Localization of ZIP14 and ZIP8 in HIBCPP Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
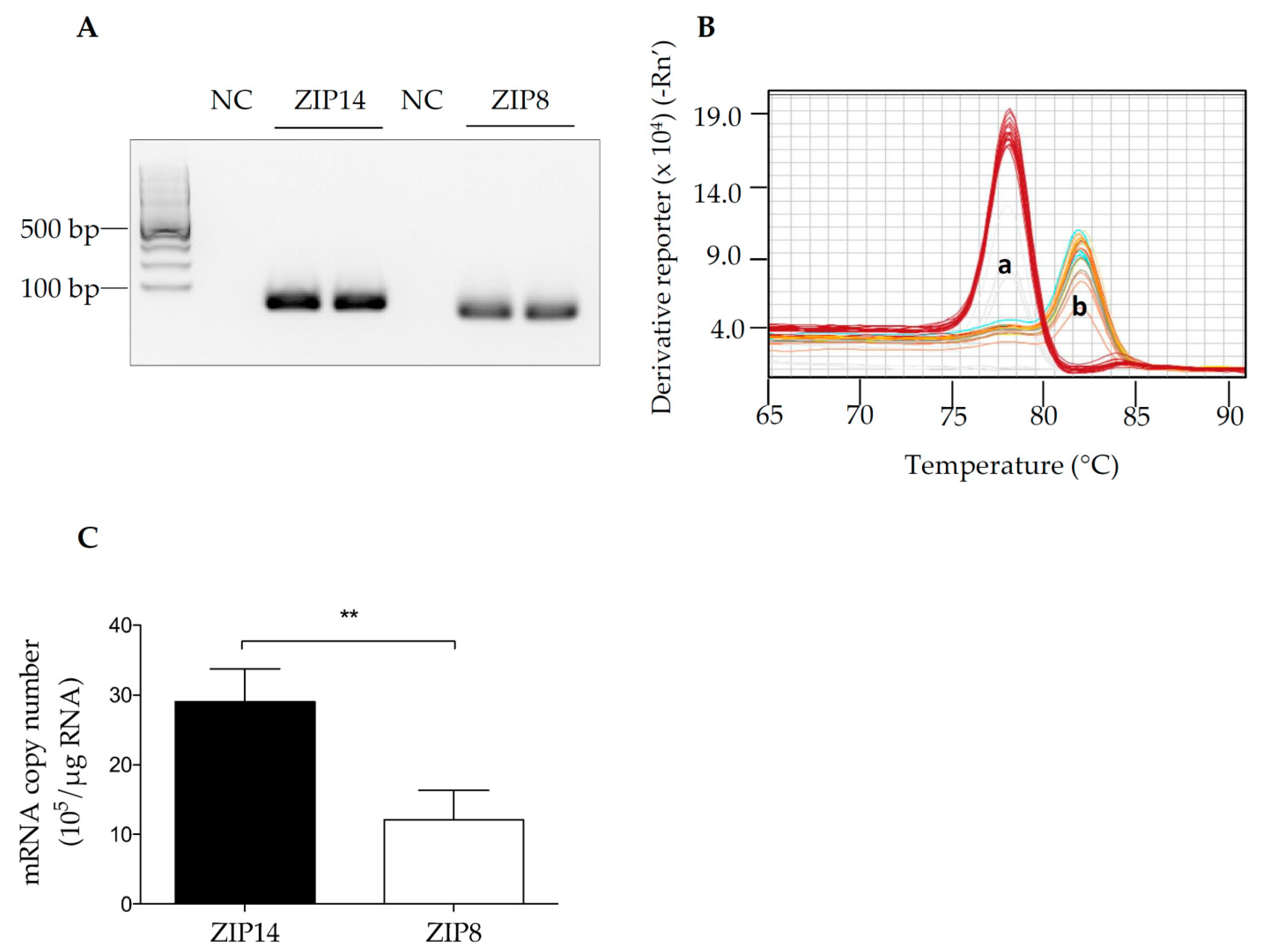
2.2. RNA Isolation, PCR, Quantitative Real-Time PCR (qRT-PCR), and Melting Curve Analysis
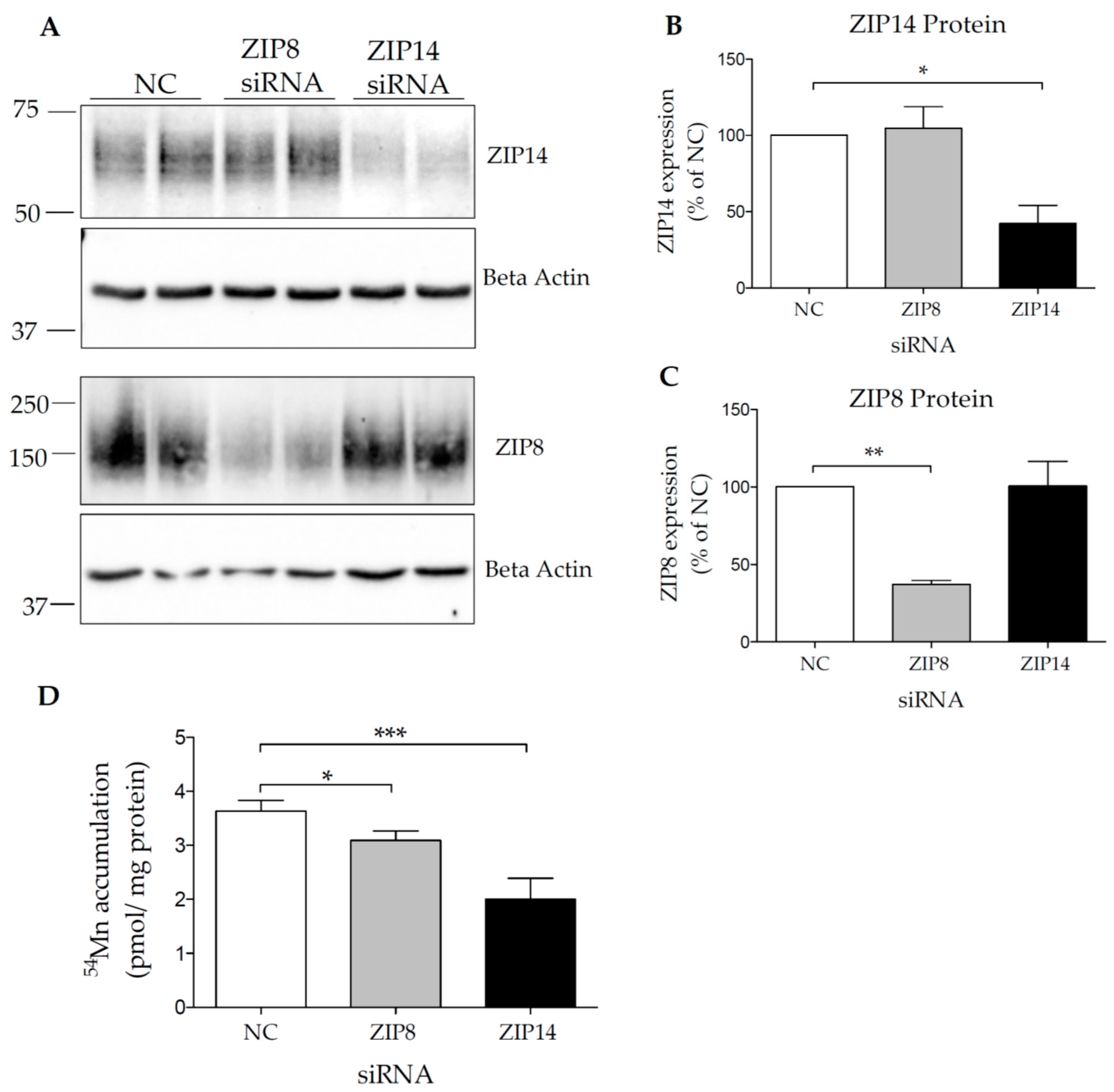
2.3. siRNA and Plasmid Transfections
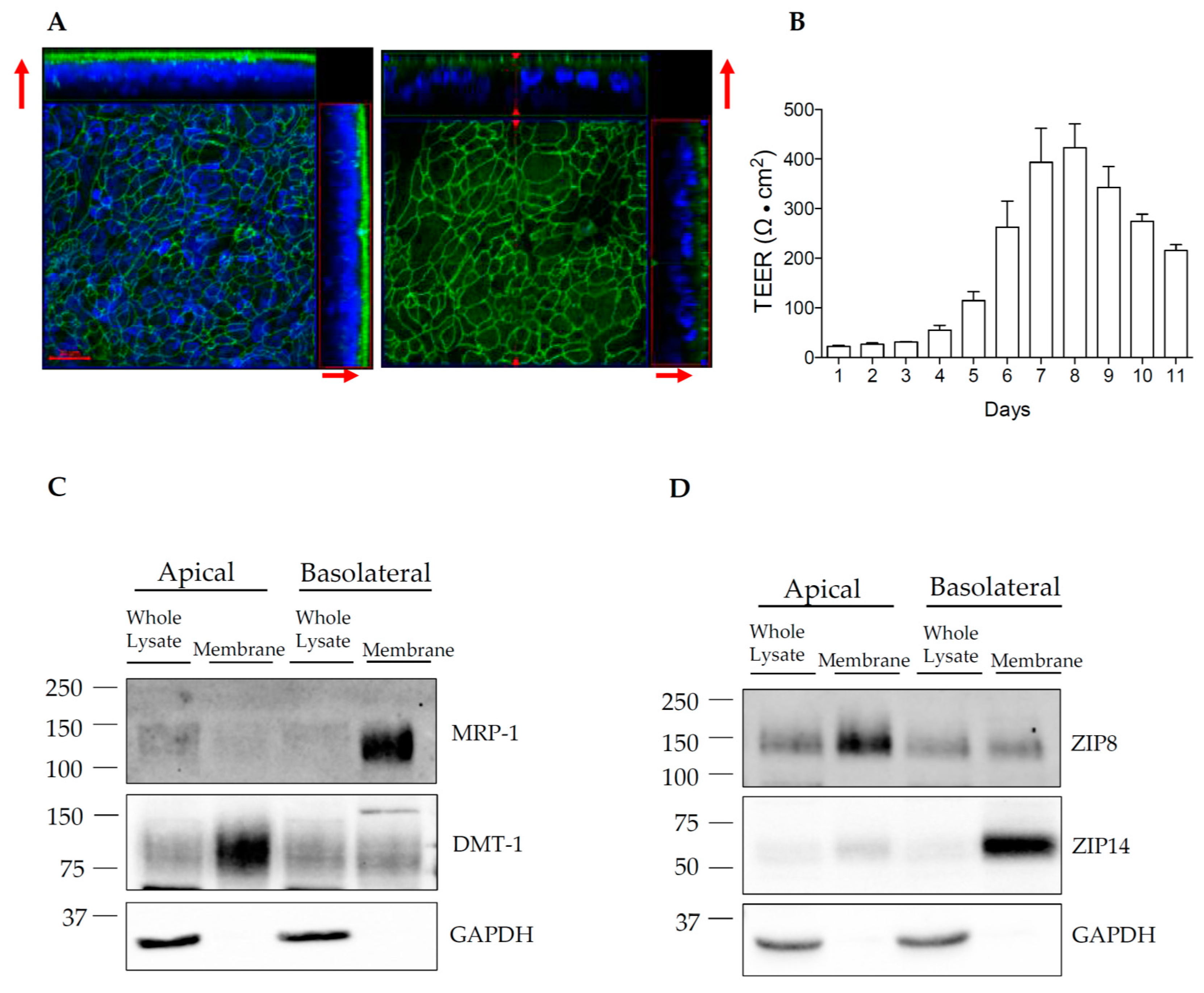
2.4. Transwell Culture
2.5. Measurement of Transepithelial Electrical Resistance (TEER)
2.6. Cell Surface Protein Biotinylation
2.7. Manganese Uptake
2.8. Immunocytochemistry and Confocal Microscopy
2.9. Western Blotting Analysis
2.10. Statistical Analysis
3. Results
3.1. The Detection of ZIP14 and ZIP8 mRNAs in HIBCPP Cells
3.2. Both ZIP14 and ZIP8 Proteins Are Present in HIBCPP Cells
3.3. Both ZIP14 and ZIP8 Contribute to Mn Accumulation in HIBCPP Cells
3.4. Establishment of HIBCPP Transwell Cultures
3.5. ZIP14 and ZIP8 Are Expressed On Opposite Sides of Polarized HIBCPP Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Majerova, P.; Michalicova, A.; Cente, M.; Hanes, J.; Vegh, J.; Kittel, A.; Kosikova, N.; Cigankova, V.; Mihaljevic, S.; Jadhav, S.; et al. Trafficking of immune cells across the blood-brain barrier is modulated by neurofibrillary pathology in tauopathies. PLoS ONE 2019, 14, e0217216. [Google Scholar] [CrossRef] [PubMed]
- Haddad-Tóvolli, R.; Dragano, N.R.V.; Ramalho, A.F.S.; Velloso, L.A. Development and function of the blood-brain barrier in the context of metabolic control. Front. Neurosci. 2017, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ghersi-Egea, J.-F. Brain Barrier Systems Play No Small Roles in Toxicant-Induced Brain Disorders. Toxicol. Sci. 2020, 175, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Bernd, A.; Ott, M.; Ishikawa, H.; Schroten, H.; Schwerk, C.; Fricker, G. Characterization of efflux transport proteins of the human choroid plexus papilloma cell line HIBCPP, a functional in vitro model of the blood-cerebrospinal fluid barrier. Pharm. Res. 2015, 32, 2973–2982. [Google Scholar] [CrossRef]
- Dinner, S.; Borkowski, J.; Stump-Guthier, C.; Ishikawa, H.; Tenenbaum, T.; Schroten, H.; Schwerk, C. A choroid plexus epithelial cell-based model of the human blood-cerebrospinal fluid barrier to study bacterial infection from the basolateral side. J. Vis. Exp. 2016, 2016. [Google Scholar] [CrossRef]
- Wiatr, M.; Stump-Guthier, C.; Latorre, D.; Uhlig, S.; Weiss, C.; Ilonen, J.; Engelhardt, B.; Ishikawa, H.; Schwerk, C.; Schroten, H.; et al. Distinct migratory pattern of naive and effector T cells through the blood-CSF barrier following Echovirus 30 infection. J. Neuroinflam. 2019, 16. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell Off. J. Hum. Cell Res. Soc. 2005, 18, 67–72. [Google Scholar] [CrossRef]
- Schneider, H.; Weber, C.E.; Schoeller, J.; Steinmann, U.; Borkowski, J.; Ishikawa, H.; Findeisen, P.; Adams, O.; Doerries, R.; Schwerk, C.; et al. Chemotaxis of T-cells after infection of human choroid plexus papilloma cells with Echovirus 30 in an in vitro model of the blood-cerebrospinal fluid barrier. Virus Res. 2012, 170, 66–74. [Google Scholar] [CrossRef]
- Roos, P.M.; Lierhagen, S.; Flaten, T.P.; Syversen, T.; Vesterberg, O.; Nordberg, M. Manganese in cerebrospinal fluid and blood plasma of patients with amyotrophic lateral sclerosis. Exp. Biol. Med. 2012, 237, 803–810. [Google Scholar] [CrossRef]
- Tayarani, I.; Cloëz, I.; Clément, M.; Bourre, J.-M. Antioxidant Enzymes and Related Trace Elements in Aging Brain Capillaries and Choroid Plexus. J. Neurochem. 1989, 53, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Bornhorst, J.; Wehe, C.A.; Hüwel, S.; Karst, U.; Galla, H.J.; Schwerdtle, T. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in Vitro. J. Biol. Chem. 2012, 287, 17140–17151. [Google Scholar] [CrossRef] [PubMed]
- Skjørringe, T.; Burkhart, A.; Johnsen, K.B.; Moos, T. Divalent metal transporter 1 (DMT1) in the brain: Implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front. Mol. Neurosci. 2015, 8, 19. [Google Scholar] [PubMed]
- Jenkitkasemwong, S.; Akinyode, A.; Paulus, E.; Weiskirchen, R.; Hojyo, S.; Fukada, T.; Giraldo, G.; Schrier, J.; Garcia, A.; Janus, C.; et al. SLC39A14 deficiency alters manganese homeostasis and excretion resulting in brain manganese accumulation and motor deficits in mice. Proc. Natl. Acad. Sci. USA 2018, 115, e1769–e1778. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Gao, H.; Wang, J.; Qiang, Y.; Imam, M.U.; Li, Y.; Wang, J.; Zhang, R.; Zhang, H.; Yu, Y.; et al. Manganese transporter Slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017, 3. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Kim, M.H.; Kim, J.; Colon-Perez, L.M.; Banan, G.; Mareci, T.H.; Febo, M.; Cousins, R.J. Metal transporter Zip14 (Slc39a14) deletion in mice increases manganese deposition and produces neurotoxic signatures and diminished motor activity. J. Neurosci. 2017, 37, 5996–6006. [Google Scholar] [CrossRef]
- Lin, W.; Vann, D.R.; Doulias, P.T.; Wang, T.; Landesberg, G.; Li, X.; Ricciotti, E.; Scalia, R.; He, M.; Hand, N.J.; et al. Hepatic metal ion transporter ZIP8 regulates manganese homeostasis and manganese-dependent enzyme activity. J. Clin. Investig. 2017, 127, 2407–2417. [Google Scholar] [CrossRef]
- Tuschl, K.; Meyer, E.; Valdivia, L.E.; Zhao, N.; Dadswell, C.; Abdul-Sada, A.; Hung, C.Y.; Simpson, M.A.; Chong, W.K.; Jacques, T.S.; et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Marti-Sanchez, L.; Ortigoza-Escobar, J.D.; Darling, A.; Villaronga, M.; Baide, H.; Molero-Luis, M.; Batllori, M.; Vanegas, M.I.; Muchart, J.; Aquino, L.; et al. Hypermanganesemia due to mutations in SLC39A14: Further insights into Mn deposition in the central nervous system. Orphanet J. Rare Dis. 2018, 13. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Cousins, R.J. The multiple faces of the metal transporter ZIP14 (SLC39A14). J. Nutr. 2018, 148, 174–184. [Google Scholar] [CrossRef]
- Scheiber, I.F.; Wu, Y.; Morgan, S.E.; Zhao, N. The intestinal metal transporter ZIP14 maintains systemic manganese homeostasis. J. Biol. Chem. 2019, 294, 9147–9160. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Hogrebe, M.; Grüneberg, M.; Duchesne, I.; Von Der Heiden, A.L.; Reunert, J.; Schlingmann, K.P.; Boycott, K.M.; Beaulieu, C.L.; Mhanni, A.A.; et al. SLC39A8 Deficiency: A Disorder of Manganese Transport and Glycosylation. Am. J. Hum. Genet. 2015, 97, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Boycott, K.M.; Beaulieu, C.L.; Kernohan, K.D.; Gebril, O.H.; Mhanni, A.; Chudley, A.E.; Redl, D.; Qin, W.; Hampson, S.; Küry, S.; et al. Autosomal-Recessive Intellectual Disability with Cerebellar Atrophy Syndrome Caused by Mutation of the Manganese and Zinc Transporter Gene SLC39A8. Am. J. Hum. Genet. 2015, 97, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.G.; Cowley, M.J.; Gayevskiy, V.; Roscioli, T.; Thorburn, D.R.; Prelog, K.; Bahlo, M.; Sue, C.M.; Balasubramaniam, S.; Christodoulou, J. A SLC39A8 variant causes manganese deficiency, and glycosylation and mitochondrial disorders. J. Inherit. Metab. Dis. 2017, 40, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Wessling-Resnick, M. ZIP14 is degraded in response to manganese exposure. BioMetals 2019, 32, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.J.; Hein, J.; Baez, A.; Sosa, J.C.; Wessling-Resnick, M. Manganese transport and toxicity in polarized WIF-B hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, g351–g363. [Google Scholar] [CrossRef] [PubMed]
- Pinilla-Tenas, J.J.; Sparkman, B.K.; Shawki, A.; Illing, A.C.; Mitchell, C.J.; Zhao, N.; Liuzzi, J.P.; Cousins, R.J.; Knutson, M.D.; Mackenzie, B. Zip14 is a complex broad-scope metal-ion transporter whose functional properties support roles in the cellular uptake of zinc and nontransferrin-bound iron. Am. J. Physiol. Cell Physiol. 2011, 301, c862. [Google Scholar] [CrossRef]
- Fujishiro, H.; Yano, Y.; Takada, Y.; Tanihara, M.; Himeno, S. Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 2012, 4, 700–708. [Google Scholar] [CrossRef]
- He, L.; Girijashanker, K.; Dalton, T.P.; Reed, J.; Li, H.; Soleimani, M.; Nebert, D.W. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: Characterization of transporter properties. Mol. Pharmacol. 2006, 70, 171–180. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef]
- Häuser, S.; Wegele, C.; Stump-Guthier, C.; Borkowski, J.; Weiss, C.; Rohde, M.; Ishikawa, H.; Schroten, H.; Schwerk, C.; Adam, R. Capsule and fimbriae modulate the invasion of Haemophilus influenzae in a human blood-cerebrospinal fluid barrier model. Int. J. Med. Microbiol. 2018, 308, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Häuser, S.; Stump-Guthier, C.; Weiss, C.; Rohde, M.; Kim, K.S.; Ishikawa, H.; Schroten, H.; Schwerk, C.; Adam, R. Virulence factor-dependent basolateral invasion of choroid plexus epithelial cells by pathogenic Escherichia coli in vitro. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, T.; Steinmann, U.; Friedrich, C.; Berger, J.; Schwerk, C.; Schroten, H. Culture models to study leukocyte trafficking across the choroid plexus. Fluids Barriers CNS 2013, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Gao, J.; Enns, C.A.; Knutson, M.D. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010, 285, 32141–32150. [Google Scholar] [CrossRef]
- Taylor, K.M.; Morgan, H.E.; Johnson, A.; Nicholson, R.I. Structure-Function analysis of a novel member of the LIV-1 subfamily of zinc transporters, ZIP14. FEBS Lett. 2005, 579, 427–432. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteomics 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Wang, B.; Schneider, S.N.; Dragin, N.; Girijashanker, K.; Dalton, T.P.; He, L.; Miller, M.L.; Stringer, K.F.; Soleimani, M.; Richardson, D.D.; et al. Enhanced cadmium-induced testicular necrosis and renal proximal tubule damage caused by gene-dose increase in a Slc39a8-transgenic mouse line. Am. J. Physiol. Cell Physiol. 2007, 292. [Google Scholar] [CrossRef]
- Troche, C.; Aydemir, T.B.; Cousins, R.J. Zinc transporter Slc39a14 regulates inflammatory signaling associated with hypertrophic adiposity. Am. J. Physiol. Endocrinol. Metab. 2016, 310, e258–e268. [Google Scholar] [CrossRef]
- Schwerk, C.; Tenenbaum, T.; Kim, K.S.; Schroten, H. The choroid plexus—A multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Griffiths, G.; Lucocq, J.M. Antibodies for immunolabeling by light and electron microscopy: Not for the faint hearted. Histochem. Cell Biol. 2014, 142, 347–360. [Google Scholar] [CrossRef]
- Mulindwa, J.; Mercé, C.; Matovu, E.; Enyaru, J.; Clayton, C. Transcriptomes of newly-isolated Trypanosoma brucei rhodesiense reveal hundreds of mRNAs that are co-regulated with stumpy-form markers. BMC Genomics 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, T.B.; Liuzzi, J.P.; McClellan, S.; Cousins, R.J. Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-γ expression in activated human T cells. J. Leukoc. Biol. 2009, 86, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kosman, D.J. Molecular mechanisms of non-transferrin-bound and transferring-bound iron uptake in primary hippocampal neurons. J. Neurochem. 2015, 133, 668–683. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Alarcon, N.O.; Zhao, N. Manganese Uptake by A549 Cells is Mediated by Both ZIP8 and ZIP14. Nutrients 2019, 11, 1473. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Cell Physiol. 2017, 312, c673–c686. [Google Scholar] [CrossRef]
- Puckett, C.A.; Ernst, R.J.; Barton, J.K. Exploring the cellular accumulation of metal complexes. Dalton Trans. 2010, 39, 1159–1170. [Google Scholar] [CrossRef]
- Cao, X.; Surma, M.A.; Simons, K. Polarized sorting and trafficking in epithelial cells. Cell Res. 2012, 22, 793–805. [Google Scholar] [CrossRef]
- Sargiacomo, M.; Lisanti, M.; Graeve, L.; Le Bivic, A.; Rodriguez-Boulan, E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J. Membr. Biol. 1989, 107, 277–286. [Google Scholar] [CrossRef]
- Caceres, P.S.; Gravotta, D.; Zager, P.J.; Dephoure, N.; Rodriguez-Boulan, E. Quantitative proteomics of MDCK cells identify unrecognized roles of clathrin adaptor AP-1 in polarized distribution of surface proteins. Proc. Natl. Acad. Sci. USA 2019, 116, 11796–11805. [Google Scholar] [CrossRef]
- Canonne-Hergaux, F.; Gros, P. Expression of the iron transporter DMT1 in kidney from normal and anemic mk mice. Kidney Int. 2002, 62, 147–156. [Google Scholar] [CrossRef]
- Wijnholds, J.; De Lange, E.C.M.; Scheffer, G.L.; Van Den Berg, D.J.; Mol, C.A.A.M.; Van Der Valk, M.; Schinkel, A.H.; Scheper, R.J.; Breimer, D.D.; Borst, P. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J. Clin. Investig. 2000, 105, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Baehr, C.; Reichel, V.; Fricker, G. Choroid plexus epithelial monolayers—A cell culture model from porcine brain. Cerebrospinal Fluid Res. 2006, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Schroten, M.; Hanisch, F.-G.; Quednau, N.; Stump, C.; Riebe, R.; Lenk, M.; Wolburg, H.; Tenenbaum, T.; Schwerk, C. A Novel Porcine In Vitro Model of the Blood-Cerebrospinal Fluid Barrier with Strong Barrier Function. PLoS ONE 2012, 7, e39835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, W.; Zhao, Q.; Graziano, J.H. Primary culture of choroidal epithelial cells: Characterization of an in vitro model of blood-CSF barrier. In Vitro Cell. Dev. Biol. Anim. 1998, 34, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Monnot, A.D.; Zheng, W. Culture of choroid plexus epithelial cells and in vitro model of blood-CSF barrier. Methods Mol. Biol. 2013, 945, 13–29. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Monuki, E.S. Proliferation of Cultured Mouse Choroid Plexus Epithelial Cells. PLoS ONE 2015, 10, e0121738. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D.; Wakefield, M.J.; Lindsay, H.; Stratzielle, N.; Ghersi-Egea, J.F.; Liddelow, S.A. Influx mechanisms in the embryonic and adult rat choroid plexus: A transcriptome study. Front. Neurosci. 2015, 9, 123. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, S.E.; Schroten, H.; Ishikawa, H.; Zhao, N. Localization of ZIP14 and ZIP8 in HIBCPP Cells. Brain Sci. 2020, 10, 534. https://doi.org/10.3390/brainsci10080534
Morgan SE, Schroten H, Ishikawa H, Zhao N. Localization of ZIP14 and ZIP8 in HIBCPP Cells. Brain Sciences. 2020; 10(8):534. https://doi.org/10.3390/brainsci10080534
Chicago/Turabian StyleMorgan, Shannon E., Horst Schroten, Hiroshi Ishikawa, and Ningning Zhao. 2020. "Localization of ZIP14 and ZIP8 in HIBCPP Cells" Brain Sciences 10, no. 8: 534. https://doi.org/10.3390/brainsci10080534
APA StyleMorgan, S. E., Schroten, H., Ishikawa, H., & Zhao, N. (2020). Localization of ZIP14 and ZIP8 in HIBCPP Cells. Brain Sciences, 10(8), 534. https://doi.org/10.3390/brainsci10080534



