Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Primary Human Astrocytes
2.3. Drug Treatment
2.4. Global DNA Methylation Analysis
2.5. RNA Extraction and Real-Time Quantitative PCR (qRT-PCR)
2.6. Western Blot Analysis
2.6.1. Total Lysates
2.6.2. Isolation of the Nuclear Fraction
2.6.3. Isolation of Mitochondrial Fraction
2.7. Immunofluorescence Staining
2.8. Multiplex PCR, Library Preparation and Sequencing for Targeted Next-Gen Bisulfite Sequencing (TNGBS)
Methylation Calculations
2.9. Statistical Analysis
3. Results
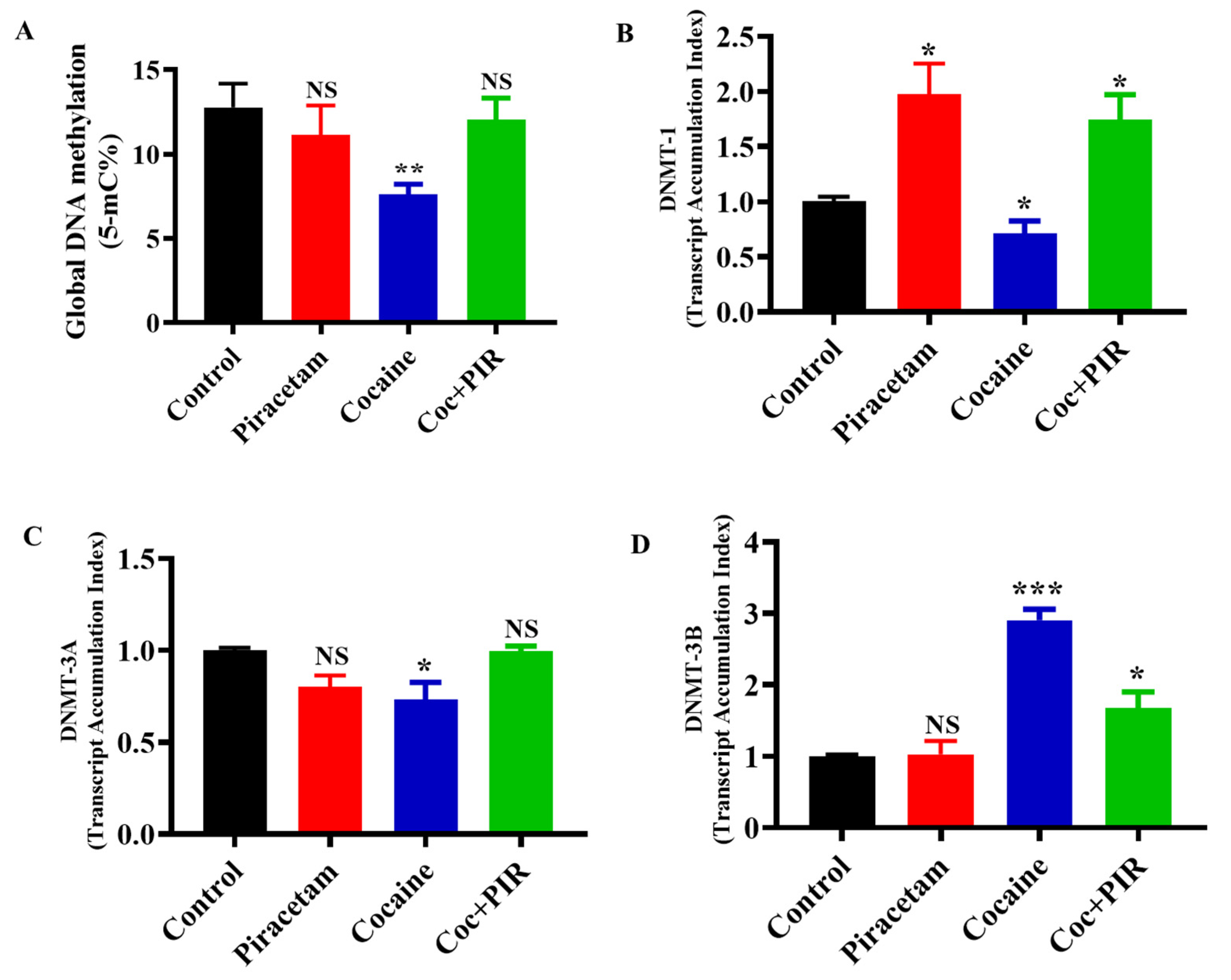
3.1. Effect of Piracetam on Cocaine-Mediated Global DNA Methylation Modifications
3.2. Protective Effect of Piracetam Against Cocaine-Induced DNMT Gene Expression in Astrocytes
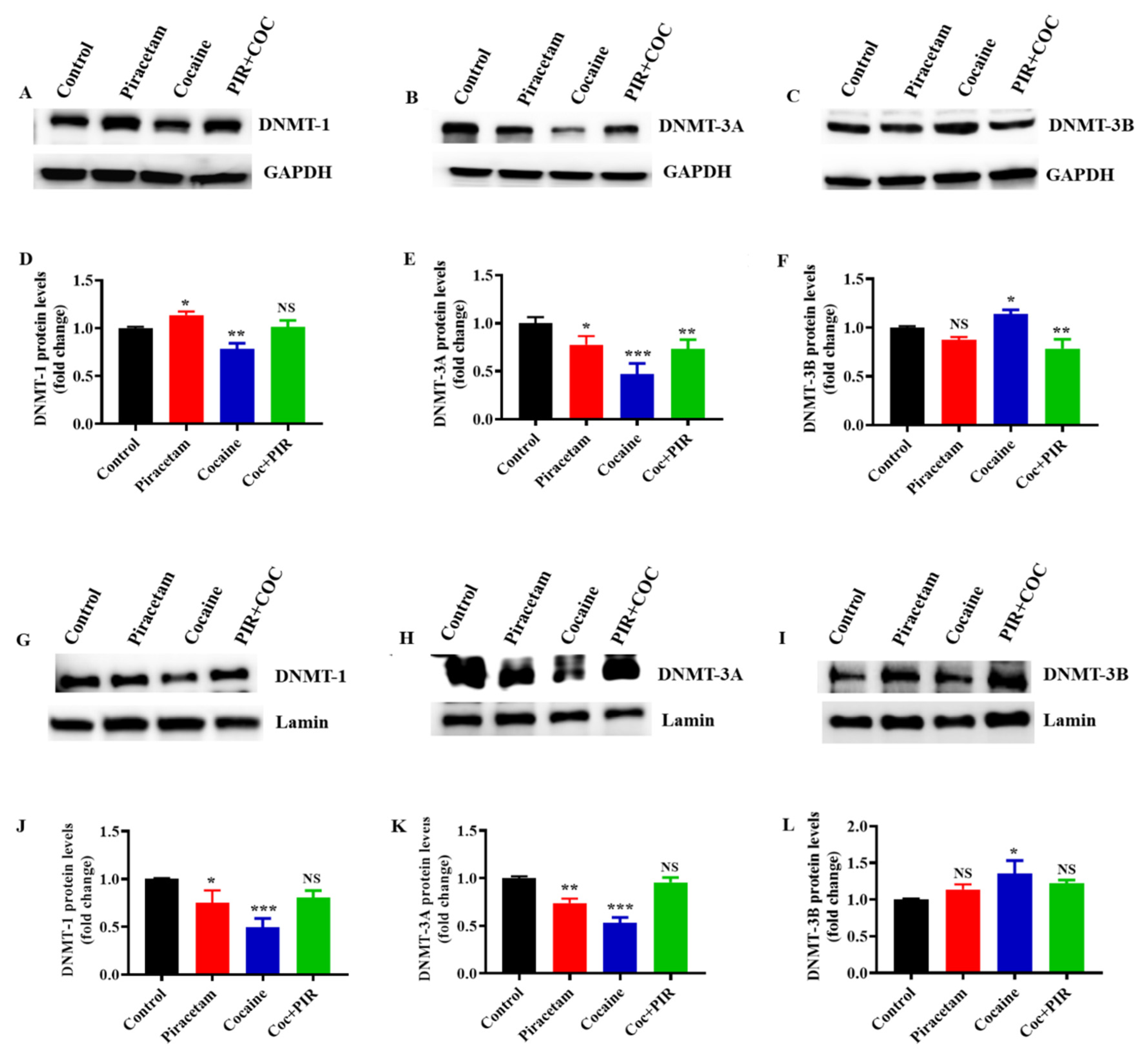
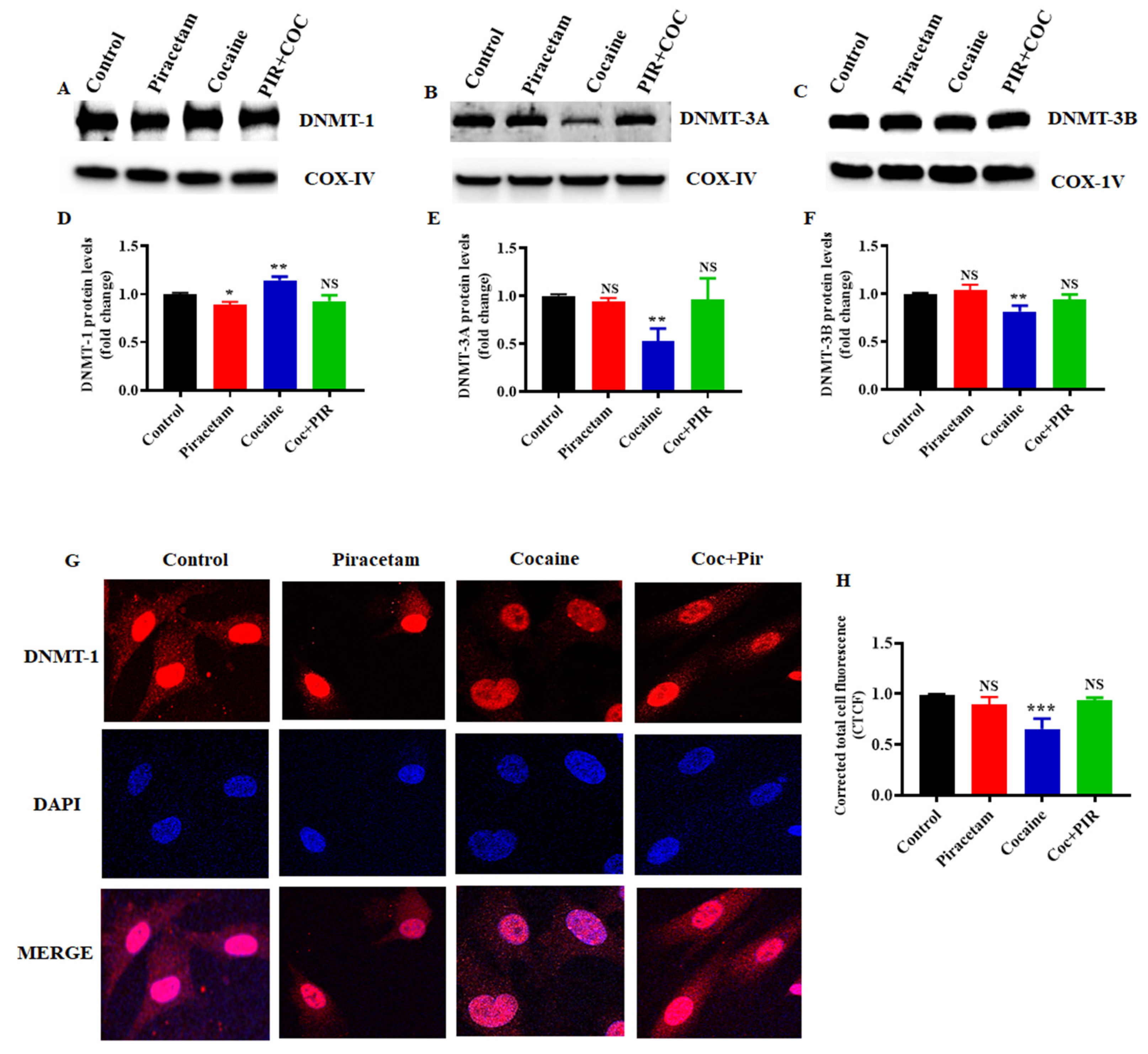
3.3. Effect of Piracetam on Cocaine-Induced DNMTs Protein Expression and Subcellular Localization (Nuclear and Mitochondria) in Astrocytes
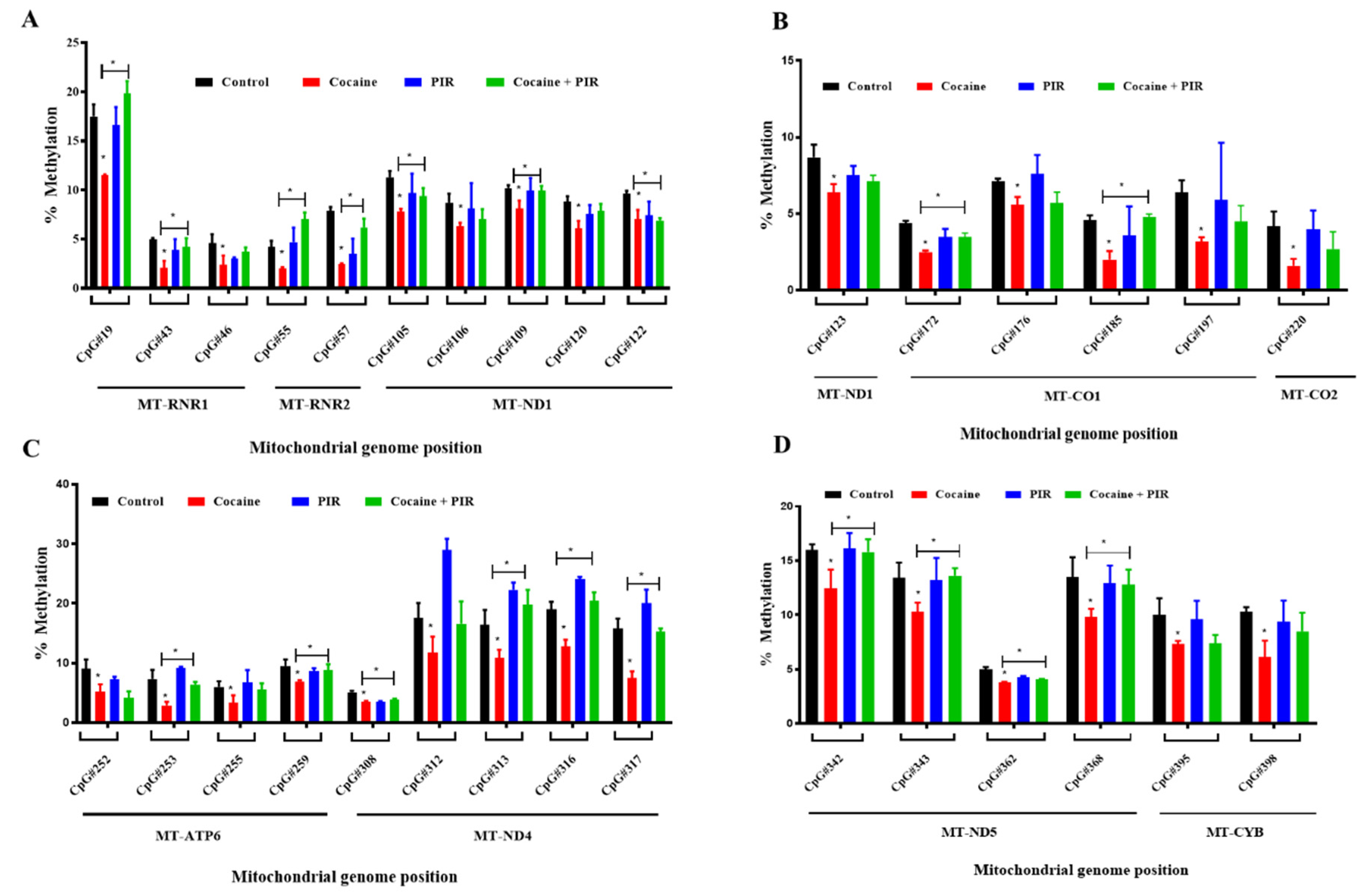
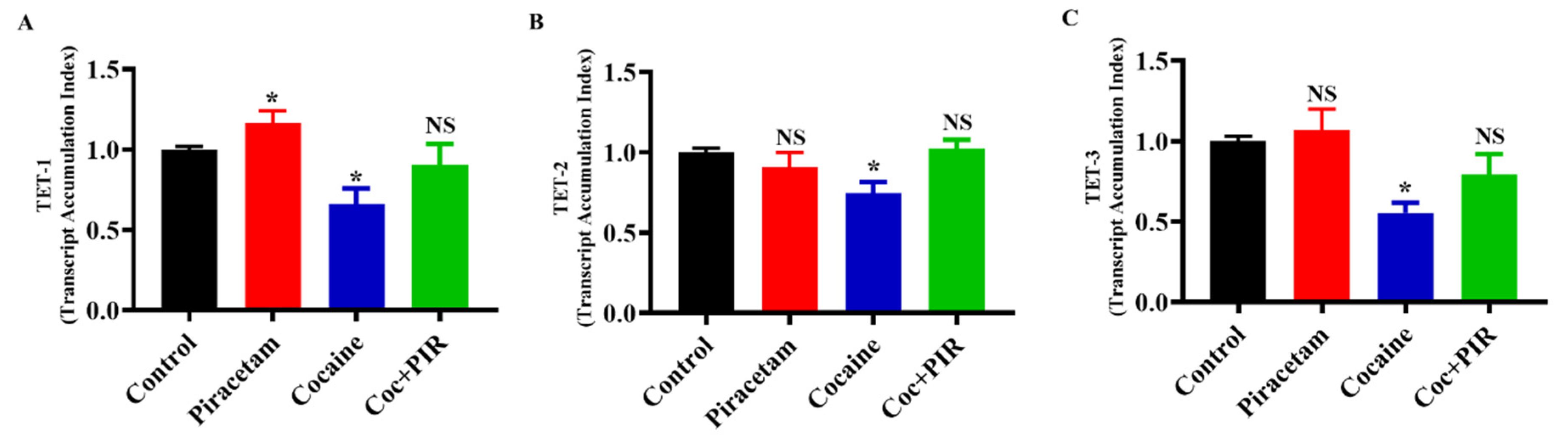
3.4. Protective Effect of Piracetam Against Cocaine-Induced DNA Demethylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Office on Drugs and Crime. Available online: https://www.unodc.org/ (accessed on 27 July 2020).
- Pimentel, E.; Sivalingam, K.; Doke, M.; Samikkannu, T. Effects of Drugs of Abuse on the Blood-Brain Barrier: A Brief Overview. Front. Neurosci. 2020, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Browne, C.J.; Godino, A.; Salery, M.; Nestler, E.J. Epigenetic Mechanisms of Opioid Addiction. Biol. Psychiatry 2020, 87, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Maze, I.; Nestler, E.J. The epigenetic landscape of addiction. Ann. N. Y. Acad. Sci. 2011, 1216, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J. Epigenetic mechanisms of drug addiction. Neuropharmacology 2014, 76, 259–268. [Google Scholar] [CrossRef]
- Ferguson, D.; Shao, N.; Heller, E.; Feng, J.; Neve, R.; Kim, H.D.; Call, T.; Magazu, S.; Shen, L.; Nestler, E.J. SIRT1-FOXO3a regulate cocaine actions in the nucleus accumbens. J. Neurosci. 2015, 35, 3100–3111. [Google Scholar] [CrossRef]
- Peng, L.; Yuan, Z.; Ling, H.; Fukasawa, K.; Robertson, K.; Olashaw, N.; Koomen, J.; Chen, J.; Lane, W.S.; Seto, E. SIRT1 Deacetylates the DNA Methyltransferase 1 (DNMT1) Protein and Alters Its Activities. Mol. Cell. Biol. 2011, 31, 4720–4734. [Google Scholar] [CrossRef]
- Okano, M.; Bell, D.W.; Haber, D.A.; Li, E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 1999, 99, 247–257. [Google Scholar] [CrossRef]
- Sharif, J.; Muto, M.; Takebayashi, S.I.; Suetake, I.; Iwamatsu, A.; Endo, T.A.; Shinga, J.; Mizutani-Koseki, Y.; Toyoda, T.; Okamura, K.; et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature 2007, 450, 908–912. [Google Scholar] [CrossRef]
- Jones, P.A.; Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 2009, 10, 805–811. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Nan, X.; Campoy, F.J.; Bird, A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 1997, 88, 471–481. [Google Scholar] [CrossRef]
- CpG Methylation Inhibits Proenkephalin Gene Expression and Binding of the Transcription Factor AP-2. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC331101/ (accessed on 27 July 2020).
- Inamdar, N.M.; Ehrlich, K.C.; Ehrlich, M. CpG methylation inhibits binding of several sequence-specific DNA-binding proteins from pea, wheat, soybean and cauliflower. Plant Mol. Biol. 1991, 17, 111–123. [Google Scholar] [CrossRef]
- Tian, W.; Zhao, M.; Li, M.; Song, T.; Zhang, M.; Quan, L.; Li, S.; Sun, Z.S. Reversal of cocaine-conditioned place preference through methyl supplementation in mice: Altering global DNA methylation in the prefrontal cortex. PLoS ONE 2012, 7, 33435. [Google Scholar] [CrossRef]
- Maresca, A.; Zaffagnini, M.; Caporali, L.; Carelli, V.; Zanna, C. DNA methyltransferase 1 mutations and mitochondrial pathology: Is mtDNA methylated? Front. Genet. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R. Pathoepigenetics: The Role of Epigenetic Biomarkers in Disease Pathogenesis. In Pharmacoepigenetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 139–189. [Google Scholar]
- Martínez-Iglesias, O.; Carrera, I.; Carril, J.C.; Fernández-Novoa, L.; Cacabelos, N.; Cacabelos, R. DNA methylation in neurodegenerative and cerebrovascular diseases disorders. Int. J. Mol. Sci. 2020, 21, 2220. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.K.; Jang, M.H.; Guo, J.U.; Kitabatake, Y.; Chang, M.L.; Pow-anpongkul, N.; Flavell, R.A.; Lu, B.; Ming, G.L.; Song, H. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 2009, 323, 1074–1077. [Google Scholar] [CrossRef]
- Lax, E.; Szyf, M. The Role of DNA Methylation in Drug Addiction: Implications for Diagnostic and Therapeutics. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2018; Volume 157, pp. 93–104. [Google Scholar]
- Winblad, B. Piracetam: A review of pharmacological properties and clinical uses. CNS Drug Rev. 2005, 11, 169–182. [Google Scholar] [CrossRef]
- Verma, D.K.; Gupta, S.; Biswas, J.; Joshi, N.; Singh, A.; Gupta, P.; Tiwari, S.; Sivarama Raju, K.; Chaturvedi, S.; Wahajuddin, M.; et al. New therapeutic activity of metabolic enhancer piracetam in treatment of neurodegenerative disease: Participation of caspase independent death factors, oxidative stress, inflammatory responses and apoptosis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2078–2096. [Google Scholar] [CrossRef]
- Stockburger, C.; Miano, D.; Pallas, T.; Friedland, K.; Müller, W.E. Enhanced Neuroplasticity by the Metabolic Enhancer Piracetam Associated with Improved Mitochondrial Dynamics and Altered Permeability Transition Pore Function. Neural Plast. 2016, 2016. [Google Scholar] [CrossRef]
- Measuring Cell Fluorescence Using ImageJ—The Open Lab Book v1.0. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 27 August 2020).
- Lu, H.; Liu, X.; Deng, Y.; Qing, H. DNA methylation, a hand behind neurodegenerative diseases. Front. Aging Neurosci. 2013, 5, 85. [Google Scholar] [CrossRef]
- O’Keefe, R.T.; Henderson, S.C.; Spector, D.L. Dynamic organization of DNA replication in mammalian cell nuclei: Spatially and temporally defined replication of chromosome-specific α- satellite DNA sequences. J. Cell Biol. 1992, 116, 1095–1110. [Google Scholar] [CrossRef] [PubMed]
- Easwaran, H.P.; Schermelleh, L.; Leonhardt, H.; Cardoso, M.C. Replication-independent chromatin loading of Dnmt1 during G2 and M phases. EMBO Rep. 2004, 5, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Szyf, M.; Tang, Y.Y.; Hill, K.G.; Musci, R. The dynamic epigenome and its implications for behavioral interventions: A role for epigenetics to inform disorder prevention and health promotion. Transl. Behav. Med. 2016, 6, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Kang, C.H.; Tsai, C.Y.; Chou, N.H.; Tu, Y.T.; Li, G.C.; Lam, H.C.; Liu, S.I.; Chang, P.M.; Lin, Y.H.; et al. Ten-eleven translocation 1 dysfunction reduces 5-hydroxymethylcytosine expression levels in gastric cancer cells. Oncol. Lett. 2018, 15, 278–284. [Google Scholar] [CrossRef]
- Xu, X.; Ji, H.; Liu, G.; Wang, Q.; Liu, H.; Shen, W.; Li, L.; Xie, X.; Zhou, W.; Duan, S. A significant association between BDNF promoter methylation and the risk of drug addiction. Gene 2016, 584, 54–59. [Google Scholar] [CrossRef]
- Wright, K.N.; Hollis, F.; Duclot, F.; Dossat, A.M.; Strong, C.E.; Chase Francis, T.; Mercer, R.; Feng, J.; Dietz, D.M.; Lobo, M.K.; et al. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J. Neurosci. 2015, 35, 8948–8958. [Google Scholar] [CrossRef]
- Robison, A.J.; Nestler, E.J. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 2011, 12, 623–637. [Google Scholar] [CrossRef]
- Vaher, K.; Anier, K.; Jürgenson, M.; Harro, J.; Kalda, A. Cocaine-induced changes in behaviour and DNA methylation in rats are influenced by inter-individual differences in spontaneous exploratory activity. J. Psychopharmacol. 2020, 34, 680–692. [Google Scholar] [CrossRef]
- Feil, J.; Sheppard, D.; Fitzgerald, P.B.; Yücel, M.; Lubman, D.I.; Bradshaw, J.L. Addiction, compulsive drug seeking, and the role of frontostriatal mechanisms in regulating inhibitory control. Neurosci. Biobehav. Rev. 2010, 35, 248–275. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Drug addiction: Updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 2016, 67, 23–50. [Google Scholar] [CrossRef]
- Fan, G.; Beard, C.; Chen, R.Z.; Csankovszki, G.; Sun, Y.; Siniaia, M.; Biniszkiewicz, D.; Bates, B.; Lee, P.P.; Kühn, R.; et al. DNA hypomethylation perturbs the function and survival of CNS neurons in postnatal animals. J. Neurosci. 2001, 21, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Malinovskaja, K.; Aonurm-Helm, A.; Zharkovsky, A.; Kalda, A. DNA methylation regulates cocaine-induced behavioral sensitization in mice. Neuropsychopharmacology 2010, 35, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Urb, M.; Niinep, K.; Matsalu, T.; Kipper, K.; Herodes, K.; Zharkovsky, A.; Timmusk, T.; Anier, K.; Kalda, A. The role of DNA methyltransferase activity in cocaine treatment and withdrawal in the nucleus accumbens of mice. Addict. Biol. 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Anier, K.; Urb, M.; Kipper, K.; Herodes, K.; Timmusk, T.; Zharkovsky, A.; Kalda, A. Cocaine-induced epigenetic DNA modification in mouse addiction-specific and non-specific tissues. Neuropharmacology 2018, 139, 13–25. [Google Scholar] [CrossRef]
- Mo, A.; Mukamel, E.A.; Davis, F.P.; Luo, C.; Henry, G.L.; Picard, S.; Urich, M.A.; Nery, J.R.; Sejnowski, T.J.; Lister, R.; et al. Epigenomic Signatures of Neuronal Diversity in the Mammalian Brain. Neuron 2015, 86, 1369–1384. [Google Scholar] [CrossRef]
- Substantial DNA Methylation Differences between Two Major Neuronal Subtypes in Human Brain. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4824074/ (accessed on 28 July 2020).
- Saini, S.K.; Mangalhara, K.C.; Prakasam, G.; Bamezai, R.N.K. DNA Methyltransferase1 (DNMT1) Isoform3 methylates mitochondrial genome and modulates its biology. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kangaspeska, S.; Stride, B.; Métivier, R.; Polycarpou-Schwarz, M.; Ibberson, D.; Carmouche, R.P.; Benes, V.; Gannon, F.; Reid, G. Transient cyclical methylation of promoter DNA. Nature 2008, 452, 112–115. [Google Scholar] [CrossRef]
- Métivier, R.; Gallais, R.; Tiffoche, C.; le Péron, C.; Jurkowska, R.Z.; Carmouche, R.P.; Ibberson, D.; Barath, P.; Demay, F.; Reid, G.; et al. Cyclical DNA methylation of a transcriptionally active promoter. Nature 2008, 452, 45–50. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Li, W.-W.; Gong, L.; Bayley, H. Single-Molecule Detection of 5-Hydroxymethylcytosine in DNA through Chemical Modification and Nanopore Analysis. Angew. Chem. Int. Ed. 2013, 52, 4350–4355. [Google Scholar] [CrossRef]
- Feng, J.; Shao, N.; Szulwach, K.E.; Vialou, V.; Huynh, J.; Zhong, C.; Le, T.; Ferguson, D.; Cahill, M.E.; Li, Y.; et al. Role of Tet1 and 5-hydroxymethylcytosine in cocaine action. Nat. Neurosci. 2015, 18, 536–544. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.L.; Roskams, A.J. Epigenetic regulation of nervous system development by DNA methylation and histone deacetylation. Prog. Neurobiol. 2009, 88, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Stresemann, C.; Lyko, F. Modes of action of the DNA methyltransferase inhibitors azacytidine and decitabine. Int. J. Cancer 2008, 123, 8–13. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivalingam, K.; Samikkannu, T. Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes. Brain Sci. 2020, 10, 611. https://doi.org/10.3390/brainsci10090611
Sivalingam K, Samikkannu T. Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes. Brain Sciences. 2020; 10(9):611. https://doi.org/10.3390/brainsci10090611
Chicago/Turabian StyleSivalingam, Kalaiselvi, and Thangavel Samikkannu. 2020. "Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes" Brain Sciences 10, no. 9: 611. https://doi.org/10.3390/brainsci10090611
APA StyleSivalingam, K., & Samikkannu, T. (2020). Neuroprotective Effect of Piracetam against Cocaine-Induced Neuro Epigenetic Modification of DNA Methylation in Astrocytes. Brain Sciences, 10(9), 611. https://doi.org/10.3390/brainsci10090611



