1. Introduction
Over the past several decades, significant advancements have taken place in the high-tech domain of glass materials exhibiting electronic conductivity. Among these, tellurite, vanadate, and bismuthate glasses have attracted scientific attention due to their distinctive structural and functional behavior. Tellurite glasses are widely acknowledged for their suitability in optical technologies, a result of their notable features such as a high refractive index, strong transmission in the infrared region, robust chemical stability, and a comparatively straightforward melting process. Meanwhile, vanadate and bismuthate glasses are especially compelling due to their inherent semiconducting and electrical properties [
1,
2,
3,
4,
5,
6,
7].
Vanadate glasses primarily consist of VO
5 and VO
4 polyhedra. Structural variations arise from the incorporation of different metal oxides (M
n+Oₘ), which either occupy interstitial positions between vanadate chains, affecting the V=O bond vibrations, or substitute directly into the vanadate framework to form V–O–M linkages. This interaction alters the local symmetry and bond strengths, causing shifts in vibrational frequencies. In glasses with increasing modifier content, VO
5 units gradually transform into VO
4 tetrahedra, signifying a shift from vanadyl-type structures toward metavanadate chain networks. Similar mechanisms apply to tellurite and bismuthate glasses, where the high polarizability and covalency of Te
4+ and Bi
3+ facilitate their integration into the glass matrix, influencing both the structural rigidity and electronic properties. These glass systems exhibit a complex balance between localized structural units and extended network connectivity, contributing to their desirable optical and semiconducting characteristics [
8].
Tellurite glasses, primarily composed of TeO
2, exhibit a distinctive structure dominated by TeO
4 trigonal bipyramids and TeO
3 trigonal pyramids. The TeO
4 units form the matrix of the glass network through corner-sharing with oxygen atoms, while TeO
3 units typically emerge as a result of modifier addition or non-bridging oxygen formation. The structural flexibility between these units contributes to the glasses’ low phonon energy, high refractive index, and excellent infrared transmittance. The presence of lone-pair electrons on Te
4+ ions plays a critical role in shaping the glass structure, introducing asymmetry and influencing the electronic polarizability. These features not only facilitate the incorporation of various functional dopants but also make tellurite glasses particularly attractive for applications in nonlinear optics, infrared photonics, and optical amplifiers. Furthermore, their relatively low melting temperatures and high chemical durability enhance their processability and suitability for advanced photonic device fabrication [
9].
The inclusion of Bi
2O
3 in glass matrices significantly influences their structural and optical characteristics, primarily due to the large atomic mass and high electronic polarizability of Bi
3+ ions. These factors lead to an elevated refractive index, which enhances the material’s suitability for use in photonic systems. From a structural standpoint, Bi
2O
3 can participate in the network as both a former and a modifier, facilitating the formation of BiO
6 coordination units. This incorporation tends to disrupt the connectivity of the glass network, increasing the population of non-bridging oxygens and thereby reducing the degree of polymerization. Such changes are evident in FTIR spectral features and are typically correlated with a narrowing of the optical band gap, which allows for broader absorption in the visible and near-infrared regions. Additionally, the introduction of Bi
2O
3 lowers the phonon energy of the glass, which contributes to improved infrared transparency—an essential property for mid-IR photonic and laser applications. The presence of stereochemically active lone-pair electrons on Bi
3+ may also enhance the nonlinear optical response of the glass. Beyond these optical benefits, Bi
2O
3 also imparts improved thermal stability and higher density, characteristics that are advantageous for applications in optoelectronic devices, protective optical components, and infrared transmission systems [
10].
The aim of this study is to synthesize and characterize glasses within the TeO2–V2O5–Bi2O3 system, with particular emphasis on elucidating the influence of structural units such as TeO4/TeO3+1/TeO3, VO5/VO4, and BiO6 on the dielectric behavior. The novelty of this work lies in establishing clear correlations between the local structural configurations and the dielectric response of these multicomponent glasses, providing new insights into the structure–property relationships governing their functional performance.
2. Materials and Methods
Two series with compositions: Series 1: (90 − x)TeO
2-xV
2O
5-10Bi
2O
3, where x = 10, 20, 30, 40, 50 mol %, and Series 2: (80 − x)TeO
2-xV
2O
5-20Bi
2O
3, where x = 20, 30, 40 mol % were synthesized (
Table 1). The initial reagents TeO
2 (Alfa Aesar (Thermo scientific), ThermoFisher, Kandel, Germany, 99.99%), Bi
2O
3 (Acros Organics BV (Thermo Fisher Scientific), Geel, Belgium, 99.7%) and V
2O
5 (Alfa Aesar (Thermo scientific), ThermoFisher, Kandel, Germany 99.2%) were homogenized in an agate mortar, after which the resulting batches were melted at 950 °C for 20 min in an electric furnace. The melts were stirred several times during heating and then melt-quenched and pressed to a thickness of 1~2 mm. The experimental density of the glasses was measured using the Archimedes principle, using a Mettler analytical balance Toledo New Classic ME 104 (Mettler Toledo, Greifensee, Switzerland), equipped with a kit for determining the density of solid samples and distilled water as an immersion liquid. For each composition, the density is measured at least ten times in at least three different samples. From the density results obtained, the molar volume and oxygen packet density were calculated. Infrared spectra were recorded in the 4000–400 cm
−1 range using the Varian 600-IR FT-IR spectrometer (Melbourne, Australia). The samples for these measurements were prepared in the form of KBr disks. The accuracy of absorption maxima is ±3 cm
−1. Raman spectra were measured with the Raman Renishaw inVia microscope (Glocestershire, UK) with Leica DM2700 M (Leica Microsystems CMS GmbH, Wetzlar, Germany). The 633 nm line of a helium-neon laser was used for excitation. To avoid local overheating, the laser power used has been reduced to 3.2 mW. An X50 lens was used to focus the laser beam and collect the scattered light in a backscatter configuration. The diameter of the illuminated spot on the surface of the specimen is about 3 μm. The studied spectral range is 90–1280 cm
−1. The accuracy of the wavenumber is 1 cm
−1. The photoelectron spectra were recorded on an ESCALAB MK II X-RAY photoelectron spectrometer (Thermoscientific, VgScientific, East Grinstead, England) with a non-monochromatic X-ray source Al under a vacuum above 10
−7 Pa at a 45-degree take-off angle and a total instrumental resolution of 1 eV. The bonding energies (BE) were determined using the C 1s line (of extra carbon) as a reference with an energy of 285.0 eV. The accuracy of the measured 18 19 connection energy is 0.2 eV. C 1s, O 1s, Te 3d, V 2p and Bi 4f photoelectron lines were recorded, and exploratory scanning was performed. The surface concentrations of the constituent elements were calculated from the peak area after subtracting the Shirley-type background. The dielectric characteristics were measured with the Zanner IM6 Impedance spectrometer (Zahner-Elektrik GmbH & Co. KG, Kronach, Germany) equipped with a standard sample holder with a two-electrode method. The samples were polished and plane-parallel, and silver electrodes were applied. Measurements of all samples were taken in the frequency range of 100 Hz–1 MHz at room temperature.
4. Discussion
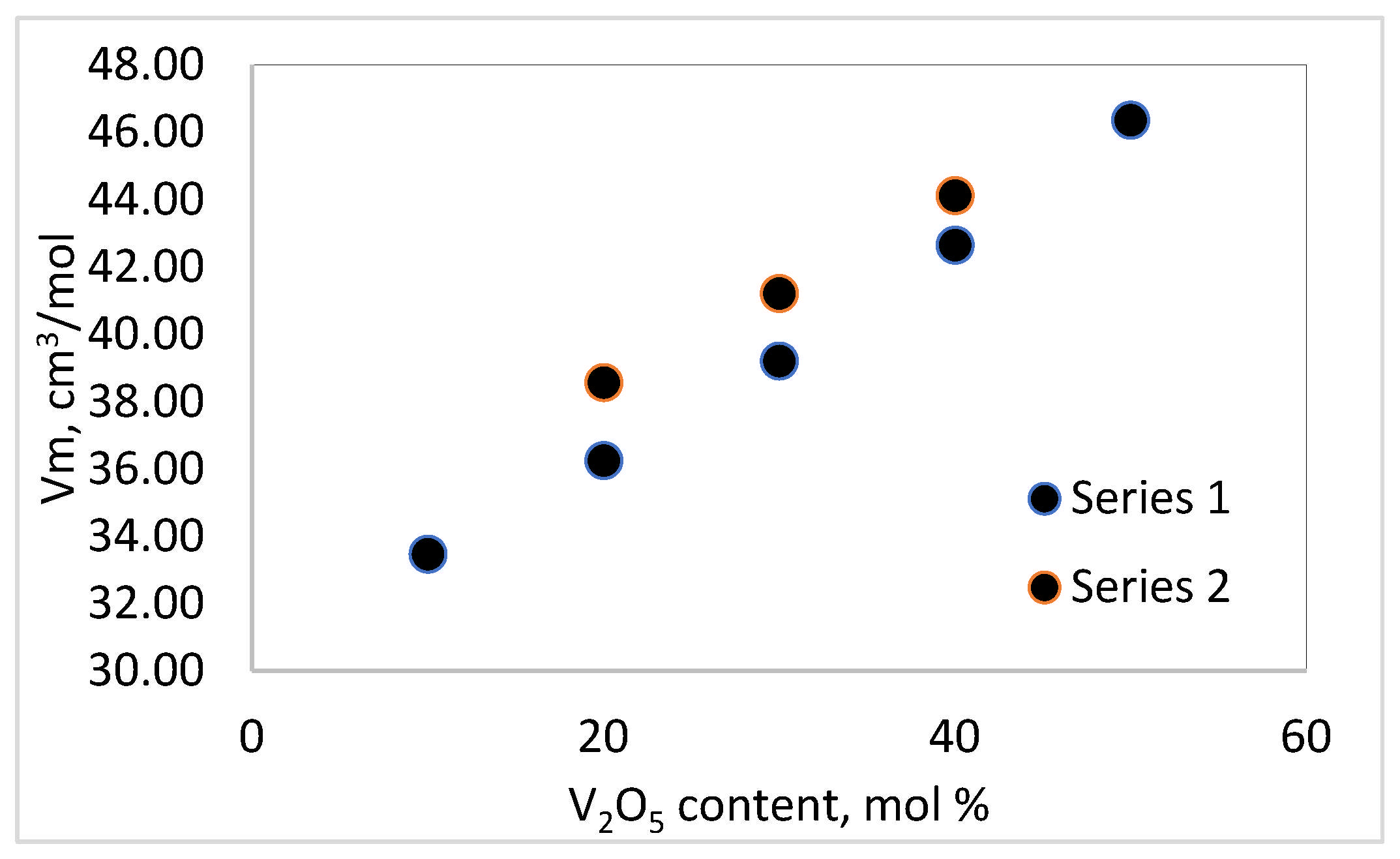
The results obtained for the density of the samples are consistent with the fact that the atomic mass of V (50.94 u.) is lower than that of Te (127.6 u.) and Bi (208.98 u.), and the densities of pure TeO
2, V
2O
5 and Bi
2O
3 are 5.67 g/cm
3, 3.36 g/cm
3 and 8.9 g/cm
3, respectively. The molar volume and the oxygen packing density increase with the increase in the content of V
2O
5 and the decrease in TeO
2. The molar volume, V
m, expresses the free space and formation of non-bridging oxygens (NBOs) in the glass structure, while the oxygen packet density (OPD) is sensitive to the tightness of the structure as a whole. An increase in molar volume implies an increase in free space and the formation of non-bridging oxygens (NBOs) in the glass structure, and an increase in oxygen packet density is due to the formation of a stronger and highly cross-linked network, resulting in a more tightly packed amorphous network [
11].
The analysis of the IR spectra was made based on the characteristic vibrations of metal-oxygen units found in various binary and ternary crystalline and amorphous materials with similar composition.
Dimitrov et al. [
12] investigated the structure of the V
2O
5-Bi
2O
3 binary system by IR spectroscopy. They found that with an increase in the content of Bi
2O
3, the characteristic band at 1020 cm
−1, associated with the vibration of the double V=O bond of VO
5, the trigonal bipyramid disappears. This can be explained by the mechanism proposed by Dimitriev et al. [
8]. According to them, Bi
3+ cations directly attack the V=O bond as a result, of which the polyhedron is transformed into a VO
4 group. The appearance of a band at 950 cm
−1 is attributed to the vibrations of free VO
2 groups of VO
4 tetrahedra forming V-O-V bridges. The bands of these V-O-V bridges appear in the region of 840–820 cm
−1. The absorption bands observed in the 760–740 cm
−1 region are assigned to the triply degenerate bending vibration νₑ(F) of isolated VO
4 tetrahedra, where each VO
4 unit exists as a distinct, non-bridging structural entity within the glass network [
12].
Dimitriev et al. [
13] investigated the IR spectra of 2TeO
2.V
2O
5 (2TV) as crystal and glass and found that the band at 950 cm
−1 is associated with the valence vibration of the double non-bridge V=O bonds in VO
5 trigonal bipyramids, and the band at 680–685 cm
−1 in the spectra of TeO
2-V
2O
5 glasses can be attributed to the valence asymmetric vibrations νdasTeO
3 of TeO
3 groups. For comparison, the band associated with the vibrations of the Te-O bond from the TeO
4 polyhedron in pure tellurite glass is located at 654 cm
−1 [
14].
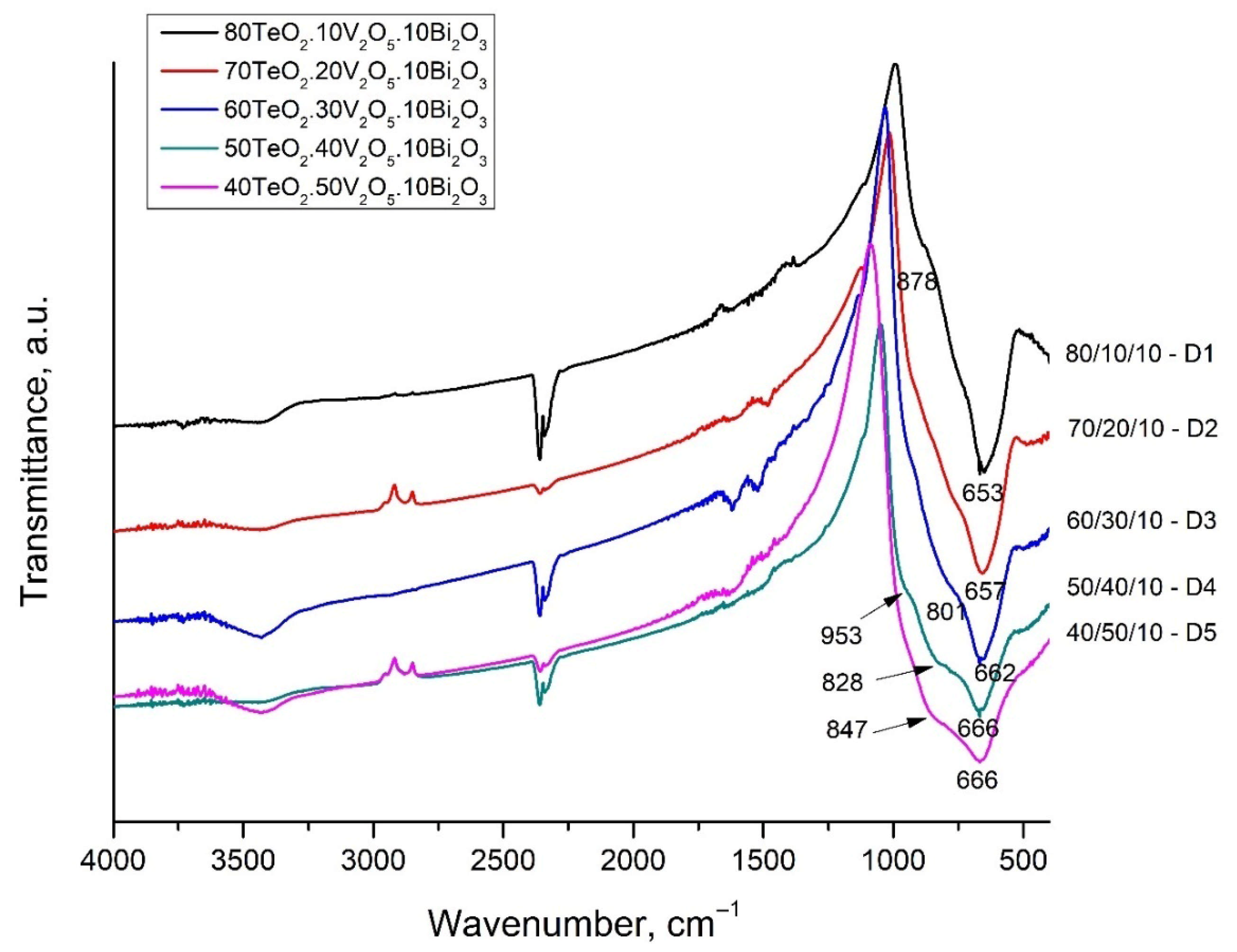
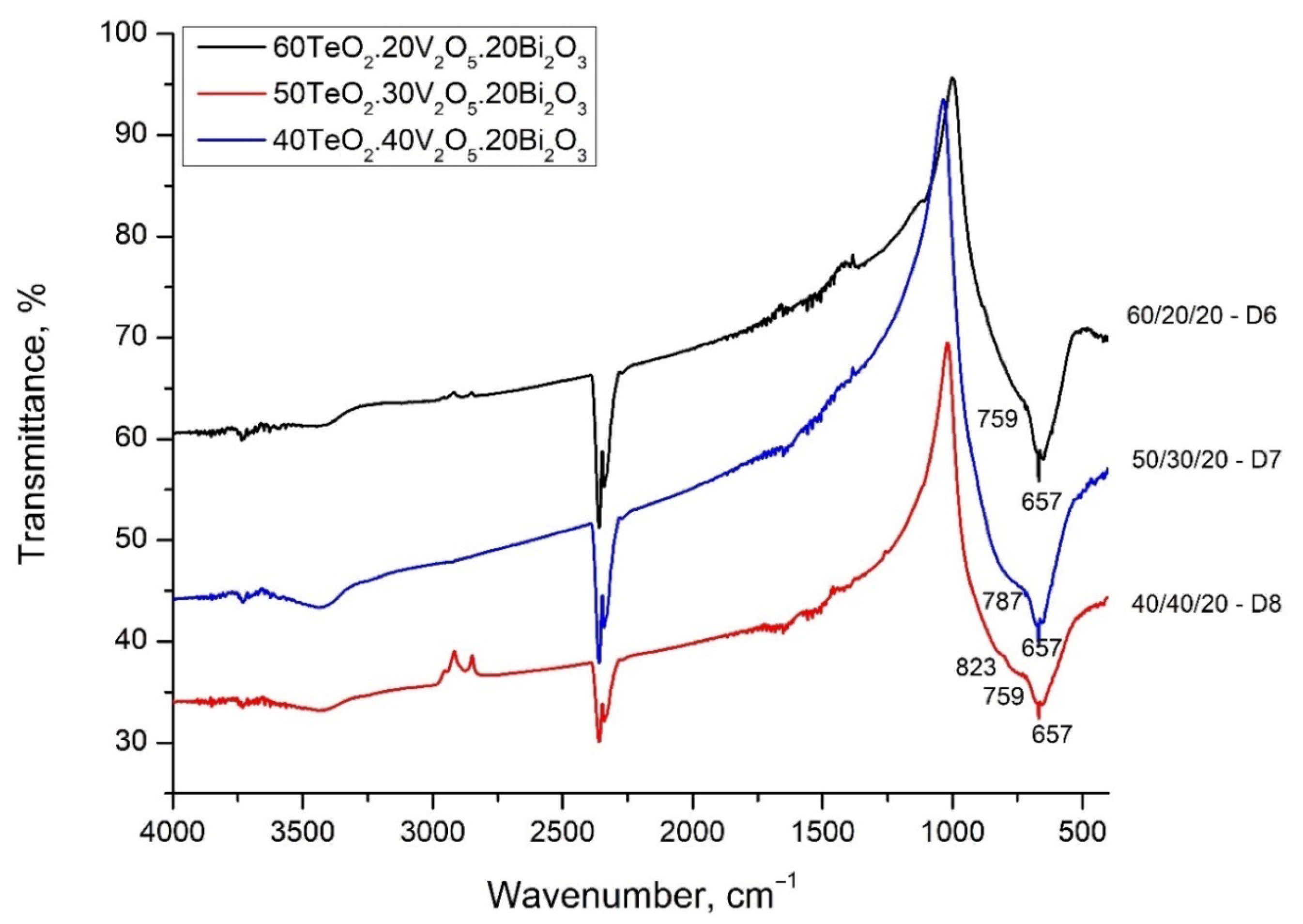
The observed displacement of the band at 653–666 cm
−1 can be explained by the transformation of the tellurite groups. The band at 653 cm
−1 in composition 80TeO
2.10V
2O
5.10Bi
2O
3 is associated with asymmetric valence vibrations of symmetric TeO
4 trigonal bipyramids and asymmetric valence vibrations of deformed TeO
4 groups (TeO
3+1), ν
asTeO
2ax. The last groups are referred to as TeO
3+1, i.e., the tellurite atom forms one bridge, two non-bridge bonds, thus presenting itself as a TeO
3 trigonal pyramid, but there is also a fourth bond, which is weak and strongly elongated. This structural group (TeO
3+1) is also distinguished by very high oxygen polarizability and optical basicity [
12]. Dimitrov and Komatsu [
15,
16] found that optical basicity in tellurite groups decreases in the order TeO
3+1 > TeO
4 > TeO
3. With a decrease in the content of TeO
2 and an increase in the content of V
2O
5, a significantly larger amount of symmetrical TeO
4 trigonal bipyramids is formed compared to the deformed TeO
3+1, as well as symmetrical TeO
3 trigonal pyramids.
Glasses with a low V2O5 content do not show a pronounced band, but two barely noticeable arms, which become better pronounced with increasing V2O5 content—at about 800–847 cm−1 and at 953 cm−1. The band at 953 cm−1 could be assigned to the vibrations of free VO2 groups of VO4 tetrahedra forming V-O-V bridges. The band of these V-O-V bridges appear in the region of 840–820 cm−1. The bands at about 760 cm−1 are attributed to the triple-degenerate valence vibrations of isolated VO4.
Tagiara et al. [
14] investigated the close order of binary and three-component tellurite glasses. In the spectrum of TeO
2 glass, the presence of a strong band at 660 cm
−1, a wide band at 700–850 cm
−1 and low-frequency vibrations at 440 and 490 cm
−1 are observed. The band at 700–850 cm
−1 corresponds to the valence vibrations of the Te-O-Te bridges connecting the TeO
4 trigonal bipyramids, as well as the vibration of the TeO
4 trigonal bipyramid itself, while the deformation vibrations of O-Te-O and Te-O-Te are active at about 440 and 490 cm
−1, respectively [
14].
In the spectrum of glass with a composition of 80TeO2.10V2O5.10Bi2O3—D1, this band is strongly pronounced and even more intense than the strip at 660 cm−1 (TeO4 trigonal bipyramids). With an increase in the content of V2O5, the weak, elongated bond of the TeO3+1 polyhedra breaks and isolated TeO3 trigonal pyramids are formed and the position of the band shifts to about 760 cm−1. The intensity of all stripes associated with the vibrations of tellurite polyhedra and bonds decreases with increasing V2O5 content.
The band at 902–914 cm
−1 in the Raman spectra of the glasses is attributed to the valence vibrations of V-O-V by VO
4 tetrahedra [
16]. The double degenerate bending vibration of the VO
4 group is active at 333–339 cm
−1 [
17].
Figure 6 shows the electron spectra in the region of 600–570 eV. This area includes the doublets attributed to the Te 3d
5/2 and Te3d
3/2 electrons in the spectra. A chemical shift in the binding energies of 575.43–577.08 eV for Te 3d
5/2 and from 585.86–587.47 eV for Te 3d
3/2 was observed, with energy differences between the two peaks of 10.43 to 10.39 eV, respectively. The tellurite ion shows no change in its oxidation state. The observed chemical shift is probably due to the change in the tellurite polyhedron.
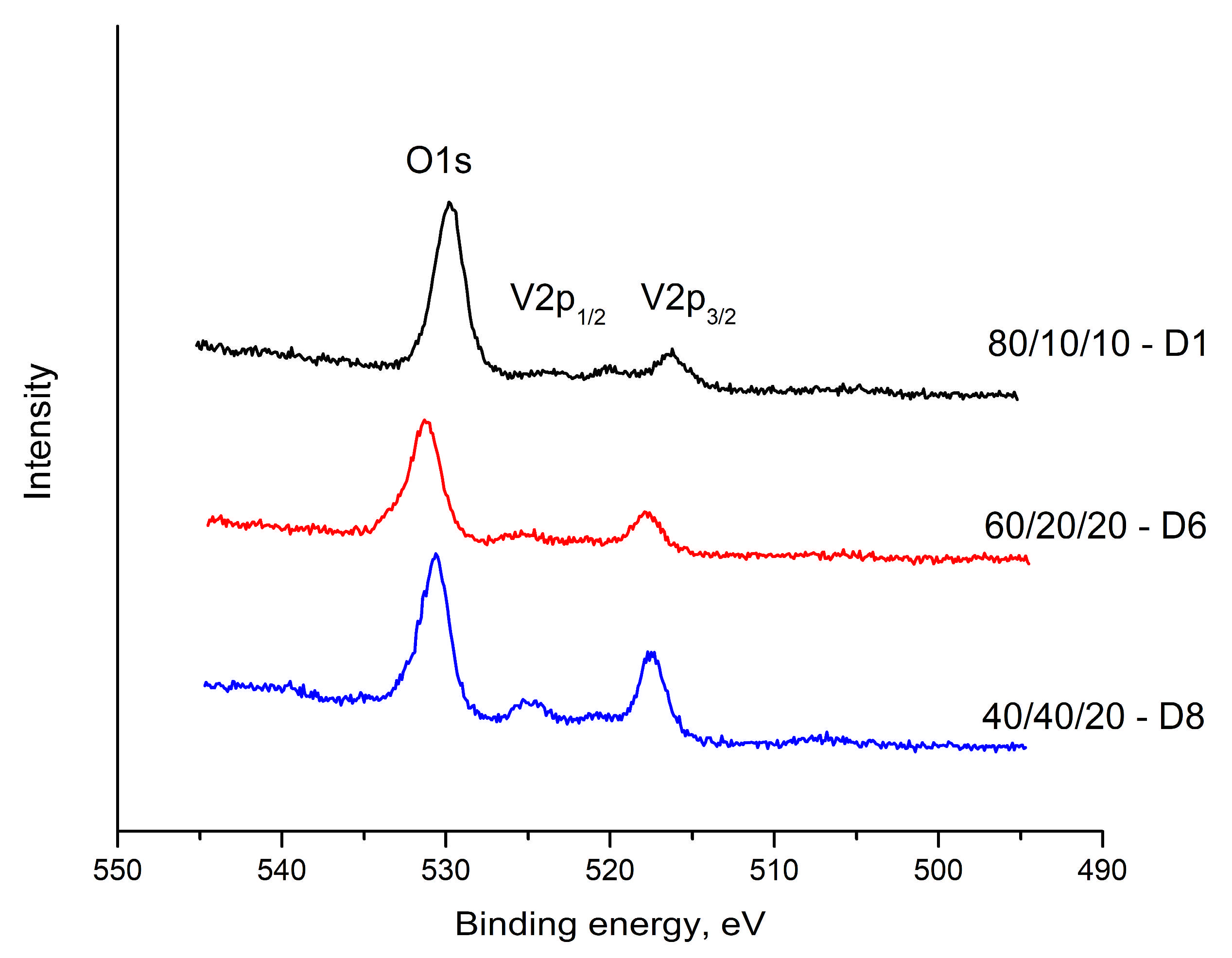
A single, well-defined peak is observed in the O1s region of the spectrum 531.20–528.59 eV (
Figure 7). A change in intensity is noteworthy, as well as the presence of a chemical shift in this peak. The chemical displacement of the O1s binding energy is a function of the ionic-covalent nature of the metal-oxygen chemical bond. As the binding energy of O 1s decreases, oxygen polarizability and the basic character of oxides increase.
The peaks at 517.81–516.36 eV and at 525.23–523.24 eV in the electron spectrum of vanadium are attributed to V 2p
1/2 and V 2p
3/2, respectively. These binding energy values are assigned to V
5+ and V
4+, respectively (
Figure 7).
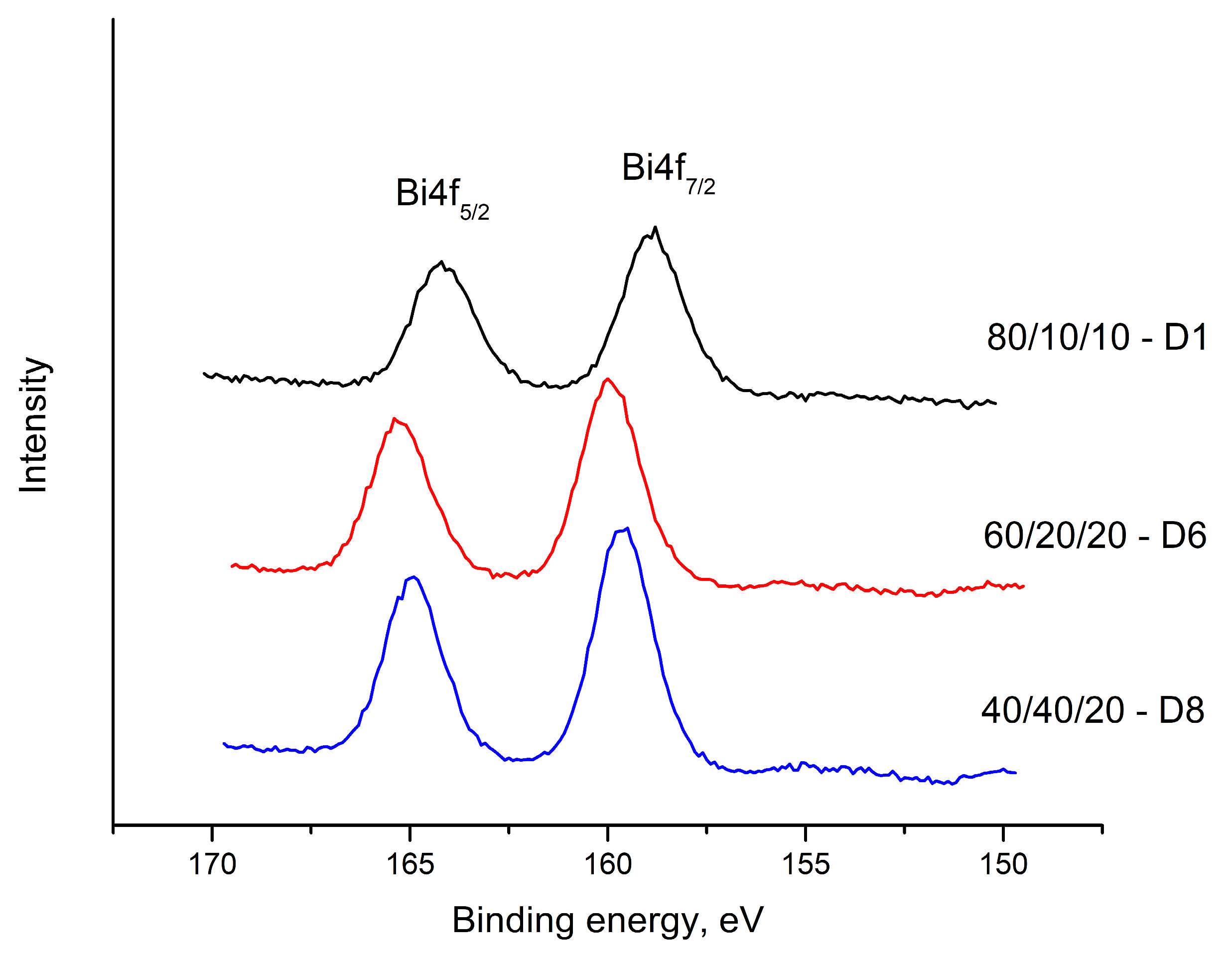
In the region of 230–240 eV, Bi 4f spin-orbital cleavage is observed, as well as chemical displacement of the binding energies, from 159.69–158.87 eV to Bi 4f7/2 and from 164.22–164.95eV to Bi 4f5/2, with an energy difference of 4.53eV to 6.08eV, respectively. The intensity of the peaks decreases with an increase in the content of V
2O
5. This energy range is characteristic of Bi
3+ (
Figure 8).
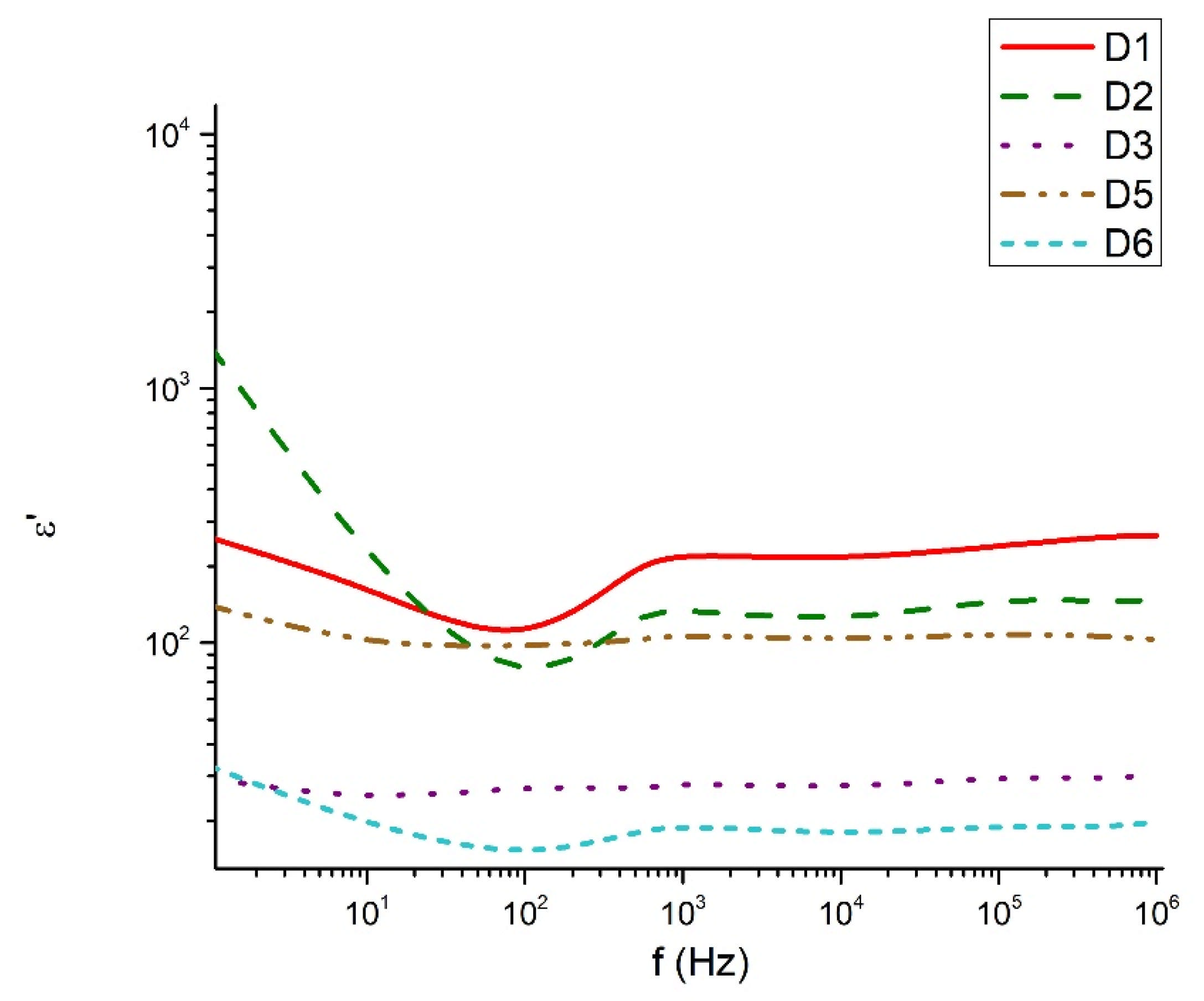
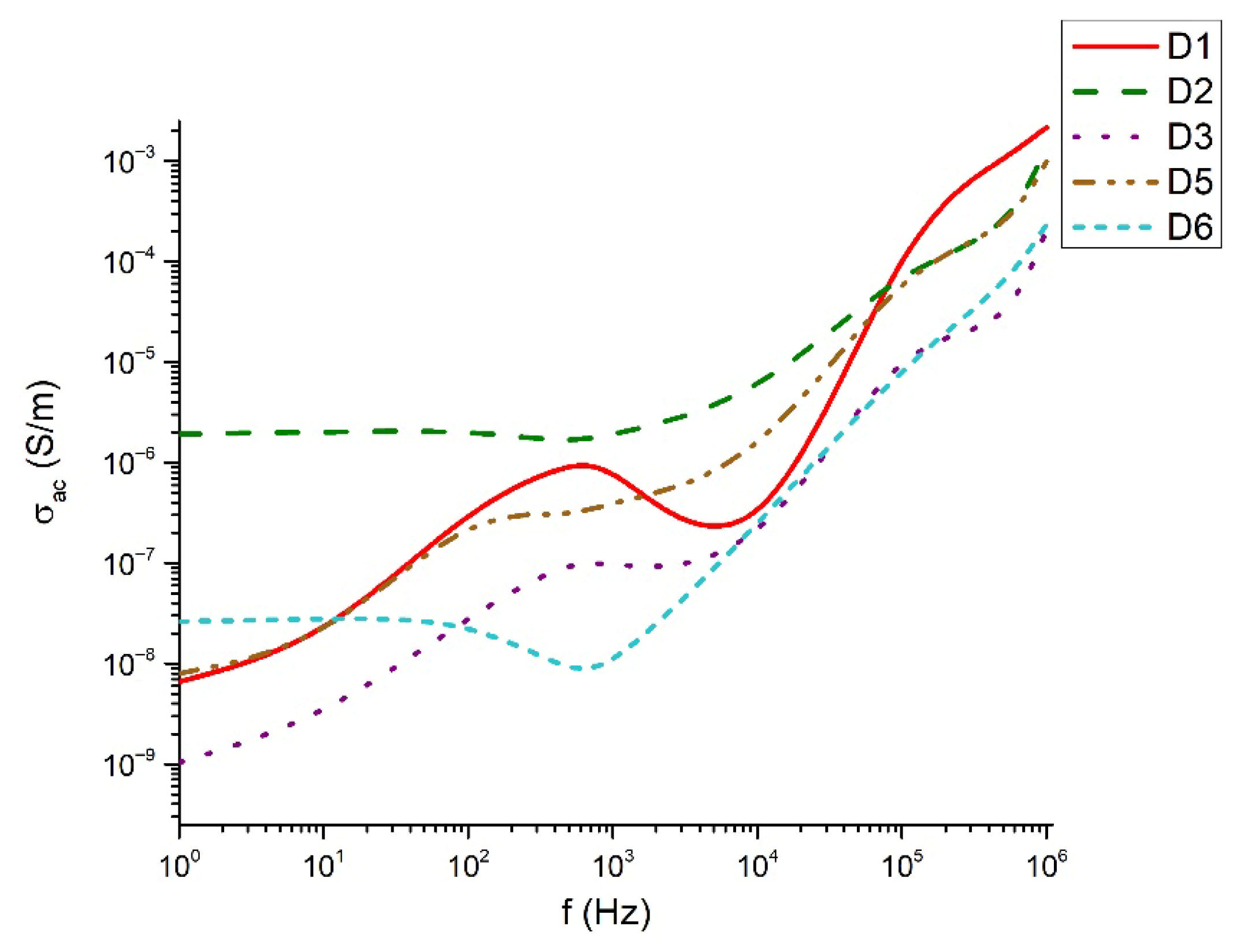
The dielectric behavior of the studied tellurite–vanadate–bismuth glasses is closely linked to their structural features. Most samples exhibit similar real permittivity (~3.102) across the measured frequency range, reflecting the dominant role of the tellurite network in establishing the base dielectric response. However, the compositions 60TeO2-30V2O5-10Bi2O3 (D3) and 50TeO2-40V2O5-10Bi2O3 (D4) show elevated permittivity values at low frequencies (<10 Hz). This enhancement is associated with the presence of highly polarized TeO3+1 units and VO4 tetrahedra, which may generate localized regions of uncompensated charges and contribute to increased dielectric losses. At high frequencies (>1 kHz), all samples exhibit a steady decrease in dielectric losses, remaining below 0.3. This behavior reflects the inability of large, highly polarizable structural units, such as TeO4 and TeO3+1 polyhedra, to follow rapid field oscillations, leading to reduced polarization and lower energy dissipation. In the intermediate frequency range (100 Hz–1 kHz), samples 60TeO2-30V2O5-10Bi2O3 (D3) and 60TeO2-20V2O5-20Bi2O3 (D6) show pronounced dielectric losses, indicating strong relaxation processes arising from structural heterogeneities, such as elongated Te–O bonds in TeO3+1 units and V–O–V linkages in VO4 tetrahedra. The variation in dielectric response can be rationalized in terms of network connectivity and polarizability. Glasses with higher V2O5 content (>40 mol%) form more symmetric TeO4 bipyramids and VO4 tetrahedra, increasing network rigidity and reducing the contribution of weakly bonded TeO3+1 units to low-frequency dielectric dispersion. In contrast, glasses with significant TeO3+1 populations (e.g., D1, D5) display reduced dielectric losses and more stable real permittivity due to the formation of isolated TeO3 trigonal pyramids, which limit charge accumulation and local polarization effects. The electrical conductivity data further support the structural interpretation. Conductivity remains negligible in the 100–2 × 104 Hz range, consistent with the insulating nature of the cross-linked tellurite-vanadate network. Beyond this frequency, a sharp increase in conductivity occurs, reflecting the frequency-dependent polarization of TeOn and VOn units and the activation of localized charge transport associated with NBOs. Thus, the dielectric properties of these glasses are strongly governed by the interplay between structural units (TeO4, TeO3+1, VO4), network connectivity, and non-bridging oxygens. Tailoring the relative contents of TeO2, V2O5, and Bi2O3 allows for precise control over the polarization mechanisms, dielectric losses, and permittivity, providing a clear structural rationale for the observed frequency-dependent behavior.
5. Conclusions
Two series of glasses were synthesized: Series 1: (90 − x)TeO2-xV2O5-10Bi2O3, where x = 10, 20, 30, 40, 50 mol %, and Series 2: (80 − x)TeO2-xV2O5–20Bi2O3, where x = 20, 30, 40 mol %.
As the content of V2O5 and Bi2O3 increases, the number of non-bridge oxygens (NBOs) and the free space of the glasses increase. In parallel, with an increase in the content of V2O5, a stronger and cross-linked structure is obtained, and the addition of Bi2O3 worsens this cross-linking in glasses with a high content of TeO2 and a content of V2O5 below 40 mol %. Above 40 mol % V2O5, the addition of Bi2O3 does not indicate such deterioration of the amorphous network.
For glasses with a content of V2O5 up to 40 mol%, the dominant glass-forming agent is TeO2, and above 40 mol %, the dominant glass-forming agent is V2O5.
Glass with the composition 80TeO2.10V2O5.10Bi2O3 (D1) is composed of highly polarized, deformed TeO4 trigonal bipyramids, as well as isolated VO4 tetrahedra. The presence of Te4+, Bi3+ and V4+ ions is observed. A decrease in the TeO2 content leads to the formation of TeO4 trigonal bipyramids, as well as weakly polarized TeO3 trigonal pyramids.
Glasses made of highly polarized structural units are characterized by the highest values of frequency-dependent dielectric constant (real and imaginary part), dielectric loss and conductivity.