Abstract
Sympathetic nervous system (SNS) imbalance is a common pathological basis for cardiovascular diseases, non-alcoholic fatty liver disease, and diabetes. This review focuses on these diseases, analyzing two core mechanisms: excessive sympathetic excitation induced by endoplasmic reticulum stress (ERS) or autophagy dysfunction in key central nuclei (e.g., hypothalamus, rostral ventrolateral medulla); and ERS/autophagy abnormalities in peripheral target organs caused by chronic SNS overactivation. Existing studies confirm that chronic SNS overactivation promotes peripheral metabolic overload via sustained catecholamine release, inducing persistent ERS and disrupting the protective unfolded protein response (UPR)–autophagy network, ultimately leading to cell apoptosis, inflammation, and fibrosis. Notably, central ERS or autophagy dysfunction further perturbs autonomic homeostasis, exacerbating sympathetic overexcitation. This review systematically elaborates on SNS overactivation as a critical bridge mediating UPR–autophagy network dysregulation in central and peripheral tissues, and explores therapeutic prospects of targeting key nodes (e.g., chemical chaperones, specific UPR modulators, nanomedicine), providing a theoretical basis for basic research and clinical translation.
1. Introduction
The high prevalence of major diseases such as chronic heart failure, obesity, and non-alcoholic fatty liver disease has become a global public health challenge. Their pathological mechanisms are closely associated with disruptions in cellular homeostasis and dysfunction of the sympathetic nervous system. Cellular homeostasis is fundamental to maintaining normal physiological functions. The endoplasmic reticulum (ER), a central site for protein synthesis and lipid metabolism, can develop functional disturbances leading to endoplasmic reticulum stress (ERS). The unfolded protein response (UPR) is activated through three sensors, including inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6), and functions to restore ER homeostasis [1,2,3]. Autophagy maintains metabolic balance by degrading damaged organelles and abnormal proteins and is transcriptionally regulated by transcription factor EB (TFEB). Its functional impairment can lead to disorders such as insulin resistance and hepatic steatosis [4,5,6]. Close crosstalk exists between the UPR and autophagy. All three branches of the UPR can activate autophagy. Furthermore, spliced X-box binding protein 1 (sXBP1) can drive autophagy-related transcriptional regulation mediated by TFEB. Together, they form a core network for maintaining cellular metabolic balance [7,8,9].
The sympathetic nervous system (SNS) regulates physiological functions such as heart rate, blood pressure, and metabolism through its multi-system innervation [10,11,12,13]. Under physiological conditions, it maintains a dynamic balance with the parasympathetic nervous system. Persistent stress or pathological stimulation can lead to excessive SNS activation [14,15]. An imbalance in central signaling is a key factor driving major diseases associated with the sympathetic nervous system. This dysregulation is particularly prevalent in critical nuclei such as the hypothalamic paraventricular nucleus (PVN), the subfornical organ (SFO), and the rostral ventrolateral medulla (RVLM). The core mechanism involves a reduction in central inhibitory regulation, which ultimately leads to excessive enhancement of sympathetic nerve activity. This further underscores the central anatomical positioning and critical physiological regulatory significance of these brain regions in the initiation and progression of sympathetic-related diseases [16,17,18]. Sympathetic imbalance also promotes cardiac structural and functional deterioration through mechanisms such as dysregulated β-adrenergic receptor signaling and abnormal cAMP compartmentalization [19]. In metabolic diseases like obesity, non-alcoholic fatty liver disease (NAFLD), and diabetes, excessive SNS activation similarly constitutes a core pathological link in inducing metabolic disorders [20,21].
Although the association between SNS imbalance and multi-organ diseases is well-established, the linking molecular pathways remain unclear. Recent studies indicate that excessive SNS activation contributes to disrupted cellular homeostasis by remodeling UPR–autophagy crosstalk, supported by preclinical evidence that SNS blockers such as stellate ganglion block and β-adrenergic receptor antagonists reverse ER stress and restore autophagic flux. However, direct causal mechanisms remain undefined. A key unanswered question is whether catecholamines bind UPR sensors or autophagy regulators. These mechanisms require validation via cell-specific knockout or conditional activation studies [22,23]. Chronic SNS activation leads to sustained catecholamine release, which induces metabolic overload and persistent ERS in target organ cells. This shifts the UPR–autophagy network toward pro-apoptotic and pro-inflammatory programs—a pathological transition that has been validated in diseases of organs such as the liver and heart [20,24,25,26]. However, a complete theoretical framework is currently lacking with regard to two core mechanisms: (1) how central UPR initiates SNS activation and drives peripheral pathologies, and (2) how SNS imbalance subsequently regulates the disorder of organ-specific UPR–autophagy networks.
This review focuses on the mechanistic role and therapeutic prospects of the UPR–autophagy crosstalk in SNS imbalance-related diseases. We will systematically outline the molecular regulation and intersectional mechanisms of ERS, the UPR, and autophagy. Specifically, this review will dissect the molecular pathways through which excessive SNS activation disrupts the UPR–autophagy network in peripheral target organs and elucidate the central mechanisms (e.g., in the hypothalamic paraventricular nucleus, PVN) where ERS or autophagy dysfunction triggers sympathetic excitation. By integrating evidence from cardiovascular and metabolic diseases, the review will summarize the disease-specific manifestations of this dysregulated network. Furthermore, it will explore the application prospects and translational potential of therapeutic strategies targeting key nodes of the UPR–autophagy axis, such as chemical chaperones and specific UPR modulators. Ultimately, this review aims to synthesize existing evidence to construct a theoretical framework linking SNS imbalance, dysregulated UPR–autophagy crosstalk, and multi-organ pathology, thereby providing a foundation for basic research and the development of novel therapeutic strategies for related diseases. Notably, the temporal sequence between SNS overactivation and ER stress—i.e., whether central ER stress precedes sympathetic excitation across all disease contexts, or if peripheral ER stress can feed back to drive central sympathetic activation—remains incompletely elucidated.
2. Synergistic Strategies in Cellular Quality Control: Endoplasmic Reticulum Stress-Mediated Selective Autophagy and Metabolic Remodeling
Cellular homeostasis in protein and organelle function is fundamental to organismal physiology. ERS, triggered by ER dysfunction, initiates the regulatory UPR. Conversely, autophagy maintains homeostasis by clearing damaged components. These two systems form a crosstalk network, working synergistically to preserve stability under physiological conditions. Their dysregulation, however, drives disease progression under pathological conditions.
2.1. Core Pathways of the UPR
When unfolded or misfolded proteins accumulate in the endoplasmic reticulum, they activate three stress sensors located on the ER membrane: IRE1, PERK, and ATF6. This activation initiates three distinct yet interconnected signaling pathways. Together, they work to restore ER functional homeostasis by regulating protein synthesis, folding, and degradation.
2.1.1. The IRE1α-XBP1 Pathway: Regulating Folding, Degradation, and Lipid Synthesis
IRE1α is the most evolutionarily conserved signaling branch of the UPR. As a transmembrane protein on the ER membrane, it possesses dual activities of serine/threonine kinase and endoribonuclease [27,28]. Upon ERS, IREα senses unfolded proteins, leading to its oligomerization and autophosphorylation, which activates its endoribonuclease activity. This activity drives two primary downstream processes. First, it specifically splices the mRNA of X-box binding protein 1 (XBP1), generating the transcriptionally active sXBP1 [29,30,31]. sXBP1 translocates to the nucleus and upregulates genes encoding ER chaperones and foldases, thereby enhancing protein folding capacity [32]. Second, it initiates the regulated IRE1-dependent decay (RIDD) pathway to degrade mRNAs encoding secreted proteins, thereby reducing the ER’s folding burden [31]. Notably, the function of IRE1α is context-dependent: beyond its protective effects, sustained ERS can activate its kinase activity to trigger the JNK pro-inflammatory pathway or degrade mRNAs of anti-apoptotic genes (e.g., Bcl-2) via RIDD, promoting cell apoptosis [31]. In NAFLD, hyperactivation of the IRE1α-XBP1 pathway exacerbates lipid accumulation by inducing fatty acid synthase expression via sXBP1, rather than merely maintaining lipid homeostasis [32,33,34,35]. An isoform, IRE1β, exists in the intestine where it regulates mucin production and secretion [36,37]. However, in the context of SNS imbalance-related metabolic and cardiovascular diseases, the IRE1α–XBP1 pathway plays a more central role.
2.1.2. The PERK–eIF2α–ATF4 Pathway: Regulating Translation, Oxidative Stress, and Autophagy
PERK is an ERS sensor. Under stress conditions, it activates via oligomerization and autophosphorylation. Its primary downstream target is eukaryotic initiation factor 2α (eIF2α) [38]. Phosphorylation of eIF2α by PERK inhibits global protein translation initiation, reducing the influx of newly synthesized peptides into the ER and mitigating folding pressure [39,40]. This translational inhibition is selective; conversely, phosphorylated eIF2α promotes the translation of activating transcription factor 4 (ATF4) mRNA. As a key transcription factor, ATF4 regulates downstream target genes. Specifically, it induces the expression of antioxidant enzymes, such as heme oxygenase-1, under oxidative stress to enhance cellular antioxidant capacity [41]. In the context of autophagy regulation, it directly binds to promoters of autophagy-related genes like Atg5 and LC3, promoting their transcription to initiate autophagy [42,43]. Furthermore, ATF4 induces the expression of C/EBP-homologous protein (CHOP). CHOP participates in adaptive responses during mild stress but triggers apoptosis under sustained stress, serving as a critical molecular switch for the physiological-to-pathological functional transition of this pathway [44].
2.1.3. The ATF6 Pathway: Transcriptional Regulatory Functions
ATF6 is a type II transmembrane transcription factor located on the ER membrane. Under resting conditions, it is kept inactive by binding to the ER chaperone protein binding immunoglobulin protein (BiP). Upon the accumulation of unfolded proteins in the ER, BiP dissociates from ATF6, allowing ATF6 to translocate to the Golgi apparatus [45]. Within the Golgi, ATF6 is sequentially cleaved by the proteases S1P and S2P. This process generates an active cytosolic fragment (ATF6f), which enters the nucleus to exert transcriptional regulation [46]. ATF6f primarily targets genes encoding ER chaperones and components of the ER-associated degradation (ERAD) system. By enhancing protein-folding capacity and the efficiency of misfolded protein clearance, it alleviates ER stress [47]. Clinical studies indicate that the ATF6 pathway is activated in the intestinal tissues of patients with inflammatory bowel disease, where it upregulates BiP to mitigate ER stress during chronic inflammation [48]. In metabolic disorders such as non-alcoholic fatty liver disease (NAFLD), abnormal activation of the ATF6 pathway is involved in regulating hepatic lipid metabolism dysregulation [49]. Furthermore, ATF6 cooperates with XBP1s to coordinately regulate ER stress-related genes, forming a collaborative network among different UPR pathways [50]. The protective and detrimental effects of the PERK-eIF2α-ATF4 pathway depend on the intensity of stress: under mild stress, ATF4 initiates protective autophagy by inducing Atg5 and LC3 [42,43]; whereas under sustained stress, excessive activation of ATF4 upregulates CHOP, ultimately triggering cell apoptosis [44]. In myocardial ischemia/reperfusion injury, hyperactivation of this pathway also drives excessive autophagy, leading to cardiomyocyte loss [51].
2.2. Core Process, Types, and Regulation of Autophagy
Autophagy is a conserved process by which cells degrade damaged organelles, misfolded proteins, and metabolic waste via lysosomes, maintaining intracellular homeostasis and contributing to cell survival and stress adaptation. Its core process includes initiation, nucleation, elongation, fusion, and degradation [52].
Autophagic flux describes the complete autophagic process—encompassing autophagosome formation, fusion with lysosomes, and substrate degradation—and reflects autophagic functional activity. Autophagosome accumulation is a phenotypic observation of increased autophagosome numbers. It can arise from two scenarios: enhanced autophagic flux with increased formation and normal degradation, or blocked autophagic flux with impaired fusion or degradation leading to buildup. Distinguishing between the two relies on combined detection of autophagy markers [53]; enhanced autophagic flux is defined by increased expression of the autophagosome marker LC3-II and decreased expression of p62/SQSTM1—a substrate marker degraded through autophagy. Blocked autophagic flux is characterized by increased LC3-II expression alongside elevated p62/SQSTM1, resulting from substrate accumulation due to impaired degradation.
2.2.1. Core Process and Regulation of Macroautophagy
Macroautophagy is the most characteristic form of autophagy, primarily responsible for degrading large organelles such as mitochondria and the ER. Its orderly progression relies on precise coordination at multiple key regulatory nodes. The phosphorylation status of the unc-51-like autophagy activating kinase 1 (ULK1) complex directly determines autophagy initiation. Under nutrient starvation or stress conditions, AMPK directly phosphorylates ULK1 at Ser317/777 sites, thereby relieving inhibition by mTOR and initiating the autophagy cascade [54]. The Beclin1–VPS34 complex catalyzes the generation of PI3P to recruit downstream ATG proteins. Concurrently, ATG9-mediated vesicle trafficking supplies the necessary membrane sources for phagophore formation, promoting autophagosome biogenesis [55,56]. The LC3 lipidation system, involving ATG7 and ATG3, facilitates the conjugation of LC3 to the autophagosome membrane, making it a core marker for autophagosomes and a key molecule for substrate recognition [57]. Recent studies have further revealed that the transcription factor TFEB upregulates the expression of ATG genes such as LC3 and Beclin1. This effectively enhances autophagic flux during lysosomal dysfunction, establishing TFEB as a crucial target in the field of transcriptional regulation of autophagy [58].
2.2.2. Other Major Types of Autophagy: Chaperone-Mediated Autophagy and Microautophagy
Chaperone-mediated autophagy (CMA) specifically degrades soluble proteins. It involves the recognition of substrates by HSPA8 (Hsc70), their binding to the lysosomal membrane protein lysosome-associated membrane protein type 2A (LAMP2A), and subsequent translocation across the lysosomal membrane for degradation [59]. CMA plays a unique role in protein quality control, and its functional decline is closely associated with aging and neurodegenerative diseases [60]. Microautophagy involves the direct engulfment of cytoplasmic components by invagination of the lysosomal membrane. It can be categorized into selective degradation via microautophagic bodies and non-selective microautophagy. This process primarily participates in lipid droplet degradation and organelle turnover, regulating lipid accumulation in hepatic lipid metabolism [61,62,63]. Unlike macroautophagy, both CMA and microautophagy do not require the formation of a double-membrane autophagosome, instead relying on the remodeling of the lysosomal membrane itself. Together, these three pathways constitute the cellular degradation network [64].
2.2.3. Physiological and Pathological Significance of Autophagy
Under physiological conditions, basal autophagy participates in organelle turnover, metabolic regulation, and immune defense. During embryonic development, autophagy promotes tissue remodeling by degrading excess cellular structures. Genetic knockout of Atg5 is embryonically lethal in mice [65]. Under pathological conditions, dysfunctional autophagy is closely linked to various diseases. Insufficient autophagy leads to protein aggregation and organelle accumulation, promoting neurodegenerative diseases and tumorigenesis [66,67]. Conversely, excessive autophagy can trigger autophagic cell death, contributing to myocardial ischemia/reperfusion injury and hepatotoxicity [68]. Furthermore, autophagy plays a dual role in metabolic diseases. Specifically, moderate autophagy relieves insulin resistance, whereas chronically high-fat diet-induced excessive autophagy exacerbates lipotoxicity in hepatocytes [69,70]. Notably, autophagy plays a biphasic role in chronic SNS activation-related diseases. Moderate autophagy exerts protective effects by clearing damaged components, while excessive or maladaptive autophagy fails to restore homeostasis and instead triggers pathological cell loss [71,72]. Excessive autophagy refers to abnormally enhanced autophagic flux exceeding cellular adaptive capacity, leading to excessive degradation of essential proteins or organelles and inducing autophagic cell death. Maladaptive autophagy denotes impaired autophagic quality such as incomplete substrate degradation or abnormal autophagosome–lysosome fusion under chronic stress, amplifying cellular damage and promoting apoptosis or necrosis.
2.3. Crosstalk Between UPR and Autophagy: Molecular Bridges
The crosstalk regulatory network between the UPR and autophagy exhibits organ- and disease-specific patterns, influencing pathogenesis through the activation or inhibition of pathways such as PERK–ATF4 and IRE1–JNK. When different organs face pathological stimuli, these regulatory axes can exert protective effects. However, under persistent stress, they may switch to destructive mechanisms, thereby driving disease progression (Table 1). It is important to emphasize that the dual nature of individual UPR branches (PERK and IRE1α) plays a critical role in disease progression. While moderate activation of these branches contributes to UPR–autophagy crosstalk and cellular homeostasis, their prolonged signaling can independently drive apoptosis and inflammation, decoupling from protective autophagic responses. This independent pathological role is distinct from the collaborative UPR–autophagy dysregulation and is widely observed in cardiovascular and metabolic diseases [9,28,44].

Table 1.
Organ-specific mechanisms of UPR–autophagy crosstalk.
2.3.1. Diabetic Nephropathy: The ATF4–HO-1 Axis Regulates Autophagy to Maintain Podocyte Homeostasis
In diabetic nephropathy, the UPR pathway plays a central role in podocyte protection by regulating autophagy. A high-glucose environment activates ERS, significantly upregulating ATF4 expression. In turn, ATF4 transcriptionally induces the expression of heme oxygenase-1 (HO-1). HO-1, initiated by ATF4, enhances autophagic flux, thereby clearing damaged organelles and inhibiting podocyte apoptosis—a process defined as protective autophagy [73]. Silencing of the ATF4 gene not only aggravates apoptosis but also blocks the HO-1-mediated protective autophagic response, confirming that the ATF4–HO-1–autophagy axis is a critical pathway for maintaining podocyte homeostasis.
Animal studies demonstrate that the HO-1 agonist hemin can significantly increase renal autophagy levels, reduce proteinuria, and ameliorate glomerulosclerosis. This mechanism provides a novel therapeutic strategy targeting ATF4/HO-1 for diabetic nephropathy.
2.3.2. Liver Disease: Core Mechanisms of UPR-Regulated Autophagy Dysfunction in Metabolic and Toxic Injury
An imbalanced UPR–autophagy network is a common mechanism in the development of liver diseases. In chronic MASLD, suppressed ATF4/CEBPG expression impairs RETREG1-mediated reticulophagy—an adaptive process that normally initiates selective ER-phagy to maintain homeostasis [74]. This defect blocks autophagic flux, causes autophagosome accumulation due to impaired degradation, and fails to attenuate persistent ERS, ultimately exacerbating lipid accumulation and inflammation. This failure to attenuate persistent ERS exacerbates lipid accumulation and inflammation.
In drug-induced liver injury, triptolide excessively activates the PERK–ATF4–CHOP pathway, driving pathological overactivation of autophagy. Conversely, catalpol inhibits this pathway to correct the autophagic imbalance [75]. Alcoholic and non-alcoholic liver diseases share a core link of ERS and autophagy dysfunction. Specifically, persistent ERS disrupts autophagic function, and impaired autophagy, in turn, aggravates ERS. This forms a vicious cycle that promotes fibrosis progression [77]. Targeting liver-specific nodes in the UPR–autophagy crosstalk has emerged as a novel therapeutic strategy for liver diseases.
2.3.3. Heart Disease: Dual Role of the UPR–Autophagy Network in Ischemic Repair and Chronic Failure
The interaction between the UPR and autophagy in heart disease exhibits dynamic evolution. During the acute ischemic phase, vascular endothelial growth factor A (VEGF-A) promotes angiogenesis via the reactive oxygen species (ROS)–ERS–autophagy axis, constituting an adaptive repair mechanism [71]. In contrast, during chronic or excessive stress, the network shifts toward pathological activation. Thrombospondin-1 (Thbs1) aberrantly activates the PERK–ATF4 pathway, driving excessive autophagy and leading to myocardial atrophy [51]. Following myocardial infarction, adenosine triphosphate (ATP) exacerbates reperfusion injury through the P2X7–NADPH oxidase 4 (Nox4)–PERK–ATF4 cascade [76].
In chronic heart failure, autoantibodies against the β1-adrenergic receptor (β1-AR) persistently activate ERS while simultaneously inhibiting autophagic flux. This “activation/inhibition” decoupling disrupts cellular homeostasis [72]. In myocardial ischemia/reperfusion injury, ERS-induced autophagy is initially protective, with CHOP serving as a critical node regulating the balance between autophagy and apoptosis [54]. These findings reveal a dynamic transition of the UPR–autophagy network from a compensatory to a decompensated state in cardiac pathologies.
2.3.4. Pancreatic Islets: Defensive Mechanisms of UPR and Autophagy and the Regulatory Role of CD47
Pancreatic β-cell homeostasis is critical for insulin secretion, and its dysfunction is closely linked to diabetes. Physiologically, UPR alleviates endoplasmic reticulum stress, while autophagy clears abnormal components, together forming a defensive barrier for β-cells. In diabetes, their dysfunction causes β-cell injury [78].
CD47 and its ligand TSP1 are upregulated under metabolic stress, which is also confirmed in clinical samples to be associated with diabetes. Inhibiting CD47 signaling improves UPR function, restores autophagic homeostasis, reduces β-cell senescence and apoptosis, and maintains insulin secretion. The CD47-TSP1 axis is key in regulating islet stress injury, and targeted intervention provides a new direction for diabetes treatment [79].
3. Central ERS Initiates SNS Excitation and Drives Peripheral Pathologies
An imbalance in the ERS and autophagy network within key nuclei of the central nervous system acts as a primary driver of SNS excitation. This dysregulation modulates neuronal circuit excitability, leading to excessive activation of the sympathetic centers and subsequently mediating the development of peripheral pathologies. Current research on the association between autophagic/ERS defects in central nuclei and sympathetic excitability primarily involves targeted intervention on key molecules in autophagy/ERS and detecting changes in peripheral sympathetic nerve activity markers, thereby confirming a causal link between the two [80,81,82]. However, these studies have not yet clarified how autophagic/ERS defects directly regulate the excitability of sympathetic neurons. The related molecular mechanisms still require further validation through techniques such as electrophysiological recordings and neuron-specific conditional knockout.
3.1. Neurogenic Hypertension: ERS and Autophagy Dysfunction in the RVLM as the Primary Central Driver of Sympathetic Overexcitation and Elevated Blood Pressure
The RVLM is the core central site for regulating sympathetic cardiovascular tone. ERS and autophagy dysfunction within the RVLM serve as a primary central mechanism for neurogenic hypertension. In the spontaneously hypertensive rat (SHR) model, markers of ERS in the RVLM are significantly upregulated even before the onset of the hypertensive phenotype. Notably, a bidirectional regulatory relationship exists between oxidative stress and ERS. Specifically, angiotensin II (Ang II)-induced oxidative stress can trigger ERS in the RVLM, whereas scavenging ROS or stabilizing ER homeostasis can reverse the hypertensive phenotype. Concurrently, autophagy in the RVLM of SHRs is overactivated, as indicated by increased expression of lysosome-associated membrane protein 2 (LAMP-2) and LC3-II. Importantly, this excessive autophagy is dependent on ERS initiation. Inhibiting autophagy or silencing the LC3-II gene in the RVLM can produce a blood pressure-lowering effect. In summary, the oxidative stress-sensitive ERS and the consequent autophagy dysregulation in the RVLM jointly drive sympathetic overexcitation and elevated blood pressure, constituting the core central mechanism of neurogenic hypertension (Figure 1) [80].
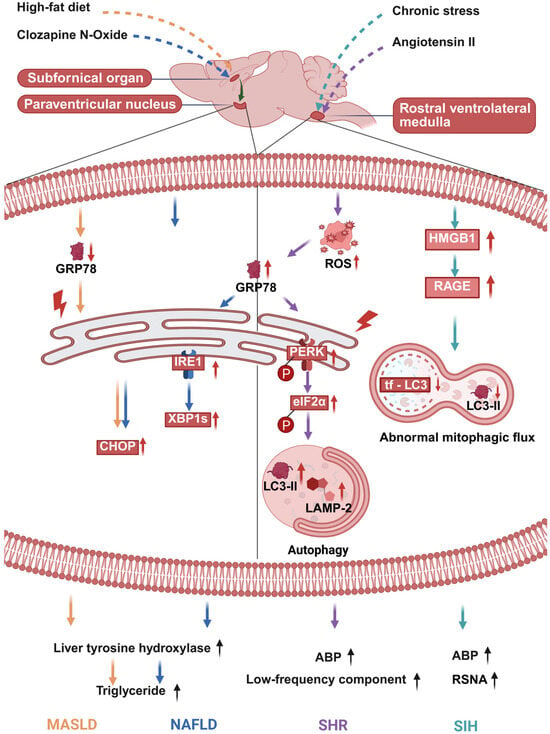
Figure 1.
Brain ERS/autophagy initiates sympathetic nervous system (SNS) hyperactivity and drives peripheral lesions. Glucose-regulated protein 78 kDa (GRP78) expression reflects endoplasmic reticulum stress (ERS). GRP78 upregulation signals that cells are counteracting ERS, indicating activated ERS but preserved cellular regulatory capacity. Adequate upregulation reduces unfolded proteins to attenuates ERS, whereas insufficient upregulation drives the progression to decompensation. In contrast, GRP78 downregulation signals the loss of cellular ability to combat ERS, which is accompanied by persistent and aggravated ERS, endoplasmic reticulum (ER) dysfunction, upregulation of pro-apoptotic molecules such as C/EBP homologous protein (CHOP), and ultimately leads to cell injury or death. Low-frequency component, it is a classic experimental indicator reflecting sympathetic neurogenic vasomotor activity. Black dotted lines within the cell membrane are used to separate the two functional parts of the figure: the left part represents PVN-associated processes, while the right part represents RVLM-associated processes. In this figure, “↑” indicates an increase in expression or activity of the corresponding molecule/pathway; meanwhile, arrows of different colors represent distinct regulatory pathways (e.g., the orange dashed arrow corresponds to the high-fat diet pathway, etc.). Created in BioRender. Bo Xu, Renjun Wang. (2025) https://BioRender.com/w55g70q (accessed on 18 December 2025). Abbreviations: ABP (arterial blood pressure); CHOP (C/EBP homologous protein); eIF2α (eukaryotic translation initiation factor 2 subunit alpha); GRP78 (glucose-regulated protein 78); HMG B1 (high-mobility group box 1); IRE1 (inositol-requiring enzyme 1); LC3-II (microtubule-associated protein 1A/1B-light chain 3-II); LAMP-2 (lysosomal-associated membrane protein 2); MASLD (metabolic dysfunction-associated steatotic liver disease); NAFLD (non-alcoholic fatty liver disease); PERK (protein kinase RNA-like endoplasmic reticulum kinase); RAGE (receptor for advanced glycation end products); ROS (reactive oxygen species); RSNA (renal sympathetic nerve activity); SHR (spontaneously hypertensive rat); SIH (stress-induced hypertension); XBP1s (spliced X-box binding protein 1).
3.2. Heart Failure: Central Nuclei-Mediated Sympathetic Imbalance via ERS and Signaling Pathways
In volume overload-induced high-output heart failure, RVLM catecholaminergic neuron activation causes autonomic imbalance. RVLM exhibits significant ERS, inflammation, and renin-angiotensin system activation, elevating the LFHRV/HFHRV ratio (sympathetic overactivation marker). Intracerebroventricular administration of ERS inhibitor TUDCA abolishes RVLM ERS, reduces arrhythmias and apneas, and alleviates cardiac hypertrophy and diastolic dysfunction, confirming RVLM ERS as a key driver of sympathetic imbalance-mediated cardiorespiratory dysfunction [83].
In myocardial infarction-induced HF, hypothalamic PVN epidermal growth factor receptor (EGFR) phosphorylation increases, activating the ERK1/2 pathway. This axis promotes PVN neuroinflammation (as reflected by elevated TNF-α and IL-1β levels), enhances RAS activity, and induces ERS (marked by increased BIP and ATF4 expression), enhancing sympathetic outflow and elevating norepinephrine levels. PVN microinjection of EGFR siRNA downregulates p-EGFR/p-ERK1/2, suppresses pathological pathways, and improves HF phenotypes, highlighting PVN EGFR-ERK1/2 signaling in sympathetic excitation [84].
3.3. Obesity: Hypothalamic POMC Neuron MANF Regulates Sympathetic Activity and BAT Thermogenesis via ERS
Obesity is tightly linked to sympathetic imbalance from hypothalamic dysfunction, with POMC neurons critical for energy homeostasis. Mesencephalic astrocyte-derived neurotrophic factor (MANF) in POMC neurons is a key regulator. MANF deletion in POMC neurons increases diet-induced obesity susceptibility by inducing hypothalamic ERS and leptin resistance, reducing POMC expression/processing, and decreasing sympathetic activity and BAT thermogenesis [85]. Conversely, MANF overexpression alleviates ERS, enhances POMC function, stimulates BAT sympathetic innervation/thermogenesis, and protects against obesity, highlighting MANF’s essential role via the ERS-POMC-sympathetic-BAT axis.
3.4. Stress-Induced Hypertension: Targeting the HMGB1/RAGE Axis in the RVLM Restores Mitophagic Flux and Inhibits Sympathetic Overexcitation
Chronic stress can induce the translocation of high-mobility group box 1 (HMGB1) from the nucleus to the cytoplasm and upregulate the expression of its receptor—namely, receptor for advanced glycation end products (RAGE)—in microglia within the RVLM. The activated HMGB1/RAGE axis serves as a key central driver of sympathetic excitation in stress-induced hypertension (SIH). This axis exerts a dual regulatory effect on mitophagic flux in microglia. It initiates autophagy during the early stress phase, but later impairs mitophagic flux by downregulating the expression of RAB7 and LAMP1 and increasing lysosomal pH, thereby compromising lysosomal function. This mitophagic flux blockade sequentially leads to mitochondrial damage, oxidative stress, and M1 polarization of microglia (Figure 1) [86]. Specific knockdown of HMGB1 or knockout of RAGE in the RVLM ameliorates mitophagic flux impairment, inhibits microglial neuroinflammation, and consequently reduces blood pressure and renal sympathetic nerve activity in mice. These findings indicate that targeting the HMGB1/RAGE axis in the RVLM can effectively suppress sympathetic overexcitation and stress-induced hypertension.
3.5. Non-Alcoholic Fatty Liver Disease: ERS in the SFO–PVN Circuit Directly Commands Hepatic Metabolic Reprogramming via the Hepatic Sympathetic Nerve
The onset and progression of non-alcoholic fatty liver disease (NAFLD) are closely associated with the regulation of forebrain–hypothalamic neural circuits. Among these, dysfunction of the subfornical organ–paraventricular nucleus (SFO–PVN) circuit serves as a key central mechanism mediating hepatic steatosis. In a normal mouse model, acute chemogenetic activation of SFO–PVN neurons can induce hepatic steatosis in a liver sympathetic nerve-dependent manner. Conversely, inhibiting this forebrain–hypothalamic circuit effectively improves obesity-associated NAFLD pathological phenotypes [81].
Further studies confirm that diet-induced NAFLD is accompanied by ultrastructural alterations in the endoplasmic reticulum (ER) and activation of ERS within the PVN. Notably, these changes can be mitigated by reducing excitatory signaling from the SFO. Similarly, specific inhibition of ERS in PVN neurons also ameliorates hepatic steatosis under obese conditions. In summary, ERS activation within the SFO–PVN circuit can directly regulate hepatocyte metabolic processes via the hepatic sympathetic nerve. This drives hepatic metabolic reprogramming and promotes abnormal lipid accumulation (Figure 1) [81]. The aberrant activation of this circuit constitutes a significant central driver of NAFLD pathogenesis. Targeted intervention against ERS in the SFO–PVN circuit may therefore provide a novel therapeutic direction for NAFLD.
3.6. Metabolic Dysfunction-Associated Steatotic Liver Disease: Neuronal ERS in the SFO-PVN Circuit Drives Hepatic Lipid Uptake via the Sympathetic Nerve
The pathological progression of MASLD is closely linked to ERS within the central SFO–PVN neural circuit. In a high-fat diet-induced obese mouse model, ERS in SFO–PVN neurons regulates hepatic lipid metabolism via the sympathetic nervous system. Blocking ERS in the neurons of this circuit not only significantly reduces hepatic triglyceride accumulation and the expression levels of genes related to lipid uptake but also decreases the content of tyrosine hydroxylase—the rate-limiting enzyme for catecholamine synthesis—in the liver. Moreover, the hepatic expression level of tyrosine hydroxylase shows a positive correlation with the lipid uptake pathway, but no significant association with the lipid breakdown pathway [82].
These results confirm that neuronal ERS in the SFO–PVN circuit upregulates hepatic tyrosine hydroxylase expression via the sympathetic nerve. This, in turn, enhances the lipid uptake capacity of hepatocytes, ultimately promoting the development of MASLD. Targeted inhibition of ERS in SFO–PVN neurons can alleviate obesity-associated hepatic steatosis by blocking the sympathetic nerve-driven stimulation of hepatic lipid uptake (Figure 1).
The reviewed evidence establishes central ERS as a convergent driver of sympathetic overexcitation across multiple diseases. A key unanswered question is whether this represents a primary cause or a secondary consequence. Current data best support a “vicious cycle” model that transcends this dichotomy: peripheral insults (hemodynamic, metabolic, or inflammatory) act as initial triggers, activating central ERS via humoral or neural signals. Once engaged, central ERS orchestrates self-amplifying responses—including neuroinflammation and neuronal excitability changes—that lock sympathetic centers into sustained overactivity. This, in turn, worsens peripheral pathology, reinforcing the feedback loop. Thus, in established disease, central ERS evolves from a triggered response into an independent, perpetuating pathological driver. This explains the efficacy of centrally targeted ERS interventions. Furthermore, future research employing temporally controlled, cell-specific manipulations is needed to dissect the precise causal weight of central ERS within this cycle, which is essential for developing stage-specific therapies. In addition, future research should also focus on neuronal subtype specificity in autonomic centers (e.g., glutamatergic and GABAergic neurons in the PVN and RVLM), exploring whether UPR/autophagy pathways exert distinct regulatory effects on different neuronal subtypes to mediate sympathetic overexcitation, which will help further clarify the precise central regulatory network.
4. SNS Imbalance Drives Organ-Specific UPR/Autophagy Network Disruption
The disruption of UPR–autophagy networks in peripheral organs by SNS imbalance involves two distinct pathways: direct regulation via adrenergic receptor binding and indirect modulation through secondary metabolic disorders. Below are organ-specific manifestations of these two pathways (Table 2).

Table 2.
Regulatory roles of sympathetic/autophagy-related factors in organ-specific pathophysiology and mechanisms.
4.1. Doxorubicin-Induced Heart Failure: Nox2-Mediated Sympathetic Nerve Terminal Dysfunction and Cardiomyocyte Autophagy Lead to Cardiac Atrophy
In wild-type mice injected with doxorubicin, sympathetic nerve terminal damage is prominent. Specifically, myocardial noradrenergic nerve fibers are reduced, and the expression of sympathetic markers such as protein gene product 9.5 (PGP9.5) and growth-associated protein 43 (GAP43) is downregulated. Concurrently, cardiomyocyte autophagy is hyperactivated, leading to reduced cell size and ultimately resulting in cardiac atrophy and functional decline. In contrast, these pathological alterations and doxorubicin-induced cardiac injury are significantly ameliorated in Nox2 knockout mice [87]. Beyond targeted modulation of Nox2, spironolactone (SP) has also been shown to ameliorate doxorubicin-induced myocardial fibrosis, apoptosis, and cardiac dysfunction, representing another potential strategy for protecting against doxorubicin cardiotoxicity [93].
In summary, Nox2 mediates oxidative stress, which triggers sympathetic nerve terminal dysfunction and directly activates cardiomyocyte autophagy (Figure 2). These two processes act synergistically to cause cardiac atrophy and progression to heart failure. Targeting Nox2 to rebalance the sympathetic nerve–autophagy axis provides a central direction for preventing and treating this form of drug-induced cardiotoxicity.
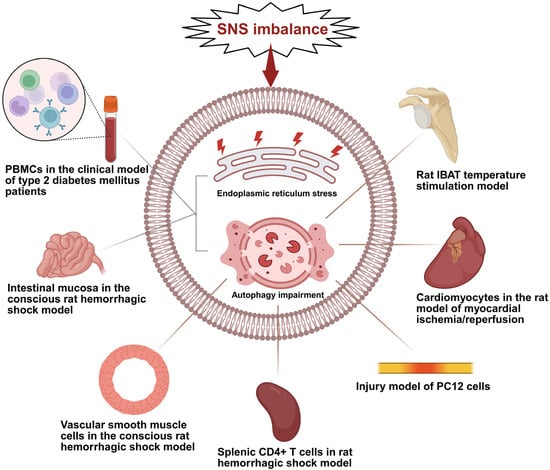
Figure 2.
Schematic diagram illustrating SNS imbalance driving organ-specific ERS and autophagy impairment. This diagram summarizes the differential pathological effects of SNS imbalance (the core upstream driver) on target cells/tissues across various models, where gray lines represent the occurrence of both ERS and autophagy impairment in the corresponding cells/tissues, and brown lines represent the occurrence of only autophagy impairment; PBMCs (Peripheral Blood Mononuclear Cells); IBAT (Interscapular Brown Adipose Tissue); PC12 (a rat pheochromocytoma cell line commonly used as a neuronal cell model in in vitro studies). Created in BioRender. Bo Xu, Renjun Wang. (2025) https://BioRender.com/mvdqtdb (accessed on 18 December 2025).
4.2. Brown Adipose Tissue Atrophy: The Sympathetic Nerve Maintains Tissue Mass by Inhibiting FoxO-Mediated Autophagy
The SNS serves as a central regulator for maintaining brown adipose tissue (BAT) mass by inhibiting forkhead box O (FoxO)-mediated autophagy. Under physiological conditions, sympathetic activation suppresses autophagy and promotes anabolism to maintain tissue homeostasis. In contrast, a lack of sympathetic tone triggers FoxO-mediated hyperactivation of autophagy, ultimately leading to tissue atrophy.
Experimental evidence shows that sympathetic denervation of the interscapular BAT in rats at 25 °C activates FoxO dephosphorylation. This drives accelerated autophagic flux and an increase in autophagosomes, culminating in tissue atrophy. Conversely, during cold stimulation at 4 °C, the sympathetic nerve is activated. This activation stimulates mechanistic target of rapamycin (mTOR) signaling via the cAMP/Epac1/Akt pathway, which promotes protein synthesis and simultaneously inhibits Unc-51 like autophagy activating kinase 1 (ULK1), a key protein for autophagy initiation, thereby blocking autophagosome formation and ultimately mediating tissue hypertrophy [88]. This mechanism clarifies the pivotal role of the sympathetic nerve–autophagy axis in BAT cold adaptation (Figure 2).
4.3. Methamphetamine-Induced Neuronal Injury Model: Norepinephrine Exerts a Protective Effect by Restoring Autophagic Flux via Activation of β2-ARs
Recent studies have confirmed that norepinephrine (NE) directly protects against methamphetamine-induced neuronal injury, an effect independent of in vivo neural circuits. In a PC12 cell model, NE reverses the autophagic dysfunction caused by methamphetamine through specific activation of plasma membrane β2-adrenergic receptors (β2-ARs) [22].
Mechanistically, methamphetamine disrupts the autophagic process, causing a blockage in autophagic flux and abnormal diffusion of LC3 protein from autophagic vesicles into the cytoplasm. Agonism of β2-ARs by NE restores autophagic flux and maintains the normal localization of LC3 to autophagic vesicles, thereby repairing autophagic function and reducing cellular damage [22]. This finding establishes a direct neuroprotective pathway: NE signaling → β2-ARs → autophagy regulation. It provides a new perspective for understanding the mechanisms of autophagy dysfunction in NE deficiency-associated neurodegenerative diseases (Figure 2). Notably, other adrenergic receptor subtypes mediate sympathetic regulation of ERS/autophagy: β1-AA (β1-AR ligand) induces myocardial injury, reversed by RD808 via enhancing protective autophagy; β3-AR activation reduces adipose GRP78 (ERS marker) and alleviates metabolic disorders. This reflects a direct adrenergic signaling effect, as NE directly binds to β2-AR on PC12 cells to restore autophagic flux, independent of systemic metabolic changes [22,94,95,96].
4.4. Organ Dysfunction Following Hemorrhagic Shock: Stellate Ganglion Blockade Achieves Multi-Target Protection by Inhibiting Autophagy/ERS
During hemorrhagic shock, the SNS is intensely activated. The massive release of catecholamines induces ERS and abnormal autophagy in multiple organs, leading to suppressed immune function, decreased vascular reactivity, and intestinal barrier injury.
4.4.1. Suppressed Splenic Immune Function: SGB Restores CD4+ T Cell Function by Inhibiting PHSML-Mediated Autophagy
Hemorrhagic shock induces the production of post-hemorrhagic shock mesenteric lymph (PHSML) via sympathetic activation. PHSML, in turn, activates autophagy in CD4+ T cells by inhibiting the PI3K/Akt pathway, resulting in impaired cell proliferation and immune function. Stellate ganglion blockade (SGB) inhibits sympathetic output, reduces PHSML generation, and blocks this autophagic signaling pathway, thereby restoring the proliferative capacity and cytokine secretion function of CD4+ T cells. This mechanism is supported by the following evidence. SGB or the autophagy inhibitor 3-MA can reverse T cell functional suppression, whereas the autophagy agonist rapamycin (RAPA) or exogenous PHSML abolishes the protective effect of SGB (Figure 2). Therefore, SGB improves post-hemorrhagic shock immune function by inhibiting the sympathetic nerve–PHSML–autophagy signaling cascade, providing a novel strategy for immunomodulatory therapy [89].
4.4.2. Vascular Hyporeactivity: SGB Maintains Contractile Phenotype by Inhibiting Autophagy-Mediated Phenotypic Switching in Vascular Smooth Muscle Cells
Hemorrhagic shock induces systemic overactivation of the SNS, which subsequently promotes the generation and reflux of PHSML. Acting as a key mediator in sympathetic-tissue crosstalk, PHSML induces excessive autophagy in vascular smooth muscle cells (VSMCs), manifesting as upregulated expression of the autophagic marker proteins LC3-II/I and Beclin1 and aberrant activation of autophagic flux [23].
This autophagic process directly drives phenotypic reprogramming of VSMCs. It switches them from a contractile phenotype, which maintains vascular tone, to a synthetic phenotype characterized by secreting extracellular matrix components and expressing matrix metalloproteinase-2 (MMP-2) [23]. This switch ultimately leads to diminished vascular contractile function and hyporeactivity. By regionally inhibiting sympathetic output, SGB can effectively suppress this process and improve vascular function (Figure 2).
4.4.3. Intestinal Mucosal Barrier Injury: SGB Repairs Barrier Function by Inhibiting Autophagy/ERS
Ischemia, hypoxia, and hypoperfusion induced by hemorrhagic shock lead to blocked autophagic flux (characterized by increased LC3-II and Beclin1, combined with elevated p62 due to impaired degradation) and hyperactivation of the ERS pathway in intestinal mucosa, both of which collectively mediate intestinal mucosal barrier damage. Relevant studies show that SGB, as a sympathetic inhibition strategy, exerts a protective effect by suppressing the ERS pathway in intestinal mucosal cells. In a conscious rat model of hemorrhagic shock, SGB downregulated the abnormal expression of key ERS molecules—ATF6α, PERK, and IRE1α—in intestinal tissue. It also reduced plasma levels of D-lactate and intestinal fatty acid-binding protein. The protective effect of SGB was comparable to that of the ERS inhibitor 4-phenylbutyric acid (4-PBA), whereas the ERS agonist tunicamycin reversed its benefits [90].
Other studies have confirmed that SGB can repair the intestinal mucosal barrier by inhibiting excessive autophagy. Specifically, SGB reversed the hemorrhagic shock-induced upregulation of autophagic markers LC3-II and Beclin1, as well as the downregulation of p62—indicating restored autophagic flux (Figure 2). It also upregulated the expression of tight junction proteins and reduced the intestinal wet-to-dry ratio and plasma fluorescein isothiocyanate–dextran 4 (FD4) levels. The effects of SGB were consistent with those of the autophagy inhibitor 3-methyladenine (3-MA), whereas the autophagy agonist rapamycin (RAPA) counteracted the beneficial effects of SGB. In summary, SGB repairs post-hemorrhagic shock intestinal mucosal barrier function by suppressing sympathetic-driven hyperactivation of both ERS and autophagy [97].
4.5. Cardiovascular Risk in Type 2 Diabetes: Sympathetic Tone Drives UPR and Autophagy Pathway Activation in Monocytes
Sympathetic overactivation in type 2 diabetes exerts secondary metabolic stress effects: sustained catecholamine release induces insulin resistance and systemic inflammation, which in turn activate UPR and autophagy pathways in monocytes (Figure 2). This is evidenced by upregulated expression of stress markers such as glucose-regulated protein 78/binding immunoglobulin protein (GRP78/BiP) and phosphorylated EIF2α, as well as activation of the autophagy-related gene BECN1. These changes subsequently trigger inflammatory pathways and oxidative stress responses, promoting the development of atherosclerosis. Clinical studies show that after 4 months of morning-timed treatment with bromocriptine-QR (3.2 mg/day), patients exhibited a significant reduction in sympathetic activity markers. Concurrently, the expression of key ERS/UPR molecules and autophagy genes in monocytes was downregulated, levels of oxidative stress and pro-inflammatory factors decreased, and systemic low-grade inflammation was ameliorated [91]. By inhibiting sympathetic activity and blocking the abnormal activation of this pathway, bromocriptine-QR represents an effective strategy for reducing cardiovascular events.
4.6. Arrhythmia: Spinal Nerve Block Restores Cardiac Homeostasis by Inhibiting Sympathetic Overshoot
In a rat model of myocardial ischemia/reperfusion (IR), intrathecal injection of bupivacaine to perform a spinal nerve block inhibited sympathetic overshoot by suppressing abnormal spinal neuronal activity. Spinal nerve block directly inhibits sympathetic overshoot and modulates cardiomyocyte autophagic balance, representing a direct regulation of adrenergic signaling on the cardiac UPR–autophagy network. Simultaneously, it significantly modulated the cardiomyocyte autophagic process and improved autophagic imbalance. This intervention not only reduced the ventricular arrhythmia score and improved heart rate variability but also alleviated IR-induced cardiac dysfunction and myocardial injury. These beneficial effects are closely associated with the restoration of connexin 43 distribution and stabilization of myocardial electrical conduction [92]. Chronic sympathetic overactivation induces sustained β-AR stimulation, driving myocardial UPR dysfunction via the IL-6/gp130/STAT3 pathway. Activated STAT3 (Y705-phosphorylated) specifically triggers PERK and IRE1α, promoting cardiomyocyte apoptosis. Inhibiting this axis reverses cardiac injury in vitro/vivo, providing direct evidence for β-AR-specific UPR sensor regulation [98]. Future studies should further validate such receptor-UPR sensor specificity across other disease contexts (e.g., metabolic diseases) and explore whether α-adrenergic receptor subtypes exhibit selective regulation of the ATF6 pathway, to refine targeted therapeutic strategies.
4.7. Recurrent Hypoglycemia: UPR-Mediated Inhibition of Neurosecretion in the Adrenal Medulla
Research on Sprague Dawley (SD) rats shows that, compared to acute hypoglycemia, recurrent hypoglycemia specifically activates the UPR pathway in the adrenal medulla. Concurrently, recurrent hypoglycemia also induces other pathway abnormalities, including enhanced neuropeptide signaling, imbalances in ion homeostasis, and downregulation of genes related to calcium-dependent vesicular exocytosis. Collectively, these alterations inhibit the neurosecretory function of the adrenal medulla, leading to impaired epinephrine secretion [99]. Thus, UPR activation in the adrenal medulla is a key mechanism by which recurrent hypoglycemia mediates hypoglycemia-associated autonomic failure (HAAF), also providing a potential target for clinical intervention.
Notably, autophagy and the UPR exhibit organ- and cell-type-specific outcomes in chronic SNS activation-related diseases, shaped by tissue/cell-specific functions. In cardiomyocytes, autophagy regulates mitochondrial quality and contractile protein turnover—excessive activation via β-AR/Nox2 signaling leads to cardiac atrophy—while UPR via PERK-ATF4 drives pathological autophagy or apoptosis [51,87,93]. In hepatocytes, autophagy focuses on lipophagy and ER homeostasis with maladaptive accumulation exacerbating steatosis, and UPR’s ATF4-CEBPG-RETREG1 axis governs reticulophagy [74,75,77]. In neurons/microglia, autophagy maintains synaptic function and neuroinflammation balance with flux impairment driving sympathetic overexcitation, and UPR activation in microglia promotes M1 polarization via HMGB1/RAGE [80,85,86]. These differences highlight the cell- and organ-specificity of UPR–autophagy crosstalk in disease progression.
5. Targeted Intervention Strategies for Sympathetic Imbalance-Related Diseases: From Molecular Regulation to Systemic Intervention
This chapter focuses on novel therapeutic strategies for diseases associated with sympathetic imbalance. Centering on the UPR/autophagy pathway as a core molecular hub, it systematically elaborates on multi-level therapeutic approaches. These range from directly modulating cellular stress pathways to indirectly intervening in neural activity to influence downstream UPR/autophagy. The discussion encompasses several key directions: pharmacological strategies that directly target cellular stress pathways [100], neural intervention methods that modulate sympathetic nervous system activity, and the foundational role of lifestyle interventions in restoring neuro-cellular homeostasis. Finally, the chapter explores the emerging potential of nanomedicine in enabling precise and synergistic therapy, as illustrated in Figure 3.
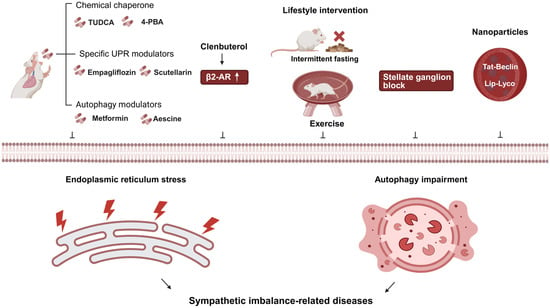
Figure 3.
Targeted intervention strategies for sympathetic imbalance-related diseases. Note: In this figure, “↑” denotes increased expression/activity; “⊥” indicates inhibition of the corresponding process (endoplasmic reticulum stress or autophagy impairment); solid black arrows represent the directional regulatory relationship (i.e., the upstream factor leads to/induces the downstream effect). Abbreviations: 4-PBA (4-phenyl butyric acid); β2-AR (beta-2 adrenergic receptor); UPR (unfolded protein response); TUDCA (taurine-conjugated ursodeoxycholic acid); Lip-Lyco (nano-liposomal formulated lycopene); Tat-Beclin (Tat-Beclin1 peptide). Created in BioRender. Bo Xu, Renjun Wang. (2025) https://BioRender.com/mvdqtdb (accessed on 23 January 2026).
5.1. Alleviating Endoplasmic Reticulum Stress: Chemical Chaperones
Studies have confirmed that the orally active chemical chaperones 4-PBA and tauroursodeoxycholic acid (TUDCA) exhibit significant ERS inhibitory effects. In both cellular and animal models, these agents directly reduce excessive ERS activation by enhancing ER adaptive capacity and reducing free cholesterol levels in adipose tissue. Intervention studies in obese diabetic mice demonstrate that these molecules not only normalize hyperglycemia and restore systemic insulin sensitivity but also ameliorate fatty liver disease and enhance insulin action in the liver, muscle, and adipose tissue [101,102]. Furthermore, chemical chaperones can correct ERS-induced adipokine secretion dysregulation, thereby breaking the vicious cycle between metabolic dysfunction and inflammation. This mechanism clarifies the central role of chemical chaperones in intervening in sympathetic imbalance-related metabolic diseases by targeted ERS alleviation, providing a novel drug target and practical rationale for treating type 2 diabetes and obesity-associated metabolic disorders [103].
5.2. Specific UPR Pathway Modulators
For myocardial I/R injury, empagliflozin inhibits the PERK/ATF4/Beclin1 pathway, blocks ERS-induced abnormal autophagy, reduces cardiomyocyte apoptosis, and improves cardiac function [9]. In NAFLD, scutellarin suppresses IRE1α/XBP1 pathway hyperactivation. This action upregulates FoxO1-mediated autophagy while reducing lipid accumulation in hepatocytes. XBP1s overexpression attenuates its protective effect, and autophagy inhibitors can negate its benefits, highlighting the regulatory value of UPR–autophagy crosstalk in this context [104]. This category of modulators achieves coordinated regulation of ERS and autophagy by precisely intervening in specific UPR branch signals. It thereby provides more targeted therapeutic directions for diseases such as myocardial injury and NAFLD.
5.3. Autophagy Modulators: Promise and Challenges
In the intervention of NAFLD/NASH, several drugs have demonstrated clear autophagy-modulating effects. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, such as empagliflozin, enhance autophagy in hepatic macrophages via the AMPK/mTOR pathway or activate autophagy through the AMPK–TFEB pathway, thereby improving hepatic lipid accumulation and fibrosis [105,106]. The anti-hepatic steatotic effect of metformin depends on TFEB-mediated autophagy activation. It can reverse high-fat diet-induced reductions in TFEB activity and insulin resistance [107]. Additionally, escin activates antioxidative and autophagic responses. Physalin B (PB) activates autophagy and antioxidative signaling; and scoparone enhances autophagic flux in macrophages. All these agents effectively ameliorate NASH-related liver injury and inflammation [108,109,110]. However, the application of autophagy modulators faces challenges. The regulatory mechanisms of autophagy vary among different cell types, necessitating the development of cell-specific targeting strategies [111].
5.4. Sympathetic Blockade: Targeting Peripheral Sympathetic Activity to Regulate ERS/Autophagy in Vascular Pathologies
Sympathetic blockade represented by SGB emerges as a promising therapeutic target for vascular-related diseases, complementing existing intervention strategies. As highlighted in Section 4.4, SGB alleviates posthemorrhagic shock-induced vascular hyporeactivity by inhibiting excessive autophagy in vascular smooth muscle cells (VSMCs), reversing PHSML-mediated phenotypic transformation. Moreover, in vascular calcification (VC), SGB suppresses sympathetic overactivation, reduces plasma norepinephrine levels, and attenuates ERS in calcified aortas, thereby ameliorating VSMC osteoblast-like transformation and calcium deposition [112,113]. The beneficial effects of SGB on VC are abolished by the ERS inducer tunicamycin but mimicked by the ERS inhibitor 4-PBA, confirming ERS as a key downstream mediator. These findings validate that targeting peripheral sympathetic activity via SGB regulates ERS/autophagy balance in VSMCs, providing a novel therapeutic direction for vascular pathologies such as VC and post-shock vascular dysfunction.
5.5. Indirect Regulation: Potential Benefits of SNS Activity Intervention on Downstream Autophagy
In the regulation of hepatic metabolism, the SNS plays a crucial role in modulating liver autophagy via the β2-AR. β2-AR agonists, such as clenbuterol and epinephrine, can significantly activate autophagy and autophagic flux in hepatocytes, hepatocellular carcinoma cells, and in vivo models. In the field of neuroprotection, NE—the core neurotransmitter of the SNS—activates β2-AR to counteract methamphetamine-induced autophagy dysfunction. Methamphetamine intoxication causes autophagic flux blockage and abnormal translocation of LC3 from autophagic vesicles to the cytoplasm. However, when NE acts directly on methamphetamine-treated PC12 cells, it restores autophagic flux homeostasis and maintains LC3 localization to autophagic vesicles through β2-AR activation. This action effectively antagonizes methamphetamine-induced neuronal injury [22,114]. This mechanism provides a therapeutic direction for degenerative diseases characterized by NE deficiency accompanied by autophagy impairment, suggesting that modulating SNS activity could restore autophagic function.
5.6. Lifestyle Interventions: Caloric Restriction and Exercise
Intermittent fasting regimens, such as alternate day fasting and the 5:2 diet, can activate autophagy. In obesity models, caloric restriction combined with exercise reduces body fat percentage and regulates skeletal muscle autophagy [115]. Short-term alternate-day fasting can activate autophagy by upregulating hepatic LC3 and downregulating p62, even without significant weight loss. This activation inhibits lipid synthesis and the expression of inflammatory and apoptotic genes, thereby ameliorating NASH-related liver injury [116]. However, the clinical efficacy of intermittent fasting for MASLD remains heterogeneous, necessitating further clinical studies to clarify its applicable scenarios [117,118].
The regulation of autophagy and UPR by exercise is type and dose dependent. Long-term moderate aerobic or resistance exercise upregulates autophagy-related proteins in aged skeletal muscle, inhibits mTOR, and delays muscle aging. Exercise intervention alone can enhance hepatic autophagy via the AMPK/mTORC1 pathway, impeding the progression from NAFL to NASH and liver fibrosis [119]. In contrast, short-term low-intensity exercise may be insufficient due to failure to reach the activation threshold, whereas high-intensity exercise risks inducing excessive autophagy and causing damage. For middle-aged and elderly populations, long-term combined training can optimize the regulation of skeletal muscle autophagy and UPR pathways, thereby improving exercise performance [120,121].
In summary, both caloric restriction and exercise improve metabolism and tissue aging by physiologically modulating the autophagy–UPR balance. They serve as foundational intervention options for sympathetic imbalance-related diseases. Their core advantages lie in safety and broad applicability. However, their protocols require precise optimization based on disease type and individual tolerance, with clear identification of suitable populations to achieve personalized intervention.
5.7. Nanomedicine Strategies: Targeted Delivery and Synergistic Therapy
To address autophagy dysfunction in NAFLD, acid-activated acid-degradable nanoparticles (acNPs) degrade specifically within the acidic environment of hepatocyte lysosomes. This action reconstructs lysosomal acidity and function, restores autophagic flux and mitochondrial homeostasis, and ultimately reverses hyperglycemia and hepatic steatosis [122]. Poly(lactic acid) nanoparticles loaded with Tat-Beclin1 peptide (NP T-B) can specifically accumulate in the liver, enabling potent and sustained autophagy induction at low doses and reducing intracellular lipid accumulation [123].
Nanocarriers also address the poor water solubility of traditional drugs, enabling multi-pathway synergistic intervention. Poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with nifedipine (NFD-NPs) achieve sustained drug release in the liver, enhancing autophagic clearance and inhibiting ERS [124]. Orally administered lycopene encapsulated in nanoliposomes (Lip-Lyco) exhibits multiple beneficial effects, including autophagy induction, antioxidant, and anti-inflammatory activities, effectively reversing hepatic steatosis and fibrosis [125]. In summary, nanomedicine, through targeted design and functional integration, efficiently regulates the UPR–autophagy axis and related pathological pathways. It overcomes the delivery limitations of conventional drugs, offering a high-potential, precision therapeutic strategy for metabolic diseases. However, its long-term safety and efficacy require further validation in clinical studies.
6. Conclusions and Perspectives
This review demonstrates that an imbalance in the SNS constitutes a common cellular axis for sympathetic imbalance-related diseases by remodeling the crosstalk network between the UPR and autophagy. Chronic SNS overactivation induces metabolic overload and persistent ERS in target organs. This shifts the initially protective UPR–autophagy network toward destructive programs that promote apoptosis, inflammation, and fibrosis. Concurrently, ERS or autophagy dysfunction within central nuclei can directly disrupt autonomic homeostasis, forming a vicious cycle. The elucidation of this mechanism provides a novel theoretical foundation for moving beyond traditional organ-specific therapies and developing synergistic strategies that target this neuro-cellular stress network. These strategies include sympathetic modulation, UPR/autophagy modulators, and nanodelivery systems. However, current mechanistic insights and therapeutic explorations are largely based on animal models such as mice. Human cohort studies and clinical trial evidence necessary for translation are lacking, representing a major bottleneck limiting the conversion of this theoretical framework into clinical applications.
Future research should focus on regulatory specificity and organ interactions. Key priorities include deciphering the direct causal mechanisms underlying SNS-UPR/autophagy crosstalk—such as validating whether catecholamines directly modulate UPR sensors (PERK/IRE1α/ATF6) or autophagy regulators (TFEB/ULK1) via adrenergic receptor-dependent or -independent pathways—and identifying key nodes such as adrenergic receptor subtypes and UPR branches. Notably, the potential sex differences in SNS regulation and ER stress responses remain an understudied area. Current evidence primarily derives from mixed-gender or gender-unspecified studies, and the extent to which biological sex modulates the SNS-UPR/autophagy axis in disease contexts has not been systematically explored. This represents a critical research gap that warrants systematic investigation to guide the development of personalized therapeutic strategies. It is also crucial to define spatiotemporal windows for intervention—for example, targeting ERS in the SFO–PVN circuit during early NAFLD versus focusing on hepatic fibrosis in later stages. Furthermore, elucidating the bidirectional feedback mechanisms between central and peripheral systems is essential to disrupt inter-organ vicious cycles. Building on this foundation, subsequent efforts should explore multi-target combination therapies, optimize nanodelivery platforms, advance clinical translation, and ultimately establish a biomarker-guided precision prevention and treatment system. This integrated approach, leveraging the UPR–autophagy network, holds promise for driving breakthroughs in chronic disease research.
Author Contributions
Conceptualization, R.W.; writing—original draft preparation, R.W. and B.X.; writing—review and editing, B.X. and R.W.; supervision, R.W.; project administration, Y.Y.; funding acquisition, R.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Development of Science and Technology of Jilin Province (grant number YDZJ202601ZYTS314 (R.W.)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dvela-Levitt, M.; Meiseles, D.; Arbeli, N.; Dvela-Levitt, M. When Proteins Go Berserk: The Unfolded Protein Response and ER Stress. Nephron 2025, 149, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rodríguez, M.; Chevet, E.; Muñoz-Pinedo, C. Glucose sensing and the unfolded protein response. FEBS J. 2025, 292, 3581–3595. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Liu, X.; Lu, C.; Li, T.; Yang, Z.; Xu, F.; Chen, S.; Yin, K.; Wang, L. Ceramide mediates cell-to-cell ER stress transmission by modulating membrane fluidity. J. Cell Biol. 2025, 224, e202405060. [Google Scholar] [CrossRef] [PubMed]
- Koutsifeli, P.; Varma, U.; Daniels, L.J.; Annandale, M.; Li, X.; Neale, J.P.H.; Hayes, S.; Weeks, K.L.; James, S.; Delbridge, L.M.D.; et al. Glycogen-autophagy: Molecular machinery and cellular mechanisms of glycophagy. J. Biol. Chem. 2022, 298, 102093. [Google Scholar] [CrossRef]
- Yan, S. Role of TFEB in Autophagy and the Pathogenesis of Liver Diseases. Biomolecules 2022, 12, 672. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, J.Q.; Wu, Y.; Zheng, P.; Wang, S.F.; Yang, M.C.; Ma, G.S.; Yao, Y.Y. Excessive or sustained endoplasmic reticulum stress: One of the culprits of adipocyte dysfunction in obesity. Ther. Adv. Endocrinol. Metab. 2024, 15, 20420188241282707. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, S.; Zhai, Y.; Wu, K.; Wang, P.; Liu, Y.; Zhang, C.; Yin, H.; Tian, Y.; Zhao, B.; et al. Endoplasmic reticulum stress regulates swainsonine-induced the autophagy in renal tubular epithelial cells through UPR signaling pathway. Sci. Total Environ. 2025, 981, 179616. [Google Scholar] [CrossRef]
- Liu, T.; Li, L.; Meng, M.; Gao, M.; Zhang, J.; Zhang, Y.; Gan, Y.; Dang, Y.; Liu, L. The protective role of the IRE1α/XBP1 signaling cascade in autophagy during ischemic stress and acute kidney injury. Cell Stress Chaperones 2025, 30, 160–171, Correction in Cell Stress Chaperones 2025, 30, 100094. [Google Scholar] [CrossRef]
- Wang, C.C.; Li, Y.; Qian, X.Q.; Zhao, H.; Wang, D.; Zuo, G.X.; Wang, K. Empagliflozin alleviates myocardial I/R injury and cardiomyocyte apoptosis via inhibiting ER stress-induced autophagy and the PERK/ATF4/Beclin1 pathway. J. Drug Target. 2022, 30, 858–872. [Google Scholar] [CrossRef]
- Wang, T.; Teng, B.; Yao, D.R.; Gao, W.; Oka, Y. Organ-specific sympathetic innervation defines visceral functions. Nature 2025, 637, 895–902. [Google Scholar] [CrossRef]
- Schaeuble, D.; Myers, B. Cortical-Hypothalamic Integration of Autonomic and Endocrine Stress Responses. Front. Physiol. 2022, 13, 820398. [Google Scholar] [CrossRef]
- Carnagarin, R.; Kiuchi, M.G.; Goh, G.; Adams, L.; Cohen, N.; Kavnoudias, H.; Gan, S.K.; Van Schie, G.; Esler, M.D.; Matthews, V.B.; et al. Role of the sympathetic nervous system in cardiometabolic control: Implications for targeted multiorgan neuromodulation approaches. J. Hypertens. 2021, 39, 1478–1489. [Google Scholar] [CrossRef]
- Jerem, P.; Romero, L.M. It’s cool to be stressed: Body surface temperatures track sympathetic nervous system activation during acute stress. J. Exp. Biol. 2023, 226, jeb246552. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Du, D.; Shan, Z.; Bi, L.; Chen, Q.H. Brain-Targeted Reactive Oxygen Species in Hypertension: Unveiling Subcellular Dynamics, Immune Cross-Talk, and Novel Therapeutic Pathways. Antioxidants 2025, 14, 408. [Google Scholar] [CrossRef]
- Chapp, A.D.; Wang, R.; Cheng, Z.J.; Shan, Z.; Chen, Q.H. Long-Term High Salt Intake Involves Reduced SK Currents and Increased Excitability of PVN Neurons with Projections to the Rostral Ventrolateral Medulla in Rats. Neural Plast. 2017, 2017, 7282834. [Google Scholar] [CrossRef]
- Wang, R.; Huang, Q.; Zhou, R.; Dong, Z.; Qi, Y.; Li, H.; Wei, X.; Wu, H.; Wang, H.; Wilcox, C.S.; et al. Sympathoexcitation in Rats With Chronic Heart Failure Depends on Homeobox D10 and MicroRNA-7b Inhibiting GABBR1 Translation in Paraventricular Nucleus. Circ. Heart Fail. 2016, 9, e002261. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, W.; Dong, Z.; Qi, Y.; Hultström, M.; Zhou, X.; Lai, E.Y. c-Jun N-terminal Kinase mediates prostaglandin-induced sympathoexcitation in rats with chronic heart failure by reducing GAD1 and GABRA1 expression. Acta Physiol. 2017, 219, 494–509. [Google Scholar] [CrossRef]
- Wang, R.J.; Zeng, Q.H.; Wang, W.Z.; Wang, W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin. Exp. Pharmacol. Physiol. 2009, 36, 516–522. [Google Scholar] [CrossRef]
- Mott, L.R.; Caldwell, J.L. Autonomic imbalance in cardiovascular disease: Molecular mechanisms and emerging therapeutics. Am. J. Physiol. Cell Physiol. 2025, 329, C1511–C1520. [Google Scholar] [CrossRef]
- Sakamoto, K.; Butera, M.A.; Zhou, C.; Maurizi, G.; Chen, B.; Ling, L.; Shawkat, A.; Patlolla, L.; Thakker, K.; Calle, V.; et al. Overnutrition causes insulin resistance and metabolic disorder through increased sympathetic nervous system activity. Cell Metab. 2025, 37, 121–137. [Google Scholar] [CrossRef]
- Xia, S.; Wu, H.; Ye, J. Sympathetic nerve activity in obesity-associated insulin resistance. Acta Pharm. Sin. B 2025, 15, 3827–3828. [Google Scholar] [CrossRef]
- Lazzeri, G.; Busceti, C.L.; Biagioni, F.; Fabrizi, C.; Morucci, G.; Giorgi, F.S.; Ferrucci, M.; Lenzi, P.; Puglisi-Allegra, S.; Fornai, F. Norepinephrine Protects against Methamphetamine Toxicity through β2-Adrenergic Receptors Promoting LC3 Compartmentalization. Int. J. Mol. Sci. 2021, 22, 7232. [Google Scholar] [CrossRef]
- Li, C.J.; Du, H.B.; Zhao, Z.A.; Sun, Q.; Li, Y.M.; Chen, S.J.; Zhang, H.; Zhang, N.; Niu, C.Y.; Zhao, Z.G. Stellate Ganglion Block Reverses Phsml-Induced Vascular Hyporeactivity Through Inhibiting Autophagy-Mediated Phenotypic Transformation of VSMCs. Shock 2024, 61, 414–423. [Google Scholar] [CrossRef]
- He, X.; Huang, S.; Yu, C.; Chen, Y.; Zhu, H.; Li, J.; Chen, S. Mst1/Hippo signaling pathway drives isoproterenol-induced inflammatory heart remodeling. Int. J. Med. Sci. 2024, 21, 1718–1729. [Google Scholar] [CrossRef]
- Maiers, J.L.; Chakraborty, S. The Cellular, Molecular, and Pathologic Consequences of Stress on the Liver. Am. J. Pathol. 2023, 193, 1353–1354. [Google Scholar] [CrossRef]
- Du, D.; Hu, L.; Wu, J.; Wu, Q.; Cheng, W.; Guo, Y.; Guan, R.; Wang, Y.; Chen, X.; Yan, X.; et al. Neuroinflammation contributes to autophagy flux blockage in the neurons of rostral ventrolateral medulla in stress-induced hypertension rats. J. Neuroinflamm. 2017, 14, 169. [Google Scholar] [CrossRef]
- Poothong, J.; Sopha, P.; Kaufman, R.J.; Tirasophon, W. IRE1α nucleotide sequence cleavage specificity in the unfolded protein response. FEBS Lett. 2018, 592, 2669. [Google Scholar] [CrossRef]
- Hetz, C.; Martinon, F.; Rodriguez, D.; Glimcher, L.H. The unfolded protein response: Integrating stress signals through the stress sensor IRE1α. Physiol. Rev. 2011, 91, 1219–1243. [Google Scholar] [CrossRef]
- Orsi, A.; van Anken, E.; Vitale, M.; Zamai, M.; Caiolfa, V.R.; Sitia, R.; Bakunts, A. Congress of multiple dimers is needed for cross-phosphorylation of IRE1α and its RNase activity. Life Sci. Alliance 2024, 7, e202302562. [Google Scholar] [CrossRef]
- Concha, N.O.; Smallwood, A.; Bonnette, W.; Totoritis, R.; Zhang, G.; Federowicz, K.; Yang, J.; Qi, H.; Chen, S.; Campobasso, N.; et al. Long-Range Inhibitor-Induced Conformational Regulation of Human IRE1α Endoribonuclease Activity. Mol. Pharmacol. 2015, 88, 1011–1023. [Google Scholar] [CrossRef]
- Le Thomas, A.; Ferri, E.; Marsters, S.; Harnoss, J.M.; Lawrence, D.A.; Zuazo-Gaztelu, I.; Modrusan, Z.; Chan, S.; Solon, M.; Chalouni, C.; et al. Decoding non-canonical mRNA decay by the endoplasmic-reticulum stress sensor IRE1α. Nat. Commun. 2021, 12, 7310. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R., 3rd; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef]
- Lee, A.H.; Scapa, E.F.; Cohen, D.E.; Glimcher, L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 2008, 320, 1492–1496. [Google Scholar] [CrossRef]
- Madhavan, A.; Kok, B.P.; Rius, B.; Grandjean, J.M.D.; Alabi, A.; Albert, V.; Sukiasyan, A.; Powers, E.T.; Galmozzi, A.; Saez, E.; et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 2022, 13, 608. [Google Scholar] [CrossRef]
- Tian, H.; Fang, Y.; Liu, W.; Wang, J.; Zhao, J.; Tang, H.; Yin, Y.; Hu, Y.; Peng, J. Inhibition on XBP1s-driven lipogenesis by Qushi Huayu Decoction contributes to amelioration of hepatic steatosis induced by fructose. J. Ethnopharmacol. 2023, 301, 115806. [Google Scholar] [CrossRef]
- Tsuru, A.; Fujimoto, N.; Takahashi, S.; Saito, M.; Nakamura, D.; Iwano, M.; Iwawaki, T.; Kadokura, H.; Ron, D.; Kohno, K. Negative feedback by IRE1β optimizes mucin production in goblet cells. Proc. Natl. Acad. Sci. USA 2013, 110, 2864–2869. [Google Scholar] [CrossRef]
- Grey, M.J.; De Luca, H.; Ward, D.V.; Kreulen, I.A.; Bugda Gwilt, K.; Foley, S.E.; Thiagarajah, J.R.; McCormick, B.A.; Turner, J.R.; Lencer, W.I. The epithelial-specific ER stress sensor ERN2/IRE1β enables host-microbiota crosstalk to affect colon goblet cell development. J. Clin. Investig. 2022, 132, e153519. [Google Scholar] [CrossRef]
- Wang, P.; Li, J.; Tao, J.; Sha, B. The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J. Biol. Chem. 2018, 293, 4110–4121. [Google Scholar] [CrossRef]
- Nghiem, T.T.; Nguyen, K.A.; Kusuma, F.; Park, S.; Park, J.; Joe, Y.; Han, J.; Chung, H.T. The PERK-eIF2α-ATF4 Axis Is Involved in Mediating ER-Stress-Induced Ferroptosis via DDIT4-mTORC1 Inhibition and Acetaminophen-Induced Hepatotoxicity. Antioxidants 2025, 14, 307. [Google Scholar] [CrossRef]
- Gordiyenko, Y.; Llácer, J.L.; Ramakrishnan, V. Structural basis for the inhibition of translation through eIF2α phosphorylation. Nat. Commun. 2019, 10, 2640. [Google Scholar] [CrossRef]
- Rzymski, T.; Milani, M.; Pike, L.; Buffa, F.; Mellor, H.R.; Winchester, L.; Pires, I.; Hammond, E.; Ragoussis, I.; Harris, A.L. Regulation of autophagy by ATF4 in response to severe hypoxia. Oncogene 2010, 29, 4424–4435. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, Y.; Yuan, F.; Xia, T.; Liu, H.; Li, Z.; Chen, S.; Liu, Z.; Ying, H.; Liu, Y.; et al. An ATF4-ATG5 signaling in hypothalamic POMC neurons regulates obesity. Autophagy 2017, 13, 1088–1089. [Google Scholar] [CrossRef]
- B’Chir, W.; Maurin, A.C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Xue, R.; Yang, Y.; Zhang, S.X.; Xiao, H.; Zhu, H.; Li, J.; Chen, G.; Ye, Y.; Yu, M.; et al. Activation of ATF4 triggers trabecular meshwork cell dysfunction and apoptosis in POAG. Aging 2021, 13, 8628–8642. [Google Scholar] [CrossRef]
- Shen, J.; Snapp, E.L.; Lippincott-Schwartz, J.; Prywes, R. Stable binding of ATF6 to BiP in the endoplasmic reticulum stress response. Mol. Cell. Biol. 2005, 25, 921–932. [Google Scholar] [CrossRef]
- Ye, J.; Rawson, R.B.; Komuro, R.; Chen, X.; Davé, U.P.; Prywes, R.; Brown, M.S.; Goldstein, J.L. ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 2000, 6, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yu, H.; Ding, S.; Liu, H.; Liu, C.; Fu, R. Molecular mechanism of ATF6 in unfolded protein response and its role in disease. Heliyon 2024, 10, e25937. [Google Scholar] [CrossRef]
- Ranjan, K.; Hedl, M.; Sinha, S.; Zhang, X.; Abraham, C. Ubiquitination of ATF6 by disease-associated RNF186 promotes the innate receptor-induced unfolded protein response. J. Clin. Investig. 2021, 131, e145472. [Google Scholar] [CrossRef]
- Li, J.; Huang, L.; Xiong, W.; Gu, C.; Zhang, S.; Xue, X. Effect of aerobic exercise on GRP78 and ATF6 expressions in mice with non-alcoholic fatty liver disease. Sports Med. Health Sci. 2023, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Vidal, R.L.; Sepulveda, D.; Troncoso-Escudero, P.; Garcia-Huerta, P.; Gonzalez, C.; Plate, L.; Jerez, C.; Canovas, J.; Rivera, C.A.; Castillo, V.; et al. Enforced dimerization between XBP1s and ATF6f enhances the protective effects of the UPR in models of neurodegeneration. Mol. Ther. 2021, 29, 1862–1882. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, Y.; Xiang, K.; Tang, Y. P2X(7) Receptor Facilitates Cardiomyocyte Autophagy After Myocardial Infarction via Nox4/PERK/ATF4 Signaling Pathway. Cell Biochem. Funct. 2025, 43, e70078. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. EMBO J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Liu, N.; Zhao, H.; Zhao, Y.G.; Hu, J.; Zhang, H. Atlastin 2/3 regulate ER targeting of the ULK1 complex to initiate autophagy. J. Cell Biol. 2021, 220, e202012091. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, M.; Ye, L.; Zhou, Q.; Duan, Y.; Jiang, H.; Wang, L.; Ouyang, Y.; Zhang, H.; Shen, Y.; et al. VHL suppresses autophagy and tumor growth through PHD1-dependent Beclin1 hydroxylation. EMBO J. 2024, 43, 931–955. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Zhao, G.; Zhao, H.; Noda, N.N.; Zhang, H. The autophagy protein ATG-9 regulates lysosome function and integrity. J. Cell Biol. 2025, 224, e202411092. [Google Scholar] [CrossRef]
- Xu, Y.; Qian, C.; Wang, Q.; Song, L.; He, Z.; Liu, W.; Wan, W. Deacetylation of ATG7 drives the induction of macroautophagy and LC3-associated microautophagy. Autophagy 2024, 20, 1134–1146. [Google Scholar] [CrossRef]
- Nader, W.O.; Brown, K.S.; Boyle, N.R.; Kaplelach, A.K.; Abdelaziz, S.M.; Davis, S.E.; Aljabi, Q.; Hakim, A.R.; Davidson, A.G.; Vollmer, G.A.; et al. TFEB overexpression alleviates autophagy-lysosomal deficits caused by progranulin insufficiency. Sci. Rep. 2025, 15, 26217. [Google Scholar] [CrossRef]
- Ikami, Y.; Terasawa, K.; Watabe, T.; Yokoyama, S.; Hara-Yokoyama, M. The two-domain architecture of LAMP2A within the lysosomal lumen regulates its interaction with HSPA8/Hsc70. Autophagy Rep. 2022, 1, 205–209. [Google Scholar] [CrossRef]
- Khawaja, R.R.; Martín-Segura, A.; Santiago-Fernández, O.; Sereda, R.; Lindenau, K.; McCabe, M.; Macho-González, A.; Jafari, M.; Scrivo, A.; Gomez-Sintes, R.; et al. Sex-specific and cell-type-specific changes in chaperone-mediated autophagy across tissues during aging. Nat. Aging 2025, 5, 691–708. [Google Scholar] [CrossRef]
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Rahman, M.A.; Kumar, R.; Sanchez, E.; Nazarko, T.Y. Lipid Droplets and Their Autophagic Turnover via the Raft-Like Vacuolar Microdomains. Int. J. Mol. Sci. 2021, 22, 8144. [Google Scholar] [CrossRef]
- Patel, T.P.; Yanovski, J.A. Hepatic MC3R is a regulator of lipid droplet autophagy and liver steatosis. Autophagy 2025, 22, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Matsui, T. Molecular Mechanisms of Macroautophagy, Microautophagy, and Chaperone-Mediated Autophagy. J. Nippon Med. Sch. 2024, 91, 2–9. [Google Scholar] [CrossRef]
- Yoshii, S.R.; Kuma, A.; Mizushima, N. Transgenic rescue of Atg5-null mice from neonatal lethality with neuron-specific expression of ATG5: Systemic analysis of adult Atg5-deficient mice. Autophagy 2017, 13, 763–764. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Y.; Huang, Z.; Jing, F.; Qiao, P.; Zou, Q.; Li, J.; Cai, Z. Impaired autophagy in amyloid-beta pathology: A traditional review of recent Alzheimer’s research. J. Biomed. Res. 2022, 37, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Poillet-Perez, L.; Xie, X.; Zhan, L.; Yang, Y.; Sharp, D.W.; Hu, Z.S.; Su, X.; Maganti, A.; Jiang, C.; Lu, W.; et al. Autophagy maintains tumour growth through circulating arginine. Nature 2018, 563, 569–573, Correction in Nature 2018, 565, E3. [Google Scholar] [CrossRef]
- Gao, B.; Gong, T.; Qi, Z.; Li, L.; Liu, P.; Xia, R.; Li, L.; Wang, J. Excessive autophagy of myocardial cells promotes ferroptosis and exacerbates heart failure in the state of myocardial infarction. Arch. Med. Sci. 2025, 21, 944–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Qiu, D.; Liang, X.; Huang, Y.; Wang, Y.; Jia, X.; Li, K.; Zhao, J.; Du, C.; Qiu, X.; et al. Lipotoxicity-induced STING1 activation stimulates MTORC1 and restricts hepatic lipophagy. Autophagy 2022, 18, 860–876. [Google Scholar] [CrossRef]
- Hou, W.; Zhao, F.; Fang, L.; Wang, X.; Wu, D.; Liu, C.; Leng, Y.; Gao, Y.; Fu, J.; Wang, J.; et al. Walnut-Derived Peptides Promote Autophagy via the Activation of AMPK/mTOR/ULK1 Pathway to Ameliorate Hyperglycemia in Type 2 Diabetic Mice. J. Agric. Food Chem. 2023, 71, 3751–3765. [Google Scholar] [CrossRef]
- Zou, J.; Fei, Q.; Xiao, H.; Wang, H.; Liu, K.; Liu, M.; Zhang, H.; Xiao, X.; Wang, K.; Wang, N. VEGF-A promotes angiogenesis after acute myocardial infarction through increasing ROS production and enhancing ER stress-mediated autophagy. J. Cell. Physiol. 2019, 234, 17690–17703. [Google Scholar] [CrossRef]
- Wang, L.; Ning, N.; Wang, C.; Hou, X.; Yuan, Y.; Ren, Y.; Sun, C.; Yan, Z.; Wang, X.; Liu, H. Endoplasmic reticulum stress contributed to β1-adrenoceptor autoantibody-induced reduction of autophagy in cardiomyocytes. Acta Biochim. Biophys. Sin. 2019, 51, 1016–1025. [Google Scholar] [CrossRef]
- Yuan, S.; Liang, X.; He, W.; Liang, M.; Jin, J.; He, Q. ATF4-dependent heme-oxygenase-1 attenuates diabetic nephropathy by inducing autophagy and inhibiting apoptosis in podocyte. Ren. Fail. 2021, 43, 968–979. [Google Scholar] [CrossRef]
- Jin, S.; Yan, M.; Liu, Y.; Zhang, S.; Song, H.; Cao, C.; Li, Y.; Sun, G.; Ye, L.; Chen, J.; et al. RETREG1-mediated reticulophagy is activated by an ATF4-CEBPG/C/EBPγ heterodimer and confers protection against lipotoxicity. Autophagy 2025, 21, 2614–2632. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.; Fu, L.; Yu, Z.; Xu, G.; Zhou, J.; Shen, M.; Feng, Z.; Zhu, H.; Xie, T.; et al. Protection of catalpol against triptolide-induced hepatotoxicity by inhibiting excessive autophagy via the PERK-ATF4-CHOP pathway. PeerJ 2022, 10, e12759. [Google Scholar] [CrossRef]
- Guan, G.; Yang, L.; Huang, W.; Zhang, J.; Zhang, P.; Yu, H.; Liu, S.; Gu, X. Mechanism of interactions between endoplasmic reticulum stress and autophagy in hypoxia/reoxygenation-induced injury of H9c2 cardiomyocytes. Mol. Med. Rep. 2019, 20, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, S.G. Endoplasmic reticulum stress and autophagy dysregulation in alcoholic and non-alcoholic liver diseases. Clin. Mol. Hepatol. 2020, 26, 715–727. [Google Scholar] [CrossRef]
- Moon, S.; Jung, H.S. Endoplasmic Reticulum Stress and Dysregulated Autophagy in Human Pancreatic Beta Cells. Diabetes Metab. J. 2022, 46, 533–542. [Google Scholar] [CrossRef]
- Kale, A.; Azar, M.; Cheng, V.; Robertson, H.; Coulter, S.; Mehta, P.M.; Julovi, S.M.; Patrick, E.; Ghimire, K.; Rogers, N.M. Regulating islet stress responses through CD47 activation. Diabetologia 2025, 68, 1279–1297. [Google Scholar] [CrossRef]
- Chao, Y.M.; Lai, M.D.; Chan, J.Y. Redox-sensitive endoplasmic reticulum stress and autophagy at rostral ventrolateral medulla contribute to hypertension in spontaneously hypertensive rats. Hypertension 2013, 61, 1270–1280. [Google Scholar] [CrossRef]
- Blackmore, K.; Houchen, C.J.; Simonyan, H.; Arestakesyan, H.; Stark, A.K.; Dow, S.A.; Kim, H.R.; Jeong, J.K.; Popratiloff, A.; Young, C.N. A forebrain-hypothalamic ER stress driven circuit mediates hepatic steatosis during obesity. Mol. Metab. 2024, 79, 101858. [Google Scholar] [CrossRef]
- Kim, H.R.; Tabiatnejad, P.; Arestakesyan, H.; Young, C.N. Modulation of liver lipid metabolic pathways by central nervous system ER stress. Am. J. Physiol. Endocrinol. Metab. 2025, 328, E833–E844. [Google Scholar] [CrossRef]
- Díaz, H.S.; Andrade, D.C.; Toledo, C.; Schwarz, K.G.; Pereyra, K.V.; Díaz-Jara, E.; Marcus, N.J.; Del Rio, R. Inhibition of Brainstem Endoplasmic Reticulum Stress Rescues Cardiorespiratory Dysfunction in High Output Heart Failure. Hypertension 2021, 77, 718–728. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, S.G.; Weiss, R.M.; Felder, R.B. Silencing Epidermal Growth Factor Receptor in Hypothalamic Paraventricular Nucleus Reduces Extracellular Signal-regulated Kinase 1 and 2 Signaling and Sympathetic Excitation in Heart Failure Rats. Neuroscience 2021, 463, 227–237. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, Q.; Li, J.; Yan, J.; Jing, X.; Zhang, J.; Xia, Y.; Xu, Y.; Li, Y.; He, J. MANF in POMC Neurons Promotes Brown Adipose Tissue Thermogenesis and Protects Against Diet-Induced Obesity. Diabetes 2022, 71, 2344–2359. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, L.; Jiang, J.; Li, H.; Wu, Q.; Ooi, K.; Wang, J.; Feng, Y.; Zhu, D.; Xia, C. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J. Neuroinflamm. 2020, 17, 15. [Google Scholar] [CrossRef]
- Ma, Y.; Zhao, H.P.; Yang, L.G.; Li, L.; Wang, A.L.; Zhang, X.J.; Wang, K.; Yang, B.; Zhu, Z.F.; Zhang, P.J.; et al. NADPH oxidase 2 mediates cardiac sympathetic denervation and myocyte autophagy, resulting in cardiac atrophy and dysfunction in doxorubicin-induced cardiomyopathy. Sci. Rep. 2024, 14, 6971. [Google Scholar] [CrossRef]
- Przygodda, F.; Lautherbach, N.; Buzelle, S.L.; Goncalves, D.A.; Assis, A.P.; Paula-Gomes, S.; Garófalo, M.A.R.; Heck, L.C.; Matsuo, F.S.; Mota, R.F.; et al. Sympathetic innervation suppresses the autophagic-lysosomal system in brown adipose tissue under basal and cold-stimulated conditions. J. Appl. Physiol. 2020, 128, 855–871. [Google Scholar] [CrossRef]
- Li, Y.; Du, H.B.; Jiang, L.N.; Wang, C.; Yin, M.; Zhang, L.M.; Zhang, H.; Zhao, Z.A.; Liu, Z.K.; Niu, C.Y.; et al. Stellate Ganglion Block Improves the Proliferation and Function of Splenic CD4 + T Cells Through Inhibition of Posthemorrhagic Shock Mesenteric Lymph-Mediated Autophagy. Inflammation 2021, 44, 2543–2553. [Google Scholar] [CrossRef]
- Yin, M.; Li, Z.H.; Wang, C.; Li, Y.; Zhang, H.; Du, H.B.; Zhao, Z.A.; Niu, C.Y.; Zhao, Z.G. Stellate Ganglion Blockade repairs Intestinal Mucosal Barrier through suppression of Endoplasmic Reticulum Stress following Hemorrhagic Shock. Int. J. Med. Sci. 2020, 17, 2147–2154. [Google Scholar] [CrossRef]
- Cincotta, A.H.; Cersosimo, E.; Alatrach, M.; Ezrokhi, M.; Agyin, C.; Adams, J.; Chilton, R.; Triplitt, C.; Chamarthi, B.; Cominos, N.; et al. Bromocriptine-QR Therapy Reduces Sympathetic Tone and Ameliorates a Pro-Oxidative/Pro-Inflammatory Phenotype in Peripheral Blood Mononuclear Cells and Plasma of Type 2 Diabetes Subjects. Int. J. Mol. Sci. 2022, 23, 8851. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wu, Y.; Luo, Z.; Zhong, M.; Hong, Z.; Wang, D. Intrathecal Anesthesia Prevents Ventricular Arrhythmias in Rats with Myocardial Ischemia/Reperfusion. Pharmacology 2024, 109, 253–265. [Google Scholar] [CrossRef]
- Liu, G.; Liu, Y.; Wang, R.; Hou, T.; Chen, C.; Zheng, S.; Dong, Z. Spironolactone Attenuates Doxorubicin-induced Cardiotoxicity in Rats. Cardiovasc. Ther. 2016, 34, 216–224. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Z.; Huang, S.; Wang, X.; He, S.; Liu, L.; Hu, Y.; Chen, L.; Chen, P.; Liu, S.; et al. Adipocyte IRE1α promotes PGC1α mRNA decay and restrains adaptive thermogenesis. Nat. Metab. 2022, 4, 1166–1184. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Fukuhara, A.; Kita, S.; Otsuki, M.; Shimomura, I. Possible Involvement of Adipose Tissue in Patients With Older Age, Obesity, and Diabetes With SARS-CoV-2 Infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of Hyperinsulinemia Management in COVID-19. Diabetes 2021, 70, 2745–2755. [Google Scholar] [CrossRef]
- Dong, Y.; Bai, Y.; Zhang, S.; Xu, W.; Xu, J.; Zhou, Y.; Zhang, S.; Wu, Y.; Yu, H.; Cao, N.; et al. Cyclic peptide RD808 reduces myocardial injury induced by β(1)-adrenoreceptor autoantibodies. Heart Vessel. 2019, 34, 1040–1051. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Sun, Q.; Suo, T.; Zhai, J.; Du, H.; Zhao, Z.; Li, F.; Niu, C.; Zhao, Z. Postconditioning of stellate ganglion block improves intestinal barrier function by inhibiting autophagy in conscious rats following hemorrhagic shock and resuscitation. Chin. Med. J. 2022, 135, 1003–1005. [Google Scholar] [CrossRef]
- Men, L.; Guo, J.; Cao, Y.; Huang, B.; Wang, Q.; Huo, S.; Wang, M.; Peng, D.; Peng, L.; Shi, W.; et al. IL-6/gp130/STAT3 signaling contributed to the activation of the PERK arm of the unfolded protein response in response to chronic β-adrenergic stimulation. Free Radic. Biol. Med. 2023, 205, 163–174. [Google Scholar] [CrossRef]
- Kim, J.L.; La Gamma, E.F.; Estabrook, T.; Kudrick, N.; Nankova, B.B. Whole genome expression profiling associates activation of unfolded protein response with impaired production and release of epinephrine after recurrent hypoglycemia. PLoS ONE 2017, 12, e0172789, Correction in PLoS ONE 2017, 12, e0173839. [Google Scholar] [CrossRef]
- Jeon, J.H.; Im, S.; Kim, H.S.; Lee, D.; Jeong, K.; Ku, J.M.; Nam, T.G. Chemical Chaperones to Inhibit Endoplasmic Reticulum Stress: Implications in Diseases. Drug Des. Dev. Ther. 2022, 16, 4385–4397. [Google Scholar] [CrossRef]
- Ozcan, U.; Yilmaz, E.; Ozcan, L.; Furuhashi, M.; Vaillancourt, E.; Smith, R.O.; Görgün, C.Z.; Hotamisligil, G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 2006, 313, 1137–1140. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Z.; Zhao, S.; Xiang, R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016, 6, 27486. [Google Scholar] [CrossRef]
- Kawamura, S.; Matsushita, Y.; Kurosaki, S.; Tange, M.; Fujiwara, N.; Hayata, Y.; Hayakawa, Y.; Suzuki, N.; Hata, M.; Tsuboi, M.; et al. Inhibiting SCAP/SREBP exacerbates liver injury and carcinogenesis in murine nonalcoholic steatohepatitis. J. Clin. Investig. 2022, 132, e151895. [Google Scholar] [CrossRef]
- Zhang, X.; Huo, Z.; Luan, H.; Huang, Y.; Shen, Y.; Sheng, L.; Liang, J.; Wu, F. Scutellarin ameliorates hepatic lipid accumulation by enhancing autophagy and suppressing IRE1α/XBP1 pathway. Phytother. Res. 2022, 36, 433–447. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 inhibitor empagliflozin negatively regulates IL-17/IL-23 axis-mediated inflammatory responses in T2DM with NAFLD via the AMPK/mTOR/autophagy pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef]
- Chun, H.J.; Kim, E.R.; Lee, M.; Choi, D.H.; Kim, S.H.; Shin, E.; Kim, J.H.; Cho, J.W.; Han, D.H.; Cha, B.S.; et al. Increased expression of sodium-glucose cotransporter 2 and O-GlcNAcylation in hepatocytes drives non-alcoholic steatohepatitis. Metabolism 2023, 145, 155612. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Y.; Liu, J.; Deng, Y.; Zhou, B.; Wen, Y.; Li, M.; Wen, D.; Ying, Y.; Luo, S.; et al. Metformin Alleviates Hepatic Steatosis and Insulin Resistance in a Mouse Model of High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease by Promoting Transcription Factor EB-Dependent Autophagy. Front. Pharmacol. 2021, 12, 689111. [Google Scholar] [CrossRef]
- Yu, H.; Yan, S.; Jin, M.; Wei, Y.; Zhao, L.; Cheng, J.; Ding, L.; Feng, H. Aescin can alleviate NAFLD through Keap1-Nrf2 by activating antioxidant and autophagy. Phytomedicine 2023, 113, 154746. [Google Scholar] [CrossRef]
- Zhang, M.H.; Li, J.; Zhu, X.Y.; Zhang, Y.Q.; Ye, S.T.; Leng, Y.R.; Yang, T.; Zhang, H.; Kong, L.Y. Physalin B ameliorates nonalcoholic steatohepatitis by stimulating autophagy and NRF2 activation mediated improvement in oxidative stress. Free Radic. Biol. Med. 2021, 164, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Deng, X.; Jiang, Q.; Li, G.; Zhang, J.; Zhang, N.; Xin, S.; Xu, K. Scoparone improves hepatic inflammation and autophagy in mice with nonalcoholic steatohepatitis by regulating the ROS/P38/Nrf2 axis and PI3K/AKT/mTOR pathway in macrophages. Biomed. Pharmacother. 2020, 125, 109895. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Wang, Y. Induction of Autophagy as a Therapeutic Breakthrough for NAFLD: Current Evidence and Perspectives. Biology 2025, 14, 989. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Yang, R.; Yang, Y.; Jin, S.; Li, Y.; Yuan, F.; Guo, Q.; Xiao, L.; Wang, X.; Wang, F.; et al. Stellate ganglion block ameliorates vascular calcification by inhibiting endoplasmic reticulum stress. Life Sci. 2018, 193, 1–8. [Google Scholar] [CrossRef]
- Wang, C.; Du, H.B.; Zhao, Z.A.; Zhai, J.Y.; Zhang, L.M.; Niu, C.Y.; Zhao, Z.G. Autophagy Is Involved in Stellate Ganglion Block Reversing Posthemorrhagic Shock Mesenteric Lymph-Mediated Vascular Hyporeactivity. Front. Physiol. 2021, 12, 728191. [Google Scholar] [CrossRef]
- Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Zhou, J.; Bay, B.H.; Yen, P.M. β-Adrenergic agonist and antagonist regulation of autophagy in HepG2 cells, primary mouse hepatocytes, and mouse liver. PLoS ONE 2014, 9, e98155. [Google Scholar] [CrossRef] [PubMed]
- Ji, F.; Kim, J.H. Muscle Type-Specific Modulation of Autophagy Signaling in Obesity: Effects of Caloric Restriction and Exercise. J. Obes. Metab. Syndr. 2025, 34, 303–314. [Google Scholar] [CrossRef]
- Elsayed, H.R.H.; El-Nablaway, M.; Khattab, B.A.; Sherif, R.N.; Elkashef, W.F.; Abdalla, A.M.; El Nashar, E.M.; Abd-Elmonem, M.M.; El-Gamal, R. Independent of Calorie Intake, Short-term Alternate-day Fasting Alleviates NASH, With Modulation of Markers of Lipogenesis, Autophagy, Apoptosis, and Inflammation in Rats. J. Histochem. Cytochem. 2021, 69, 575–596. [Google Scholar] [CrossRef] [PubMed]
- Varady, K.A.; Cienfuegos, S.; Ezpeleta, M.; Gabel, K. Clinical application of intermittent fasting for weight loss: Progress and future directions. Nat. Rev. Endocrinol. 2022, 18, 309–321. [Google Scholar] [CrossRef]
- Różański, G.; Pheby, D.; Newton, J.L.; Murovska, M.; Zalewski, P.; Słomko, J. Effect of Different Types of Intermittent Fasting on Biochemical and Anthropometric Parameters among Patients with Metabolic-Associated Fatty Liver Disease (MAFLD)-A Systematic Review. Nutrients 2021, 14, 91. [Google Scholar] [CrossRef]
- Guarino, M.; Kumar, P.; Felser, A.; Terracciano, L.M.; Guixé-Muntet, S.; Humar, B.; Foti, M.; Nuoffer, J.M.; St-Pierre, M.V.; Dufour, J.F. Exercise Attenuates the Transition from Fatty Liver to Steatohepatitis and Reduces Tumor Formation in Mice. Cancers 2020, 12, 1407. [Google Scholar] [CrossRef]
- Hentilä, J.; Hulmi, J.J.; Laakkonen, E.K.; Ahtiainen, J.P.; Suominen, H.; Korhonen, M.T. Sprint and Strength Training Modulates Autophagy and Proteostasis in Aging Sprinters. Med. Sci. Sports Exerc. 2020, 52, 1948–1959. [Google Scholar] [CrossRef]
- Wang, C.; Liang, J.; Ren, Y.; Huang, J.; Jin, B.; Wang, G.; Chen, N. A Preclinical Systematic Review of the Effects of Chronic Exercise on Autophagy-Related Proteins in Aging Skeletal Muscle. Front. Physiol. 2022, 13, 930185. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Acin-Perez, R.; Assali, E.A.; Martin, A.; Brownstein, A.J.; Petcherski, A.; Fernández-Del-Rio, L.; Xiao, R.; Lo, C.H.; Shum, M.; et al. Restoration of lysosomal acidification rescues autophagy and metabolic dysfunction in non-alcoholic fatty liver disease. Nat. Commun. 2023, 14, 2573. [Google Scholar] [CrossRef]
- Zagkou, S.; Marais, V.; Zeghoudi, N.; Guillou, E.L.; Eskelinen, E.L.; Panasyuk, G.; Verrier, B.; Primard, C. Design and Evaluation of Autophagy-Inducing Particles for the Treatment of Abnormal Lipid Accumulation. Pharmaceutics 2022, 14, 1379. [Google Scholar] [CrossRef]
- Lee, S.; Han, D.; Kang, H.G.; Jeong, S.J.; Jo, J.E.; Shin, J.; Kim, D.K.; Park, H.W. Intravenous sustained-release nifedipine ameliorates nonalcoholic fatty liver disease by restoring autophagic clearance. Biomaterials 2019, 197, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Salem, G.A.; Mohamed, A.A.; Khater, S.I.; Noreldin, A.E.; Alosaimi, M.; Alansari, W.S.; Shamlan, G.; Eskandrani, A.A.; Awad, M.M.; El-Shaer, R.A.A.; et al. Enhancement of biochemical and genomic pathways through lycopene-loaded nano-liposomes: Alleviating insulin resistance, hepatic steatosis, and autophagy in obese rats with non-alcoholic fatty liver disease: Involvement of SMO, GLI-1, and PTCH-1 genes. Gene 2023, 883, 147670. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.