- Article
Notch Signalling Plays a Role in Patterning the Ventral Mesoderm During Early Embryogenesis in Drosophila melanogaster
- Marvel Megaly,
- Gregory Foran and
- Aleksandar Necakov
- + 6 authors
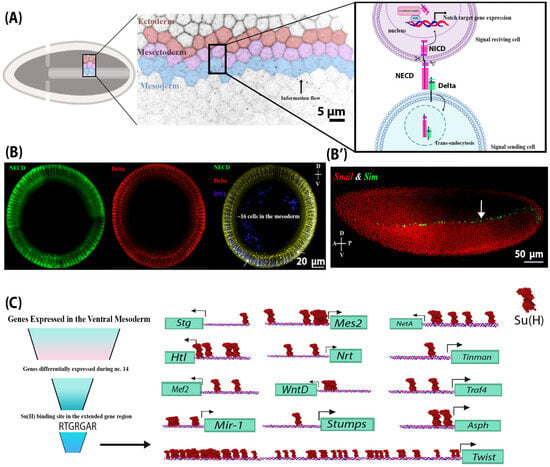
Notch signalling is a critical regulator of multiple developmental processes through its ability to control gene expression and thereby influence cell fate specification and cell proliferation through direct cell–cell communication. Although Notch signalling has been implicated in myogenesis during late embryogenesis, its role in early mesoderm development has been largely unexplored. Endocytosis of the Notch ligand Delta and the Notch receptor extracellular domain, a critical step in Notch pathway activation, has been extensively observed in the ventral mesoderm of the early Drosophila embryo, indicating a potential for Notch signalling activity in this early germ layer. Here, we present evidence that genes critical to mesoderm development require and are responsive to Notch signalling activity. Using a novel light-inducible Optogenetic variant of the Notch intracellular domain (OptoNotch), which affords precise spatial and temporal control over ectopic activation of Notch signalling, in combination with high-resolution fluorescent RNA in situ hybridization and qPCR, we identified a set of mesodermal genes whose expression is directly regulated by Notch signalling. We also provide evidence that Notch signalling indirectly regulates the dorsal–ventral patterning program mediated by the Toll signalling pathway through the Dorsal/Twist/Snail gene network. Our findings demonstrate that Notch signalling regulates ventral mesoderm patterning and is critical for establishing the mesoderm–mesectoderm–ectoderm boundary by regulating gene expression patterns and providing negative feedback on the upstream patterning network.
27 January 2026