Oxide |
| SC | Supercapacitor |
| SS | Stainless Steal |
| SWCNT | Single-Walled Carbon Nanotube |
| TEM | Transmission Electron Microscopy |
| Ti | Titanium |
| V2O5 | Vanadium(V) Oxide |
| VACNT | Vertically Aligned Carbon Nanotube |
| Vo | Operating Voltage |
References
- Sadiq, M.; Nawaz, M.A.; Chien, F.; Sharif, A.; Hanif, S. Enhancing Environmental Quality and Mitigating Climate Change: A Renewable Energy Policy Perspective Based on Evidence from Most Polluted European Countries. Gondwana Res. 2025, 148, 96–105. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y. Economic and Environmental Assessment of Different Energy Storage Methods for Hybrid Energy Systems. Sci. Rep. 2025, 15, 25592. [Google Scholar] [CrossRef] [PubMed]
- Sultanova, G.; Naser, H. Navigating Sustainability: How Export Diversification Influences Ecological Footprints in Developed and Developing Countries. Discov. Sustain. 2025, 6, 316. [Google Scholar] [CrossRef]
- Rezaei, S.; Hormaza Mejia, A.; Wu, Y.; Reed, J.; Brouwer, J. Global Warming Impacts of the Transition from Fossil Fuel Conversion and Infrastructure to Hydrogen. Appl. Energy 2025, 397, 126363. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Chen, X. Supercapacitors for Renewable Energy Applications: A Review. Micro Nano Eng. 2023, 21, 100229. [Google Scholar] [CrossRef]
- Argyrou, M.C.; Christodoulides, P.; Kalogirou, S.A. Energy Storage for Electricity Generation and Related Processes: Technologies Appraisal and Grid Scale Applications. Renew. Sustain. Energy Rev. 2018, 94, 804–821. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Huhn, E.; Braxtan, N.; Chen, S.-E.; Bombik, A.; Zhao, T.; Ma, L.; Sherman, J.; Roghani, S. Lithium-Ion Battery Thermal Runaway Suppression Using Water Spray Cooling. Energies 2025, 18, 2709. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, C.; Li, X.; Zhang, H.; Li, P. Energy Management Strategy of Fuel Cell/Battery/Supercapacitors Hybrid Storage System Based on Adaptive Real-Time Wavelets-Fuzzy Logic. IFAC-PapersOnLine 2024, 58, 373–378. [Google Scholar] [CrossRef]
- Akin, M.; Zhou, X. Recent Advances in Solid-state Supercapacitors: From Emerging Materials to Advanced Applications. Int. J. Energy Res. 2022, 46, 10389–10452. [Google Scholar] [CrossRef]
- Volfkovich, Y.M. High Power Supercapacitors. Review. J. Electroanal. Chem. 2024, 963, 118290. [Google Scholar] [CrossRef]
- Khan, K.; Tareen, A.K.; Aslam, M.; Mahmood, A.; khan, Q.; Zhang, Y.; Ouyang, Z.; Guo, Z.; Zhang, H. Going Green with Batteries and Supercapacitor: Two Dimensional Materials and Their Nanocomposites Based Energy Storage Applications. Prog. Solid. State Chem. 2020, 58, 100254. [Google Scholar] [CrossRef]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super Capacitors for Energy Storage: Progress, Applications and Challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D.; et al. Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Libich, J.; Sedlaříková, M.; Máca, J.; Čudek, P.; Kazda, T.; Fafilek, G.; Rodríguez, J.J.S. Supercapacitors vs. Lithium-ion Batteries: Properties and Applications. Chem. Ing. Tech. 2024, 96, 279–285. [Google Scholar] [CrossRef]
- Jayananda, D.; Kularatna, N.; Steyn-Ross, D.A. Supercapacitor-assisted LED (SCALED) Technique for Renewable Energy Systems: A Very Low Frequency Design Approach with Short-term DC-UPS Capability Eliminating Battery Banks. IET Renew. Power Gener. 2020, 14, 1559–1570. [Google Scholar] [CrossRef]
- R-Smith, N.A.-Z.; Moertelmaier, M.; Gramse, G.; Kasper, M.; Ragulskis, M.; Groebmeyer, A.; Jurjovec, M.; Brorein, E.; Zollo, B.; Kienberger, F. Fast Method for Calibrated Self-Discharge Measurement of Lithium-Ion Batteries Including Temperature Effects and Comparison to Modelling. Energy Rep. 2023, 10, 3394–3401. [Google Scholar] [CrossRef]
- Kowal, J.; Avaroglu, E.; Chamekh, F.; Šenfelds, A.; Thien, T.; Wijaya, D.; Sauer, D.U. Detailed Analysis of the Self-Discharge of Supercapacitors. J. Power Sources 2011, 196, 573–579. [Google Scholar] [CrossRef]
- Lee, N.; Nee, C.H.; Yap, S.S.; Tham, K.K.; You, A.H.; Yap, S.L.; Mohd Arof, A.K.B. Capacity Sizing of Embedded Control Battery–Supercapacitor Hybrid Energy Storage System. Energies 2022, 15, 3783. [Google Scholar] [CrossRef]
- Ariyarathna, T.; Kularatna, N.; Gunawardane, K.; Jayananda, D.; Steyn-Ross, D.A. Development of Supercapacitor Technology and Its Potential Impact on New Power Converter Techniques for Renewable Energy. IEEE J. Emerg. Sel. Top. Ind. Electron. 2021, 2, 267–276. [Google Scholar] [CrossRef]
- Subasinghage, K.; Gunawardane, K.; Padmawansa, N.; Kularatna, N.; Moradian, M. Modern Supercapacitors Technologies and Their Applicability in Mature Electrical Engineering Applications. Energies 2022, 15, 7752. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature Effect and Thermal Impact in Lithium-Ion Batteries: A Review. Prog. Nat. Sci. Mater. Int. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Singha Roy, P.K.; Karayaka, H.B.; He, J.; Yu, Y.-H. Economic Comparison between Battery and Supercapacitor for Hourly Dispatching Wave Energy Converter Power. In Proceedings of the 2020 52nd North American Power Symposium (NAPS), Tempe, AZ, USA, 11–13 April 2021; IEEE: New York, NY, USA, 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Xu, T.; Wang, Y.; Liu, K.; Zhao, Q.; Liang, Q.; Zhang, M.; Si, C. Ultralight MXene/Carbon Nanotube Composite Aerogel for High-Performance Flexible Supercapacitor. Adv. Compos. Hybrid. Mater. 2023, 6, 108. [Google Scholar] [CrossRef]
- Loh, K.H.; Liew, J.; Liu, L.; Goh, Z.L.; Pershaanaa, M.; Kamarulazam, F.; Bashir, S.; Ramesh, K.; Ramesh, S. A Comprehensive Review on Fundamentals and Components of Zinc-Ion Hybrid Supercapacitors. J. Energy Storage 2024, 81, 110370. [Google Scholar] [CrossRef]
- Goikolea, E.; Mysyk, R. Nanotechnology in Electrochemical Capacitors. In Emerging Nanotechnologies in Rechargeable Energy Storage Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–169. [Google Scholar] [CrossRef]
- Dissanayake, K.; Kularatna-Abeywardana, D. A Review of Supercapacitors: Materials, Technology, Challenges, and Renewable Energy Applications. J. Energy Storage 2024, 96, 112563. [Google Scholar] [CrossRef]
- Pandya, D.J.; Muthu Pandian, P.; Kumar, I.; Parmar, A.; Sravanthi; Singh, N.; Abd Al-saheb, A.J.; Arun, V. Supercapacitors: Review of Materials and Fabrication Methods. Mater. Today Proc. 2023, in press. [Google Scholar] [CrossRef]
- Behzadi pour, G.; Fekri aval, L.; Kianfar, E. Comparative Studies of Nanosheet-Based Supercapacitors: A Review of Advances in Electrodes Materials. Case Stud. Chem. Environ. Eng. 2024, 9, 100584. [Google Scholar] [CrossRef]
- Iversen, P.; Lacks, D.J. A Life of Its Own: The Tenuous Connection between Thales of Miletus and the Study of Electrostatic Charging. J. Electrost. 2012, 70, 309–311. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Heilbron, J.L. Electricity in the 17th and 18th Centuries; University of California Press: Oakland, CA, USA, 1979. [Google Scholar] [CrossRef]
- Kurzweil, P.; Schottenbauer, J.; Schell, C. Past, Present and Future of Electrochemical Capacitors: Pseudocapacitance, Aging Mechanisms and Service Life Estimation. J. Energy Storage 2021, 35, 102311. [Google Scholar] [CrossRef]
- Rashid Khan, H.; Latif Ahmad, A. Supercapacitors: Overcoming Current Limitations and Charting the Course for next-Generation Energy Storage. J. Ind. Eng. Chem. 2025, 141, 46–66. [Google Scholar] [CrossRef]
- Miao, L.; Song, Z.; Zhu, D.; Li, L.; Gan, L.; Liu, M. Recent Advances in Carbon-Based Supercapacitors. Mater. Adv. 2020, 1, 945–966. [Google Scholar] [CrossRef]
- Poonam; Sharma, K.; Arora, A.; Tripathi, S.K. Review of Supercapacitors: Materials and Devices. J. Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Becker, H.I. Low Voltage Electrolytic Capacitor. U.S. Patent US2800616A, 23 June 1957. [Google Scholar]
- Pershaanaa, M.; Bashir, S.; Ramesh, S.; Ramesh, K. Every Bite of Supercap: A Brief Review on Construction and Enhancement of Supercapacitor. J. Energy Storage 2022, 50, 104599. [Google Scholar] [CrossRef]
- Khan, H.A.; Tawalbeh, M.; Aljawrneh, B.; Abuwatfa, W.; Al-Othman, A.; Sadeghifar, H.; Olabi, A.G. A Comprehensive Review on Supercapacitors: Their Promise to Flexibility, High Temperature, Materials, Design, and Challenges. Energy 2024, 295, 131043. [Google Scholar] [CrossRef]
- Spyker, R.L.; Nelms, R.M. Optimization of Double-Layer Capacitor Arrays. IEEE Trans. Ind. Appl. 2000, 36, 194–198. [Google Scholar] [CrossRef]
- Namisnyk, A.M.; Zhu, J.G. A Survey of Electrochemical Supercapacitor Technology. In Proceedings of the Australian Universities Power Engineering Conference, Christchurch, New Zealand, 28 September 2003; University of Canterbury: Christchurch, New Zealand, 2003; pp. 51–56. [Google Scholar]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” Behavior in Electrochemical Energy Storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Liu, Y.; Shearing, P.R.; He, G.; Brett, D.J.L. Supercapacitors: History, Theory, Emerging Technologies, and Applications. In Advances in Sustainable Energy; Springer International Publishing: Cham, Switzerland, 2021; pp. 417–449. [Google Scholar] [CrossRef]
- Dutta, A.; Mitra, S.; Basak, M.; Banerjee, T. A Comprehensive Review on Batteries and Supercapacitors: Development and Challenges since Their Inception. Energy Storage 2023, 5, e339. [Google Scholar] [CrossRef]
- Yaseen, M.; Khattak, M.A.K.; Humayun, M.; Usman, M.; Shah, S.S.; Bibi, S.; Hasnain, B.S.U.; Ahmad, S.M.; Khan, A.; Shah, N.; et al. A Review of Supercapacitors: Materials Design, Modification, and Applications. Energies 2021, 14, 7779. [Google Scholar] [CrossRef]
- Shah, S.S.; Niaz, F.; Ehsan, M.A.; Das, H.T.; Younas, M.; Khan, A.S.; Rahman, H.U.; Nayem, S.M.A.; Oyama, M.; Aziz, M.A. Advanced Strategies in Electrode Engineering and Nanomaterial Modifications for Supercapacitor Performance Enhancement: A Comprehensive Review. J. Energy Storage 2024, 79, 110152. [Google Scholar] [CrossRef]
- Huang, J.; Xie, Y.; You, Y.; Yuan, J.; Xu, Q.; Xie, H.; Chen, Y. Rational Design of Electrode Materials for Advanced Supercapacitors: From Lab Research to Commercialization. Adv. Funct. Mater. 2023, 33, 2213095. [Google Scholar] [CrossRef]
- Ahmad, F.; Zahid, M.; Jamil, H.; Khan, M.A.; Atiq, S.; Bibi, M.; Shahbaz, K.; Adnan, M.; Danish, M.; Rasheed, F.; et al. Advances in Graphene-Based Electrode Materials for High-Performance Supercapacitors: A Review. J. Energy Storage 2023, 72, 108731. [Google Scholar] [CrossRef]
- Manfo, T.A.; Laaksonen, H. A Review of Carbon-Based Hybrid Materials for Supercapacitors. New Carbon. Mater. 2025, 40, 81–110. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Ogawa, T.; Zada, L.; Marwat, M.A.; Abdullah, S.M.; Khan, A.J.; Usman, M.; Khan, I.; Said, Z.; et al. Revolutionary NiCo Layered Double Hydroxide Electrodes: Advances, Challenges, and Future Prospects for High-Performance Supercapacitors. Mater. Sci. Eng. R Rep. 2025, 166, 101041. [Google Scholar] [CrossRef]
- He, X.; Zhang, X. A Comprehensive Review of Supercapacitors: Properties, Electrodes, Electrolytes and Thermal Management Systems Based on Phase Change Materials. J. Energy Storage 2022, 56, 106023. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Hwang, G.; Lee, K.; Kim, J.; Lee, K.-J.; Lee, S.; Kim, M. Energy Management Optimization of Series Hybrid Electric Bus Using an Ultra-Capacitor and Novel Efficiency Improvement Factors. Sustainability 2020, 12, 7354. [Google Scholar] [CrossRef]
- Varghese, A.M.; Pradhan, R.P. A Comprehensive Review and Research Agenda on the Adoption, Transition, and Procurement of Electric Bus Technologies into Public Transportation. Sustain. Energy Technol. Assess. 2025, 75, 104218. [Google Scholar] [CrossRef]
- Choi, Y.; Bhakta, S. Optimal Sizing of Grid-Tied Hybrid Solar Tracking Photovoltaic/Hydrogen Fuel Cell Energy Systems for Electric Vehicle Charging Stations in South Korea: A Techno-Economic Study. J. Clean. Prod. 2025, 486, 144511. [Google Scholar] [CrossRef]
- Tong, H.Y. Development of a Driving Cycle for a Supercapacitor Electric Bus Route in Hong Kong. Sustain. Cities Soc. 2019, 48, 101588. [Google Scholar] [CrossRef]
- Du, J.; Li, F.; Li, J.; Wu, X.; Song, Z.; Zou, Y.; Ouyang, M. Evaluating the Technological Evolution of Battery Electric Buses: China as a Case. Energy 2019, 176, 309–319. [Google Scholar] [CrossRef]
- Cai, Y.; Yang, F.; Ouyang, M.G. Impact of Control Strategy on Battery Degradation for a Plug-in Hybrid Electric City Bus in China. Energy 2016, 116, 1020–1030. [Google Scholar] [CrossRef]
- Chemali, E.; Preindl, M.; Malysz, P.; Emadi, A. Electrochemical and Electrostatic Energy Storage and Management Systems for Electric Drive Vehicles: State-of-the-Art Review and Future Trends. IEEE J. Emerg. Sel. Top. Power Electron. 2016, 4, 1117–1134. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Li, Y.; Li, L.; Ji, H.; Feteira, A.; Zhou, D.; Wang, D.; Zhang, S.; Reaney, I.M. Electroceramics for High-Energy Density Capacitors: Current Status and Future Perspectives. Chem. Rev. 2021, 121, 6124–6172. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhao, K.; Chi, H.-Y.; Shen, Y.; Song, S.; Hsu, K.-J.; Chevalier, M.; Shi, W.; Agrawal, K.V. Electrochemical-Repaired Porous Graphene Membranes for Precise Ion-Ion Separation. Nat. Commun. 2024, 15, 4006. [Google Scholar] [CrossRef]
- Umoru, S.E.; Osafile, O.E. A Comparative Study of the Electrochemical Performance of Spinels and Chalcogenides in Supercapacitors. Proc. Indian. Natl. Sci. Acad. 2025. [Google Scholar] [CrossRef]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Wayu, M. Manganese Oxide Carbon-Based Nanocomposite in Energy Storage Applications. Solids 2021, 2, 232–248. [Google Scholar] [CrossRef]
- Pameté, E.; Köps, L.; Kreth, F.A.; Pohlmann, S.; Varzi, A.; Brousse, T.; Balducci, A.; Presser, V. The Many Deaths of Supercapacitors: Degradation, Aging, and Performance Fading. Adv. Energy Mater. 2023, 13, 2301008. [Google Scholar] [CrossRef]
- Dubey, P.; Shrivastav, V.; Maheshwari, P.H.; Sundriyal, S. Recent Advances in Biomass Derived Activated Carbon Electrodes for Hybrid Electrochemical Capacitor Applications: Challenges and Opportunities. Carbon 2020, 170, 1–29. [Google Scholar] [CrossRef]
- Ghosh, A.; Lee, Y.H. Carbon-Based Electrochemical Capacitors. ChemSusChem 2012, 5, 480–499. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.V.T.; Bin Faheem, A.; Kwak, K.; Lee, K.-K. Propionitrile as a Single Organic Solvent for High Voltage Electric Double-Layer Capacitors. J. Power Sources 2020, 463, 228134. [Google Scholar] [CrossRef]
- Thakur, A.K.; Majumder, M.; Patole, A.S.; Patole, S.P. Commercial Aspects, Safety Regulations, Environmental and Health Impacts, and Recycling Strategies of Supercapacitors. In Low-Carbon Supercapacitors; Royal Society of Chemistry: London, UK, 2023; pp. 477–498. [Google Scholar] [CrossRef]
- Kharabati, S.; Saedodin, S. A Systematic Review of Thermal Management Techniques for Electric Vehicle Batteries. J. Energy Storage 2024, 75, 109586. [Google Scholar] [CrossRef]
- Chu, B.; Liu, S.; You, L.; Liu, D.; Huang, T.; Li, Y.; Yu, A. Enhancing the Cycling Stability of Ni-Rich LiNi0.6Co0.2Mn0.2O2 Cathode at a High Cutoff Voltage with Ta Doping. ACS Sustain. Chem. Eng. 2020, 8, 3082–3090. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Okoye, P.U.; Alegre, C. A Review on Carbon Materials for Electrochemical Energy Storage Applications: State of the Art, Implementation, and Synergy with Metallic Compounds for Supercapacitor and Battery Electrodes. J. Power Sources 2024, 617, 235140. [Google Scholar] [CrossRef]
- Mitali, J.; Dhinakaran, S.; Mohamad, A.A. Energy Storage Systems: A Review. Energy Storage Sav. 2022, 1, 166–216. [Google Scholar] [CrossRef]
- Querne, C.; Vignal, T.; Pinault, M.; Banet, P.; Mayne-L’Hermite, M.; Aubert, P.-H. A Comparative Study of High Density Vertically Aligned Carbon Nanotubes Grown onto Different Grades of Aluminum—Application to Supercapacitors. J. Power Sources 2023, 553, 232258. [Google Scholar] [CrossRef]
- Vessally, E.; Rzayev, R.M.; Niyazova, A.A.; Aggarwal, T.; Rahimova, K.E. Overview of Recent Developments in Carbon-Based Nanocomposites for Supercapacitor Applications. RSC Adv. 2024, 14, 40141–40159. [Google Scholar] [CrossRef]
- Şahin, M.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Waqas Hakim, M.; Fatima, S.; Rizwan, S.; Mahmood, A. Pseudo-Capacitors: Introduction, Controlling Factors and Future; Springer: Berlin/Heidelberg, Germany, 2022; pp. 53–70. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Env. Sci. 2014, 7, 1597. [Google Scholar] [CrossRef]
- Huang, H.; Niederberger, M. Towards Fast-Charging Technologies in Li+/Na+ Storage: From the Perspectives of Pseudocapacitive Materials and Non-Aqueous Hybrid Capacitors. Nanoscale 2019, 11, 19225–19240. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Augustyn, V.; Dunn, B. The Effect of Crystallinity on the Rapid Pseudocapacitive Response of Nb2O5. Adv. Energy Mater. 2012, 2, 141–148. [Google Scholar] [CrossRef]
- Conway, B.E.; Angerstein-Kozlowska, H. The Electrochemical Study of Multiple-State Adsorption in Monolayers. Acc. Chem. Res. 1981, 14, 49–56. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef]
- Wu, J. Understanding the Electric Double-Layer Structure, Capacitance, and Charging Dynamics. Chem. Rev. 2022, 122, 10821–10859. [Google Scholar] [CrossRef]
- Beknalkar, S.A.; Teli, A.M.; Bhat, T.S.; Pawar, K.K.; Patil, S.S.; Harale, N.S.; Shin, J.C.; Patil, P.S. Mn3O4 Based Materials for Electrochemical Supercapacitors: Basic Principles, Charge Storage Mechanism, Progress, and Perspectives. J. Mater. Sci. Technol. 2022, 130, 227–248. [Google Scholar] [CrossRef]
- Panchu, S.J.; Raju, K.; Swart, H.C. Emerging Two–Dimensional Intercalation Pseudocapacitive Electrodes for Supercapacitors. ChemElectroChem 2024, 11, e202300810. [Google Scholar] [CrossRef]
- Gao, L.; Li, C.; Huang, W.; Mei, S.; Lin, H.; Ou, Q.; Zhang, Y.; Guo, J.; Zhang, F.; Xu, S.; et al. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. [Google Scholar] [CrossRef]
- Tundwal, A.; Kumar, H.; Binoj, B.J.; Sharma, R.; Kumar, G.; Kumari, R.; Dhayal, A.; Yadav, A.; Singh, D.; Kumar, P. Developments in Conducting Polymer-, Metal Oxide-, and Carbon Nanotube-Based Composite Electrode Materials for Supercapacitors: A Review. RSC Adv. 2024, 14, 9406–9439. [Google Scholar] [CrossRef] [PubMed]
- Girirajan, M.; Bojarajan, A.K.; Pulidindi, I.N.; Hui, K.N.; Sangaraju, S. An Insight into the Nanoarchitecture of Electrode Materials on the Performance of Supercapacitors. Coord. Chem. Rev. 2024, 518, 216080. [Google Scholar] [CrossRef]
- Thomas, S.A.; Cherusseri, J.; Rajendran, D.N. 2D Nickel Sulfide Electrodes with Superior Electrochemical Thermal Stability along with Long Cyclic Stability for Supercapatteries. Energy Technol. 2024, 12, 2301641. [Google Scholar] [CrossRef]
- Maria Mahimai, B.; Li, E.; Pang, J.; Zhang, J.; Zhang, J. Interface Engineering in Conducting Polymers-Based Supercapacitor. J. Energy Storage 2024, 96, 112598. [Google Scholar] [CrossRef]
- Shchegolkov, A.V.; Lipkin, M.S.; Shchegolkov, A.V. Preparation of WO3 Films on Titanium and Graphite Foil for Fuel Cell and Supercapacitor Applications by Electrochemical (Cathodic) Deposition Method. Russ. J. Gen. Chem. 2022, 92, 1161–1167. [Google Scholar] [CrossRef]
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643. [Google Scholar] [CrossRef]
- Schoetz, T.; Gordon, L.W.; Ivanov, S.; Bund, A.; Mandler, D.; Messinger, R.J. Disentangling Faradaic, Pseudocapacitive, and Capacitive Charge Storage: A Tutorial for the Characterization of Batteries, Supercapacitors, and Hybrid Systems. Electrochim. Acta 2022, 412, 140072. [Google Scholar] [CrossRef]
- Sahoo, S.; Kumar, R.; Joanni, E.; Singh, R.K.; Shim, J.-J. Advances in Pseudocapacitive and Battery-like Electrode Materials for High Performance Supercapacitors. J. Mater. Chem. A Mater. 2022, 10, 13190–13240. [Google Scholar] [CrossRef]
- Surendran, V.; Arya, R.S.; Vineesh, T.V.; Babu, B.; Shaijumon, M.M. Engineered Carbon Electrodes for High Performance Capacitive and Hybrid Energy Storage. J. Energy Storage 2021, 35, 102340. [Google Scholar] [CrossRef]
- Himadri Reddy, P.C.; Amalraj, J.; Ranganatha, S.; Patil, S.S.; Chandrasekaran, S. A Review on Effect of Conducting Polymers on Carbon-Based Electrode Materials for Electrochemical Supercapacitors. Synth. Met. 2023, 298, 117447. [Google Scholar] [CrossRef]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage—A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Kularatna, N.; Subasinghage, K.; Gunawardane, K.; Jayananda, D.; Ariyarathna, T. Supercapacitor-Assisted Techniques and Supercapacitor-Assisted Loss Management Concept: New Design Approaches to Change the Roadmap of Power Conversion Systems. Electronics 2021, 10, 1697. [Google Scholar] [CrossRef]
- Saleh, H.M.; Hassan, A.I. Synthesis and Characterization of Nanomaterials for Application in Cost-Effective Electrochemical Devices. Sustainability 2023, 15, 10891. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and Challenges of Hydrothermal Technique for Synthesis of Transition Metal Oxides and Composites as Supercapacitor Electrode Materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Hardianto, Y.P.; Shah, S.S.; Shuaibu, A.D.; Mohamed, M.; Sarker, S.; Alzahrani, A.S.; Aziz, M.A. Modeling Supercapacitors with the Simplified Randles Circuit: Analyzing Electrochemical Behavior through Cyclic Voltammetry and Galvanostatic Charge-Discharge. Electrochim. Acta 2025, 513, 145552. [Google Scholar] [CrossRef]
- Zeng, L.; Wu, T.; Ye, T.; Mo, T.; Qiao, R.; Feng, G. Modeling Galvanostatic Charge–Discharge of Nanoporous Supercapacitors. Nat. Comput. Sci. 2021, 1, 725–731. [Google Scholar] [CrossRef]
- Gaire, M.; Liang, K.; Luo, S.; Subedi, B.; Adireddy, S.; Schroder, K.; Farnsworth, S.; Chrisey, D.B. Nanostructured Manganese Oxides Electrode with Ultra-Long Lifetime for Electrochemical Capacitors. RSC Adv. 2020, 10, 16817–16825. [Google Scholar] [CrossRef]
- Permatasari, F.A.; Irham, M.A.; Bisri, S.Z.; Iskandar, F. Carbon-Based Quantum Dots for Supercapacitors: Recent Advances and Future Challenges. Nanomaterials 2021, 11, 91. [Google Scholar] [CrossRef]
- Vivier, V.; Orazem, M.E. Impedance Analysis of Electrochemical Systems. Chem. Rev. 2022, 122, 11131–11168. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Balducci, A.; Belanger, D.; Brousse, T.; Long, J.W.; Sugimoto, W. Perspective—A Guideline for Reporting Performance Metrics with Electrochemical Capacitors: From Electrode Materials to Full Devices. J. Electrochem. Soc. 2017, 164, A1487–A1488. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards Establishing Standard Performance Metrics for Batteries, Supercapacitors and Beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate Methods for Evaluating the Efficiency and Capacitive Behavior of Different Types of Supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, N. Supercapacitors Performance Evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Park, H.W.; Roh, K.C. Recent Advances in and Perspectives on Pseudocapacitive Materials for Supercapacitors–A Review. J. Power Sources 2023, 557, 232558. [Google Scholar] [CrossRef]
- Patel, A.; Patel, S.K.; Singh, R.S.; Patel, R.P. Review on Recent Advancements in the Role of Electrolytes and Electrode Materials on Supercapacitor Performances. Discov. Nano 2024, 19, 188. [Google Scholar] [CrossRef]
- Fialkov, A.S. Carbon Application in Chemical Power Sources. Russ. J. Electrochem. 2000, 36, 345–366. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Li, B.; Dai, F.; Xiao, Q.; Yang, L.; Shen, J.; Zhang, C.; Cai, M. Nitrogen-Doped Activated Carbon for a High Energy Hybrid Supercapacitor. Energy Env. Sci. 2016, 9, 102–106. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and Characterisations of Various Activated Carbons Used for Carbon/Carbon Supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmed, A.; Rafat, M. Supercapacitor Performance of Activated Carbon Derived from Rotten Carrot in Aqueous, Organic and Ionic Liquid Based Electrolytes. J. Saudi Chem. Soc. 2018, 22, 993–1002. [Google Scholar] [CrossRef]
- Shamsuddin, M.S.; Yusoff, N.R.N.; Sulaiman, M.A. Synthesis and Characterization of Activated Carbon Produced from Kenaf Core Fiber Using H3PO4 Activation. Procedia Chem. 2016, 19, 558–565. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural Bio-Waste Materials as Potential Sustainable Precursors Used for Activated Carbon Production: A Review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Balathanigaimani, M.S.; Shim, W.-G.; Lee, M.-J.; Kim, C.; Lee, J.-W.; Moon, H. Highly Porous Electrodes from Novel Corn Grains-Based Activated Carbons for Electrical Double Layer Capacitors. Electrochem. Commun. 2008, 10, 868–871. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Rocha, G.J.M.; Órfão, J.J.M.; Teixeira, J.A.; Roberto, I.C. Production, Characterization and Application of Activated Carbon from Brewer’s Spent Grain Lignin. Bioresour. Technol. 2010, 101, 2450–2457. [Google Scholar] [CrossRef]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. Chempluschem 2024, 89, e202400408. [Google Scholar] [CrossRef]
- Rajasekaran, S.J.; Grace, A.N.; Jacob, G.; Alodhayb, A.; Pandiaraj, S.; Raghavan, V. Investigation of Different Aqueous Electrolytes for Biomass-Derived Activated Carbon-Based Supercapacitors. Catalysts 2023, 13, 286. [Google Scholar] [CrossRef]
- Temesgen, T.; Bekele, E.T.; Gonfa, B.A.; Tufa, L.T.; Sabir, F.K.; Tadesse, S.; Dessie, Y. Advancements in Biomass Derived Porous Carbon Materials and Their Surface Influence Effect on Electrode Electrochemical Performance for Sustainable Supercapacitors: A Review. J. Energy Storage 2023, 73, 109293. [Google Scholar] [CrossRef]
- Iijima, S. Direct Observation of the Tetrahedral Bonding in Graphitized Carbon Black by High Resolution Electron Microscopy. J. Cryst. Growth 1980, 50, 675–683. [Google Scholar] [CrossRef]
- Kaur, S.; Krishnan, A.; Chakraborty, S. Recent Advances and Challenges of Carbon Nano Onions (CNOs) for Application in Supercapacitor Devices (SCDs). J. Energy Storage 2023, 71, 107928. [Google Scholar] [CrossRef]
- Xiao, J.; Ouyang, G.; Liu, P.; Wang, C.X.; Yang, G.W. Reversible Nanodiamond-Carbon Onion Phase Transformations. Nano Lett. 2014, 14, 3645–3652. [Google Scholar] [CrossRef]
- Muduli, S.; Pani, T.K.; Garlapati, K.K.; Martha, S.K. Carbon Nano-Onions Triggering the Supercapacitive Performance of PEDOT-Wrapped MoO3 Microstructures in Hybrid Ultracapacitors. J. Energy Storage 2024, 95, 112396. [Google Scholar] [CrossRef]
- Almeida Gonzalez, H.D.; Hernandez Ojeda, J.; Corcho-Valdés, A.L.; Padron-Ramirez, I.; Perez Cruz, M.; Iriarte-Mesa, C.; Desdin-Garcia, L.F.; Gobbo, P.; Antuch, M. The Promise of Carbon Nano-Onions: Preparation, Characterization and Their Application in Electrochemical Sensing. Anal. Sens. 2025, 5, e202400035. [Google Scholar] [CrossRef]
- Pakpum, C.; Wongpanya, N.; Newyawong, P.; Saensuk, O.; Budkhod, P.; Butburee, P.; Wanachantararak, P.; Kanchiang, K. Synthesis and Characterization of Carbon Nano-Onions-like Structure Produced by C2H2 Microwave Plasma and Application in Bio-Imaging. Mater. Chem. Phys. 2025, 343, 131033. [Google Scholar] [CrossRef]
- Anjos, D.M.; McDonough, J.K.; Perre, E.; Brown, G.M.; Overbury, S.H.; Gogotsi, Y.; Presser, V. Pseudocapacitance and Performance Stability of Quinone-Coated Carbon Onions. Nano Energy 2013, 2, 702–712. [Google Scholar] [CrossRef]
- Matsumoto, N.; Kinoshita, H.; Choi, J.; Kato, T. Formation of Large Area Closely Packed Carbon Onions Film by Plasma-Based Ion Implantation. Sci. Rep. 2020, 10, 10037. [Google Scholar] [CrossRef]
- McDonough, J.K.; Gogotsi, Y. Carbon Onions: Synthesis and Electrochemical Applications. Interface Mag. 2013, 22, 61–66. [Google Scholar] [CrossRef]
- Pérez-Ojeda, M.E.; Castro, E.; Kröckel, C.; Lucherelli, M.A.; Ludacka, U.; Kotakoski, J.; Werbach, K.; Peterlik, H.; Melle-Franco, M.; Chacón-Torres, J.C.; et al. Carbon Nano-Onions: Potassium Intercalation and Reductive Covalent Functionalization. J. Am. Chem. Soc. 2021, 143, 18997–19007. [Google Scholar] [CrossRef]
- Mohamed, N.B.; El-Kady, M.F.; Kaner, R.B. Macroporous Graphene Frameworks for Sensing and Supercapacitor Applications. Adv. Funct. Mater. 2022, 32, 2203101. [Google Scholar] [CrossRef]
- Ran, J.; Liu, Y.; Feng, H.; Shi, H.; Ma, Q. A Review on Graphene-Based Electrode Materials for Supercapacitor. J. Ind. Eng. Chem. 2024, 137, 106–121. [Google Scholar] [CrossRef]
- Lakra, R.; Kumar, R.; Sahoo, P.K.; Thatoi, D.; Soam, A. A Mini-Review: Graphene Based Composites for Supercapacitor Application. Inorg. Chem. Commun. 2021, 133, 108929. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Takakura, A.; Beppu, K.; Nishihara, T.; Fukui, A.; Kozeki, T.; Namazu, T.; Miyauchi, Y.; Itami, K. Strength of Carbon Nanotubes Depends on Their Chemical Structures. Nat. Commun. 2019, 10, 3040. [Google Scholar] [CrossRef]
- Krishnan, A.; Dujardin, E.; Ebbesen, T.W.; Yianilos, P.N.; Treacy, M.M.J. Young’s Modulus of Single-Walled Nanotubes. Phys. Rev. B 1998, 58, 14013–14019. [Google Scholar] [CrossRef]
- Kaneko, K.; Li, M.; Noda, S. Appropriate Properties of Carbon Nanotubes for the Three-Dimensional Current Collector in Lithium-Ion Batteries. Carbon. Trends 2023, 10, 100245. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, G.J. Electrical Conductivity of Carbon Nanotube- and Graphene-Based Nanocomposites. In Micromechanics and Nanomechanics of Composite Solids; Springer International Publishing: Cham, Switzerland, 2018; pp. 123–156. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Cai, N.; Liu, Q.; Wu, C.; Yang, H. Preparation of Easily-Stripped and High-Purity Carbon Nanotubes from Various Waste Plastics. J. Energy Inst. 2024, 116, 101740. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Gao, Z.; Hou, P.; Xu, L.; Shi, C.; Liang, Y.; Wang, Y.; Liu, C. Highly Conductive Double-Wall Carbon Nanotube Fibers Produced by Dry-Jet Wet Spinning. Adv. Funct. Mater. 2024, 34, 2404538. [Google Scholar] [CrossRef]
- Snarski, L.; Biran, I.; Bendikov, T.; Pinkas, I.; Iron, M.A.; Kaplan-Ashiri, I.; Weissman, H.; Rybtchinski, B. Highly Conductive Robust Carbon Nanotube Networks for Strong Buckypapers and Transparent Electrodes. Adv. Funct. Mater. 2024, 34, 2309742. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, B.; Zhu, H.; Yuan, G.; Li, B.; Guo, J.; Li, X.; Cong, Y.; Zhang, J. A Review of Aligned Carbon Nanotube Arrays and Carbon/Carbon Composites: Fabrication, Thermal Conduction Properties and Applications in Thermal Management. New Carbon. Mater. 2021, 36, 873–892. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.-W. Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Li, W.Z.; Xie, S.S.; Qian, L.X.; Chang, B.H.; Zou, B.S.; Zhou, W.Y.; Zhao, R.A.; Wang, G. Large-Scale Synthesis of Aligned Carbon Nanotubes. Science (1979) 1996, 274, 1701–1703. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass. Science (1979) 1998, 282, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Nwanno, C.E.; Gotame, R.C.; Watt, J.; Kuo, W.; Li, W. Vertically Aligned Carbon Nanotubes Grown on Copper Foil as Electrodes for Electrochemical Double Layer Capacitors. Nanomaterials 2025, 15, 1506. [Google Scholar] [CrossRef]
- Zhan, Y.; Hu, Y.; Chen, Y.; Yang, Q.; Shi, Z.; Xiong, C. In-Situ Synthesis of Flexible Nanocellulose/Carbon Nanotube/Polypyrrole Hydrogels for High-Performance Solid-State Supercapacitors. Cellulose 2021, 28, 7097–7108. [Google Scholar] [CrossRef]
- Szabó, A.; Perri, C.; Csató, A.; Giordano, G.; Vuono, D.; Nagy, J.B. Synthesis Methods of Carbon Nanotubes and Related Materials. Materials 2010, 3, 3092–3140. [Google Scholar] [CrossRef]
- Quinton, B.T.; Barnes, P.N.; Varanasi, C.V.; Burke, J.; Tsao, B.-H.; Yost, K.J.; Mukhopadhyay, S.M. A Comparative Study of Three Different Chemical Vapor Deposition Techniques of Carbon Nanotube Growth on Diamond Films. J. Nanomater. 2013, 2013, 356259. [Google Scholar] [CrossRef]
- Szabó, A.; Nánai, L.; Tóth, Z.R.; Hernadi, K. Simplification of the CCVD Method Used in the Growth of Carbon Nanotube Forests on Titanium Substrate. Solid. State Sci. 2021, 117, 106648. [Google Scholar] [CrossRef]
- Goto, S.; Nakano, T.; Sugime, H.; Inoue, Y. Enhanced Growth of Ultra-High Density Carbon Nanotube Forests via Fe and Al Vapor Addition in a CVD Process. Carbon 2025, 243, 120537. [Google Scholar] [CrossRef]
- Thapa, A.; Guo, J.; Jungjohann, K.L.; Wang, X.; Li, W. Density Control of Vertically Aligned Carbon Nanotubes and Its Effect on Field Emission Properties. Mater. Today Commun. 2020, 22, 100761. [Google Scholar] [CrossRef]
- Hiraoka, T.; Yamada, T.; Hata, K.; Futaba, D.N.; Kurachi, H.; Uemura, S.; Yumura, M.; Iijima, S. Synthesis of Single- and Double-Walled Carbon Nanotube Forests on Conducting Metal Foils. J. Am. Chem. Soc. 2006, 128, 13338–13339. [Google Scholar] [CrossRef]
- Wang, B.; Liu, X.; Liu, H.; Wu, D.; Wang, H.; Jiang, J.; Wang, X.; Hu, P.; Liu, Y.; Zhu, D. Controllable Preparation of Patterns of Aligned Carbon Nanotubes on Metals and Metal-Coated Silicon Substrates. J. Mater. Chem. 2003, 13, 1124–1126. [Google Scholar] [CrossRef]
- Gao, L.; Peng, A.; Wang, Z.Y.; Zhang, H.; Shi, Z.; Gu, Z.; Cao, G.; Ding, B. Growth of Aligned Carbon Nanotube Arrays on Metallic Substrate and Its Application to Supercapacitors. Solid. State Commun. 2008, 146, 380–383. [Google Scholar] [CrossRef]
- Moyer-Vanderburgh, K.; Park, S.J.; Fornasiero, F. Growth of Carbon Nanotube Forests on Flexible Metal Substrates: Advances, Challenges, and Applications. Carbon 2023, 206, 402–421. [Google Scholar] [CrossRef]
- Lepró, X.; Lima, M.D.; Baughman, R.H. Spinnable Carbon Nanotube Forests Grown on Thin, Flexible Metallic Substrates. Carbon 2010, 48, 3621–3627. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, G.P.; Yang, Y.S. Using a Cut–Paste Method to Prepare a Carbon Nanotube Fur Electrode. Nanotechnology 2007, 18, 195607. [Google Scholar] [CrossRef]
- Liao, X.Z.; Serquis, A.; Jia, Q.X.; Peterson, D.E.; Zhu, Y.T.; Xu, H.F. Effect of Catalyst Composition on Carbon Nanotube Growth. Appl. Phys. Lett. 2003, 82, 2694–2696. [Google Scholar] [CrossRef]
- Sakurai, S.; Tsuji, T.; He, J.; Yamada, M.; Futaba, D.N. Double-Layer Support Strategy for Enhancing the Lifetime of Fe Catalyst Nanoparticles in Synthesis of Single-Walled Carbon Nanotubes. ACS Appl. Nano Mater. 2024, 7, 12745–12751. [Google Scholar] [CrossRef]
- Seman, R.N.A.R.; Azam, M.A.; Mohamed, M.A. Highly Efficient Growth of Vertically Aligned Carbon Nanotubes on Fe–Ni Based Metal Alloy Foils for Supercapacitors. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 045016. [Google Scholar] [CrossRef]
- Moon, S.Y.; Kang, I.J.; Kim, S.M.; Kim, W.S. Influence of the Sulfur Content Catalyst on the Packing Density of Carbon Nanotube Forests. Nanomaterials 2019, 9, 889. [Google Scholar] [CrossRef]
- Yemini, R.; Muallem, M.; Sharabani, T.; Teblum, E.; Gofer, Y.; Nessim, G.D. Patterning of Forests of Carbon Nanotubes (CNTs) Using Copper Overlayers as Iron Catalyst Deactivators. J. Phys. Chem. C 2016, 120, 12242–12248. [Google Scholar] [CrossRef]
- Yemini, R.; Itzhak, A.; Gofer, Y.; Sharabani, T.; Drela, M.; Nessim, G.D. Nickel Overlayers Modify Precursor Gases To Pattern Forests of Carbon Nanotubes. J. Phys. Chem. C 2017, 121, 11765–11772. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Luo, B.; Wang, Q.; Attarilar, S. High-Performance Nanoscale Metallic Multilayer Composites: Techniques, Mechanical Properties and Applications. Materials 2024, 17, 2124. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, C.; Esconjauregui, S.; Xie, R.; Zhong, G.; Bhardwaj, S.; Cepek, C.; Robertson, J. Carbon Nanotube Forests Growth Using Catalysts from Atomic Layer Deposition. J. Appl. Phys. 2014, 115, 144303. [Google Scholar] [CrossRef]
- Bulyarskiy, S.; Dudin, A.; L’vov, P.; Grishin, T.; Volkova, L.; Poliakov, M.; Mikhailov, I.; Rudakov, G. Formation of Catalyst Particles for the CNT Growth from Thin Films: Experiment and Simulation. Chem. Phys. 2024, 579, 112202. [Google Scholar] [CrossRef]
- Chatterjee, K.; Kumar, V.; Agnihotri, P.K.; Basu, S.; Gupta, N. On the Importance of the Metal Catalyst Layer to the Performance of CNT-Based Supercapacitor Electrodes. IEEE Trans. Nanotechnol. 2025, 24, 48–53. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. Functionalization of Carbon Nanotubes. In Functional Molecular Nanostructures; Springer: Berlin/Heidelberg, Germany, 2005; pp. 193–237. [Google Scholar] [CrossRef]
- Van Hooijdonk, E.; Bittencourt, C.; Snyders, R.; Colomer, J.-F. Functionalization of Vertically Aligned Carbon Nanotubes. Beilstein J. Nanotechnol. 2013, 4, 129–152. [Google Scholar] [CrossRef] [PubMed]
- John, A.R.; Arumugam, P. Open Ended Nitrogen-Doped Carbon Nanotubes for the Electrochemical Storage of Energy in a Supercapacitor Electrode. J. Power Sources 2015, 277, 387–392. [Google Scholar] [CrossRef]
- Patiño, J.; López-Salas, N.; Gutiérrez, M.C.; Carriazo, D.; Ferrer, M.L.; del Monte, F. Phosphorus-Doped Carbon–Carbon Nanotube Hierarchical Monoliths as True Three-Dimensional Electrodes in Supercapacitor Cells. J. Mater. Chem. A Mater. 2016, 4, 1251–1263. [Google Scholar] [CrossRef]
- Nonomura, R.; Itoh, T.; Sato, Y.; Yokoyama, K.; Yamamoto, M.; Nishida, T.; Motomiya, K.; Tohji, K.; Sato, Y. Electrochemical Capacitors Using Nitrogen-Doped Vertically Aligned Multi-Walled Carbon Nanotube Electrodes Prepared by Defluorination. Carbon 2018, 132, 539–547. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Li, Y.; Wang, Y.; Zhang, J.; Guan, G.; Pan, Z.; Zheng, G.; Peng, H. Nitrogen-Doped Core-Sheath Carbon Nanotube Array for Highly Stretchable Supercapacitor. Adv. Energy Mater. 2017, 7, 1601814. [Google Scholar] [CrossRef]
- Jiang, J.; Li, Y.; Liu, J.; Huang, X.; Yuan, C.; Lou, X.W. Recent Advances in Metal Oxide-based Electrode Architecture Design for Electrochemical Energy Storage. Adv. Mater. 2012, 24, 5166–5180. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, L.; Vellacheri, R.; Lei, Y. Recent Advances in Designing and Fabricating Self-Supported Nanoelectrodes for Supercapacitors. Adv. Sci. 2017, 4, 1700188. [Google Scholar] [CrossRef]
- Jha, S.; Qin, Y.; Chen, Y.; Song, Z.; Miao, L.; Lv, Y.; Gan, L.; Liu, M. Hydrogen-Bond-Guided Micellar Self-Assembly-Directed Carbon Superstructures for High-Energy and Ultralong-Life Zinc-Ion Hybrid Capacitors. J. Mater. Chem. A Mater. 2025, 13, 15101–15110. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, C.; Huang, Q.; Lv, Y.; Song, Z.; Gan, L.; Liu, M. Hydrogen-Bonded Interfacial Super-Assembly of Spherical Carbon Superstructures for High-Performance Zinc Hybrid Capacitors. Nanomicro Lett. 2026, 18, 38. [Google Scholar] [CrossRef]
- Shi, T.; Song, Z.; Lv, Y.; Zhu, D.; Miao, L.; Gan, L.; Liu, M. Hierarchical Porous Carbon Guided by Constructing Organic-Inorganic Interpenetrating Polymer Networks to Facilitate Performance of Zinc Hybrid Supercapacitors. Chin. Chem. Lett. 2025, 36, 109559. [Google Scholar] [CrossRef]
- Zhou, Q.; Yao, H. Recent Development of Carbon Electrode Materials for Electrochemical Supercapacitors. Energy Rep. 2022, 8, 656–661. [Google Scholar] [CrossRef]
- Kim, T.; Subedi, S.; Dahal, B.; Chhetri, K.; Mukhiya, T.; Muthurasu, A.; Gautam, J.; Lohani, P.C.; Acharya, D.; Pathak, I.; et al. Homogeneous Elongation of N-Doped CNTs over Nano-Fibrillated Hollow-Carbon-Nanofiber: Mass and Charge Balance in Asymmetric Supercapacitors Is No Longer Problematic. Adv. Sci. 2022, 9, e2200650. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q.; Wang, L.; Yang, X.; Yang, W.; Zheng, J.; Hou, H. Advanced Supercapacitors Based on Porous Hollow Carbon Nanofiber Electrodes with High Specific Capacitance and Large Energy Density. ACS Appl. Mater. Interfaces 2020, 12, 4777–4786. [Google Scholar] [CrossRef]
- Szabó, A.; Bakos, L.P.; Karajz, D.; Gyulavári, T.; Tóth, Z.-R.; Pap, Z.; Szilágyi, I.M.; Igricz, T.; Parditka, B.; Erdélyi, Z.; et al. Decoration of Vertically Aligned Carbon Nanotubes with Semiconductor Nanoparticles Using Atomic Layer Deposition. Materials 2019, 12, 1095. [Google Scholar] [CrossRef]
- Rahat, S.M.S.M.; Hasan, K.M.Z.; Mondol, M.M.H.; Mallik, A.K. A Comprehensive Review of Carbon Nanotube-Based Metal Oxide Nanocomposites for Supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-Dimensional NiCo2O4@NiWO4 Core–Shell Nanowire Arrays for High Performance Supercapacitors. J. Mater. Chem. A Mater. 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Reddy, A.L.M.; Gowda, S.R.; Shaijumon, M.M.; Ajayan, P.M. Hybrid Nanostructures for Energy Storage Applications. Adv. Mater. 2012, 24, 5045–5064. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, T.; Ma, Y. Advancements in the Utilization of Nanocarbon Sphere Composites in Supercapacitor. Adv. Compos. Hybrid. Mater. 2025, 8, 103. [Google Scholar] [CrossRef]
- Younas, U.; Imtiaz, S.; Naz, S.; Anjam, M.N.; Saleem, A.; Ali, A.; Ali, F.; Khan, M.I.; Ashraf, A.; Pervaiz, M. Facile Synthesis of Zinc Aluminate (ZnAl2O4) Decorated CNTs as a Multi-Functional Material for Energy Storage and Photocatalysis. Diam. Relat. Mater. 2025, 157, 112557. [Google Scholar] [CrossRef]
- Jung, M.; Sivakumar, P.; Park, H.S. Carbon Nanotube Branch-Grown Nickel Nanoparticles/Graphene Composites for a High-Capacitance Electrode. J. Phys. Energy 2023, 5, 025005. [Google Scholar] [CrossRef]
- Bhagwan, J.; Han, J.I. Facile Synthesis of CdO/CdFe2O4/MWCNT Nanocomposite for Aqueous Hybrid Supercapacitor. J. Energy Storage 2025, 118, 116232. [Google Scholar] [CrossRef]
- Wu, X.; Han, Z.; Zheng, X.; Yao, S.; Yang, X.; Zhai, T. Core-Shell Structured Co3O4@NiCo2O4 Electrodes Grown on Flexible Carbon Fibers with Superior Electrochemical Properties. Nano Energy 2017, 31, 410–417. [Google Scholar] [CrossRef]
- Hwang, S.-G.; Ryu, S.-H.; Yun, S.-R.; Ko, J.M.; Kim, K.M.; Ryu, K.-S. Behavior of NiO–MnO2/MWCNT Composites for Use in a Supercapacitor. Mater. Chem. Phys. 2011, 130, 507–512. [Google Scholar] [CrossRef]
- Roguai, S.; Djelloul, A. Gold Coated Vertically Aligned Carbon Nanotubes as Electrode for Electrochemical Capacitors. Thin Solid. Film. 2023, 777, 139894. [Google Scholar] [CrossRef]
- Vignal, T.; Banet, P.; Pinault, M.; Lafourcade, R.; Descarpentries, J.; Darchy, L.; Hauf, H.; Reynaud, C.; Mayne-L’Hermite, M.; Aubert, P.-H. Electropolymerized Poly(3-Methylthiophene) onto High Density Vertically Aligned Carbon Nanotubes Directly Grown on Aluminum Substrate: Application to Electrochemical Capacitors. Electrochim. Acta 2020, 350, 136377. [Google Scholar] [CrossRef]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/Vertically-Aligned Carbon Nanotubes as Negative Electrode for Asymmetric Supercapacitor in Neutral Aqueous Electrolyte. J. Colloid. Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Park, S.K.; Sure, J.; Vishnu, D.S.M.; Jo, S.J.; Lee, W.C.; Ahmad, I.A.; Kim, H.-K. Nano-Fe3O4/Carbon Nanotubes Composites by One-Pot Microwave Solvothermal Method for Supercapacitor Applications. Energies 2021, 14, 2908. [Google Scholar] [CrossRef]
- Abouali, S.; Akbari Garakani, M.; Xu, Z.-L.; Kim, J.-K. NiCo2O4/CNT Nanocomposites as Bi-Functional Electrodes for Li Ion Batteries and Supercapacitors. Carbon 2016, 102, 262–272. [Google Scholar] [CrossRef]
- Arsyad, A.; Saaid, F.I.; Najihah, M.Z.; Hisam, R.; Woo, H.J.; Tseng, T.-Y.; Winie, T. CNT-RGO-Wrapped FeCo2O4 for Asymmetric Supercapacitor with Enhanced Power Density and Rate Capability. J. Mater. Sci. Mater. Electron. 2024, 35, 2114. [Google Scholar] [CrossRef]
- Karaman, O.; Afşin Kariper, İ.; Korkmaz, S.; Karimi-Maleh, H.; Usta, M.; Karaman, C. Irradiated RGO Electrode-Based High-Performance Supercapacitors: Boosting Effect of GO/RGO Mixed Nanosheets on Electrochemical Performance. Fuel 2022, 328, 125298. [Google Scholar] [CrossRef]
- Arshad, N.; Usman, M.; Adnan, M.; Ahsan, M.T.; Rehman, M.R.; Javed, S.; Ali, Z.; Akram, M.A.; Demopoulos, G.P.; Mahmood, A. Nanoengineering of NiO/MnO2/GO Ternary Composite for Use in High-Energy Storage Asymmetric Supercapacitor and Oxygen Evolution Reaction (OER). Nanomaterials 2022, 13, 99. [Google Scholar] [CrossRef]
- Elessawy, N.A.; El Nady, J.; Wazeer, W.; Kashyout, A.B. Development of High-Performance Supercapacitor Based on a Novel Controllable Green Synthesis for 3D Nitrogen Doped Graphene. Sci. Rep. 2019, 9, 1129. [Google Scholar] [CrossRef]
- Xu, S.-W.; Zhang, M.-C.; Zhang, G.-Q.; Liu, J.-H.; Liu, X.-Z.; Zhang, X.; Zhao, D.-D.; Xu, C.-L.; Zhao, Y.-Q. Temperature-Dependent Performance of Carbon-Based Supercapacitors with Water-in-Salt Electrolyte. J. Power Sources 2019, 441, 227220. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Li, A.; Li, H.; Cui, D.; Dong, M.; Lin, J.; Fan, J.; Zhang, J.; Hou, H.; et al. Advanced Porous Hierarchical Activated Carbon Derived from Agricultural Wastes toward High Performance Supercapacitors. J. Alloys Compd. 2020, 820, 153111. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Yan, L.; Gong, Q.; Chen, G.; Yang, L.; Wu, Q.; Wang, X.; Hu, Z. The Composite-Template Method to Construct Hierarchical Carbon Nanocages for Supercapacitors with Ultrahigh Energy and Power Densities. Small 2022, 18, e2107082. [Google Scholar] [CrossRef]
- Kariper, İ.A.; Korkmaz, S.; Karaman, C.; Karaman, O. High Energy Supercapacitors Based on Functionalized Carbon Nanotubes: Effect of Atomic Oxygen Doping via Various Radiation Sources. Fuel 2022, 324, 124497. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Y.; Wang, H.; Zhao, P.; Liu, W.; Wang, Y.; Yang, J. N-Doped Porous Carbon Anchoring on Carbon Nanotubes Derived from ZIF-8/Polypyrrole Nanotubes for Superior Supercapacitor Electrodes. Appl. Surf. Sci. 2018, 457, 1018–1024. [Google Scholar] [CrossRef]
- Lal, M.S.; Badam, R.; Matsumi, N.; Ramaprabhu, S. Hydrothermal Synthesis of Single-Walled Carbon Nanotubes/TiO2 for Quasi-Solid-State Composite-Type Symmetric Hybrid Supercapacitors. J. Energy Storage 2021, 40, 102794. [Google Scholar] [CrossRef]
- Mendoza, R.; Al-Sardar, M.; Oliva, A.I.; Robledo-Trujillo, G.; Rodriguez-Gonzalez, V.; Zakhidov, A.; Oliva, J. Improving the Electrochemical Performance of Flexible Carbon Nanotubes Based Supercapacitors by Depositing Ni@TiO2:W Nanoparticles on Their Anodes. J. Phys. Chem. Solids 2021, 155, 110128. [Google Scholar] [CrossRef]
- Isacfranklin, M.; Rathinam, Y.; Ganesan, R.; Velauthapillai, D. Direct Growth of Binder-Free CNTs on a Nickel Foam Substrate for Highly Efficient Symmetric Supercapacitors. ACS Omega 2023, 8, 11700–11708. [Google Scholar] [CrossRef]
- Ojeda, L.; Oliva, J.; Encinas, A.; Mtz-Enriquez, A.I.; del Castillo, P.C.H.; Quintero, J.P.; Muñoz-Sandoval, E. Increasing the Capacitance of Flexible Supercapacitors by Adding Spongy-like CNTs on Their Electrodes and Application of CNTs to Remove Oil/Microplastics from Tap Water. J. Appl. Electrochem. 2025. [Google Scholar] [CrossRef]
- Nepal, M.; Gudavalli, G.S.; Dhakal, T.P. MnO2 Nanoflowers Electrodeposited on Vertically Aligned CNTs as Binder-Free Electrodes for High-Rate Supercapacitors. ACS Omega 2025, 10, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, F.; Wang, G.; Xiao, M.; Meng, Y.; Zhang, Y. Co3O4/Carbon Nano-Onions Composite as Supercapacitor Electrode and Its Excellent Electrochemical Performance. Int. J. Mater. Res. 2018, 109, 873–879. [Google Scholar] [CrossRef]
- Siemiaszko, G.; Breczko, J.; Hryniewicka, A.; Ilnicka, A.; Markiewicz, K.H.; Terzyk, A.P.; Plonska-Brzezinska, M.E. Composites Containing Resins and Carbon Nano-Onions as Efficient Porous Carbon Materials for Supercapacitors. Sci. Rep. 2023, 13, 6606. [Google Scholar] [CrossRef]
- Jiang, K.; Gerhardt, R.A. Fabrication and Supercapacitor Applications of Multiwall Carbon Nanotube Thin Films. C 2021, 7, 70. [Google Scholar] [CrossRef]
- Bu, F.; Zhou, W.; Xu, Y.; Du, Y.; Guan, C.; Huang, W. Recent Developments of Advanced Micro-Supercapacitors: Design, Fabrication and Applications. npj Flex. Electron. 2020, 4, 31. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, G.; Shi, X.; Qiao, Y.; Liu, J.; Liu, H.; Ganesh, A.; Li, L. Direct Graphene-Carbon Nanotube Composite Ink Writing All-Solid-State Flexible Microsupercapacitors with High Areal Energy Density. Adv. Funct. Mater. 2020, 30, 1907284. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Cao, Y.; Du, J.; Gu, J.; Liu, Q. Building Porous yet Robust Binders towards Fast-Rate Flexible Supercapacitors Based on Activated Carbons. Electrochim. Acta 2025, 538, 147030. [Google Scholar] [CrossRef]
- Boruah, B.D.; Nandi, S.; Misra, A. Layered Assembly of Reduced Graphene Oxide and Vanadium Oxide Heterostructure Supercapacitor Electrodes with Larger Surface Area for Efficient Energy-Storage Performance. ACS Appl. Energy Mater. 2018, 1, 1567–1574. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; An, Z.; Wang, Z.; Toda, M.; Ono, T. Constructing In-Chip Micro-Supercapacitors of 3D Graphene Nanowall/Ruthenium Oxides Electrode through Silicon-Based Microfabrication Technique. J. Power Sources 2018, 401, 204–212. [Google Scholar] [CrossRef]
- Li, J.; Sollami Delekta, S.; Zhang, P.; Yang, S.; Lohe, M.R.; Zhuang, X.; Feng, X.; Östling, M. Scalable Fabrication and Integration of Graphene Microsupercapacitors through Full Inkjet Printing. ACS Nano 2017, 11, 8249–8256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Wu, Y.; Chen, Y.; Zhang, Y.; Lai, W.; Huang, W. Inkjet-Printed High-Performance Flexible Micro-Supercapacitors with Porous Nanofiber-Like Electrode Structures. Small 2019, 15, 1901830. [Google Scholar] [CrossRef] [PubMed]
- Vyas, A.; Hajibagher, S.Z.; Méndez-Romero, U.; Thurakkal, S.; Li, Q.; Haque, M.; Azega, R.K.; Wang, E.; Zhang, X.; Lundgren, P.; et al. Spin-Coated Heterogenous Stacked Electrodes for Performance Enhancement in CMOS-Compatible On-Chip Microsupercapacitors. ACS Appl. Energy Mater. 2022, 5, 4221–4231. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Neff, T.; Day, A.; Krueger, A. Scalable Fabrication of Flexible Supercapacitor Electrodes Using Sustainable Water-Based Onion-Like Carbon Inks. Batter. Supercaps 2024, 7, e202400203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).