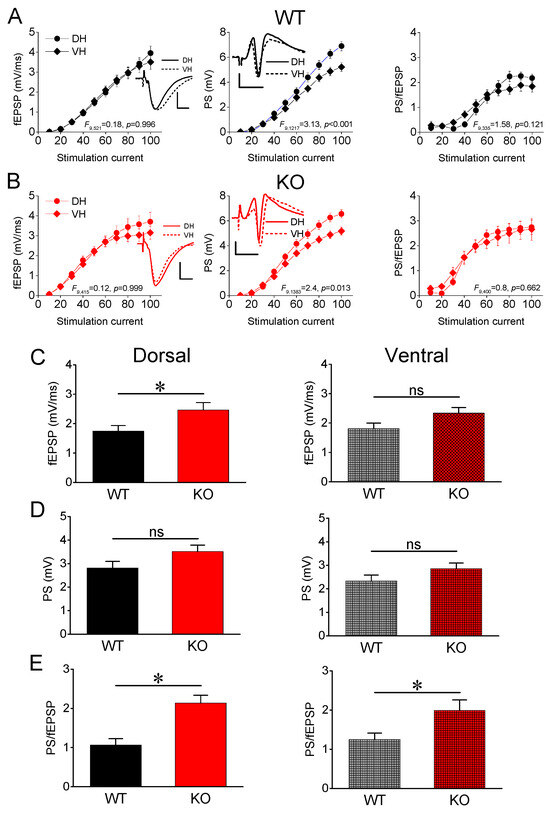
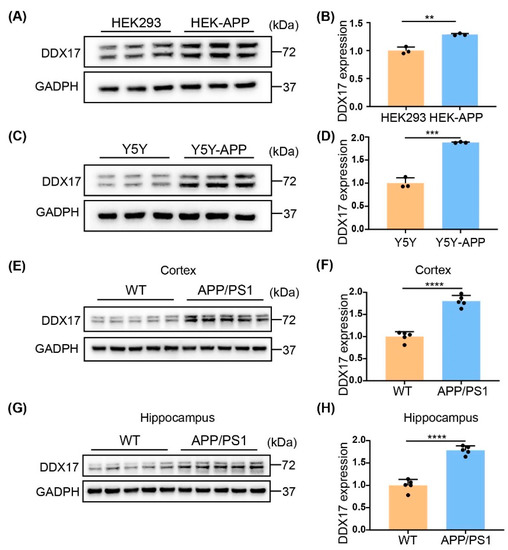
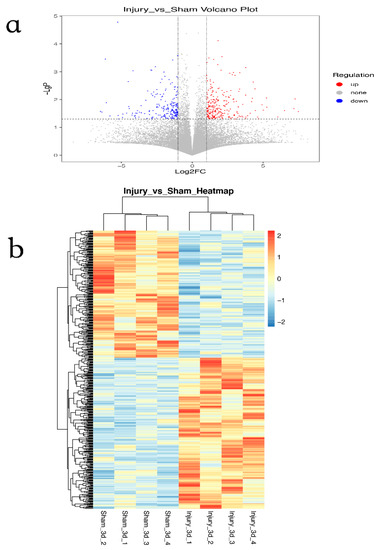
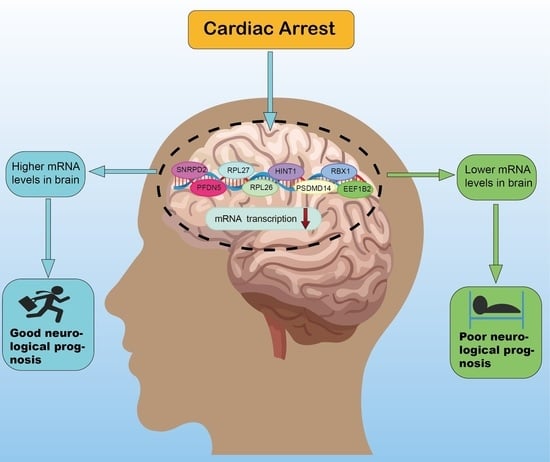
Collection on Molecular and Cellular Neuroscience
A topical collection in Brain Sciences (ISSN 2076-3425). This collection belongs to the section "Molecular and Cellular Neuroscience".
Viewed by 118346Editor
Interests: translational neuroscience; neurorepair; pharmacotherapies; neurorehabilitation; behavioural sciences
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
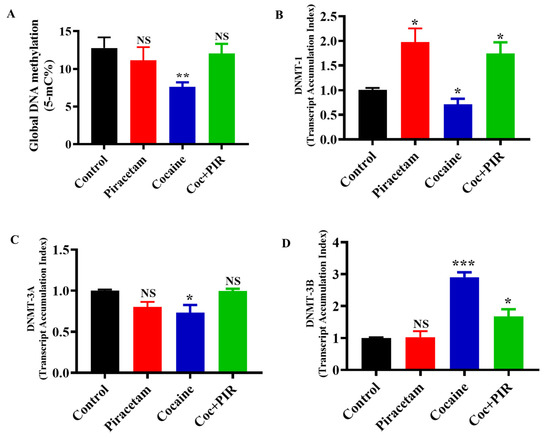
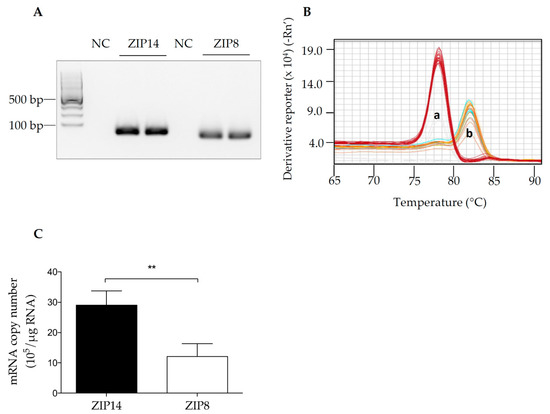
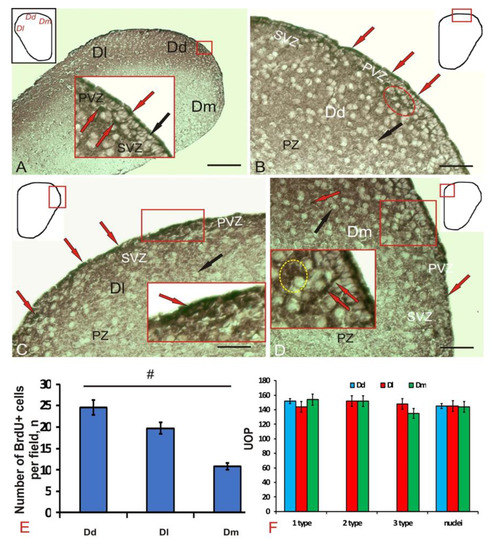
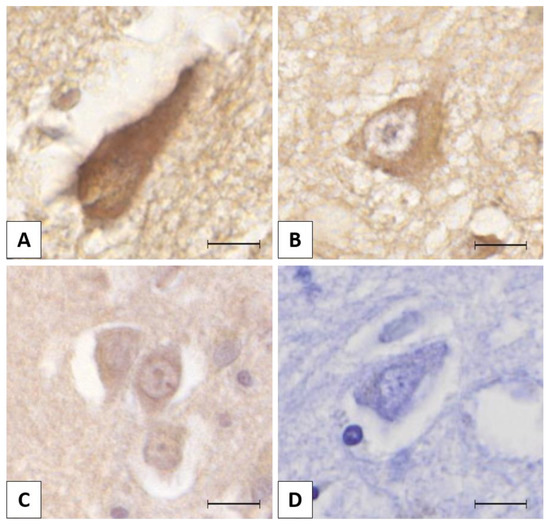
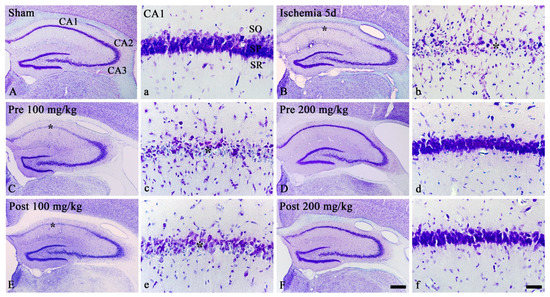
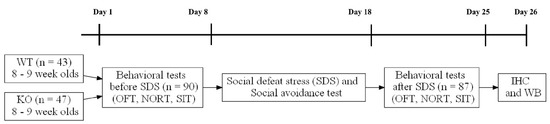
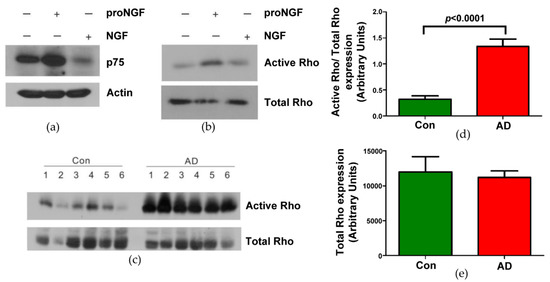
The mission of the collection on Molecular and Cellular Neuroscience is to publish cutting-edge original articles and critical reviews on the molecular and cellular basis of neurological disorders. In particular, the journal covers all aspects of molecular and cellular neuroscience, from genetic analyses of human populations to tissue culture and animal models of neurological disorders. Coverage includes experimental analyses of molecular and cellular events underpinning both developmental and adult plasticity mechanisms of the normal nervous system and occurring as a consequence of neurological dysfunction and in neuronal degeneration and repair. In particular, in relation to the latter subject, this journal section focuses on synaptic maintenance, synaptic de- and reorganization, neuron–glia communication, de- and regenerative neurobiology, molecular genetics, signal transduction, synaptic plasticity, and cell death. Of particular interest are studies using animal models of disease with translational prospects and experimental approaches utilising a bedside-to-bench approach to validate disease signatures from human patients.
Dr. Andrew Clarkson
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Brain Sciences is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2200 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.