Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Study Design
2.3. Behavioral Measures
2.3.1. Social Defeat Stress (SDS)
2.3.2. Social Avoidance Test
2.3.3. Open Field Test (OFT)
2.3.4. Novel Object Recognition Test (NORT)
2.3.5. Social Interaction Test
2.4. Immunohistochemistry (IHC)
2.5. Western Blotting
2.5.1. Preparation of Brain Tissue for Western Blot
2.5.2. Total STMN1 and STMN1 Phosphorylated at Serine 16 (pS16-STMN1)
2.5.3. Short (D2S) and Long (D2L) Forms of The Dopamine D2 Receptor (D2R)
2.5.4. Total Dopamine- and Cyclic Adenosine 3′,5′-Monophosphate-regulated phosphoprotein-32 (DARPP-32), DARPP-32 Phosphorylated at Threonine 34 (pDARPP-32 Thr34), and DARPP-32 Phosphorylated at Threonine 75 (pDARPP-32 Thr75)
2.6. Statistical Analysis
3. Results
3.1. Social Avoidance Test (Table 1)
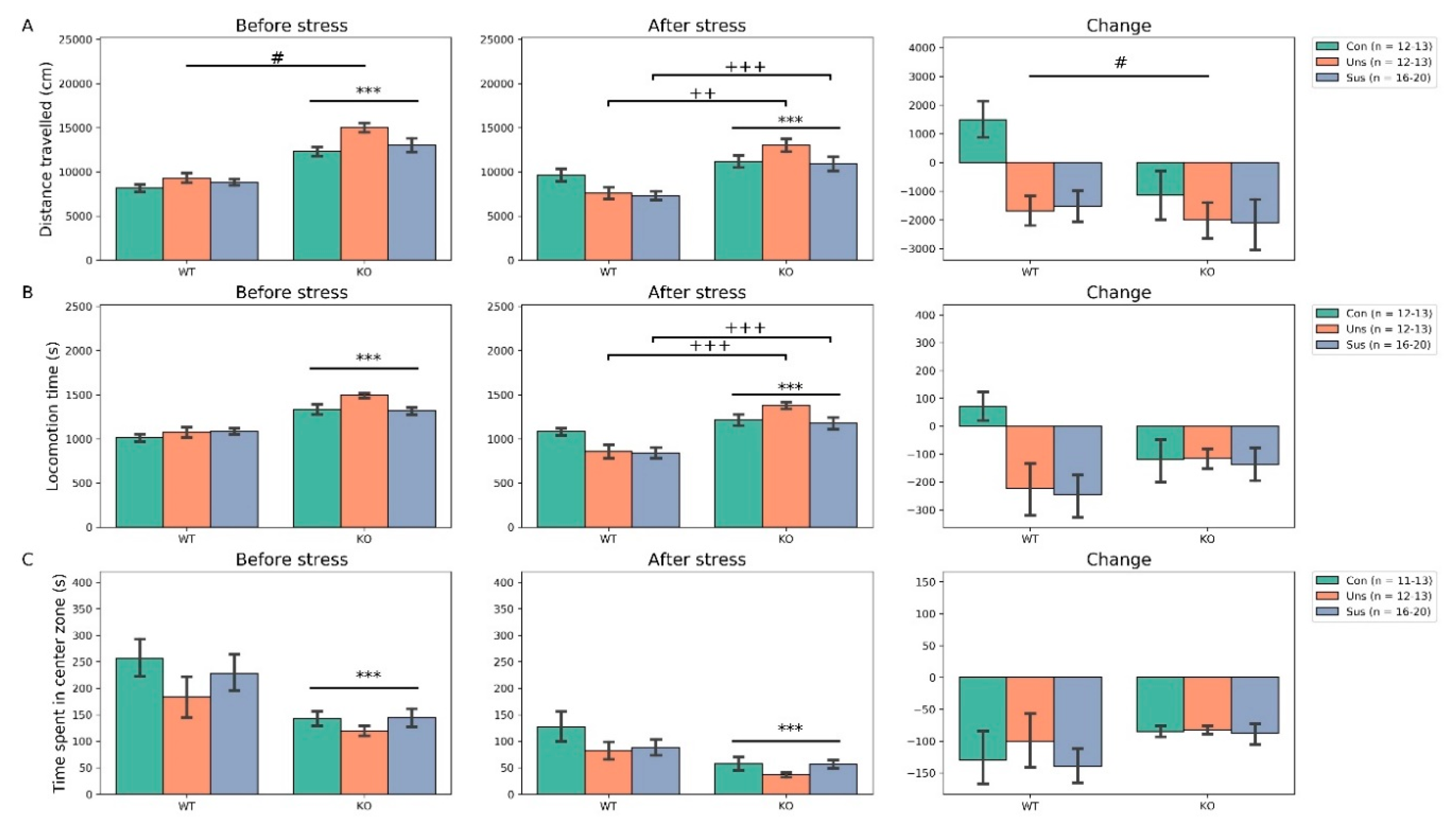
3.2. Open Field Test (Figure 2)
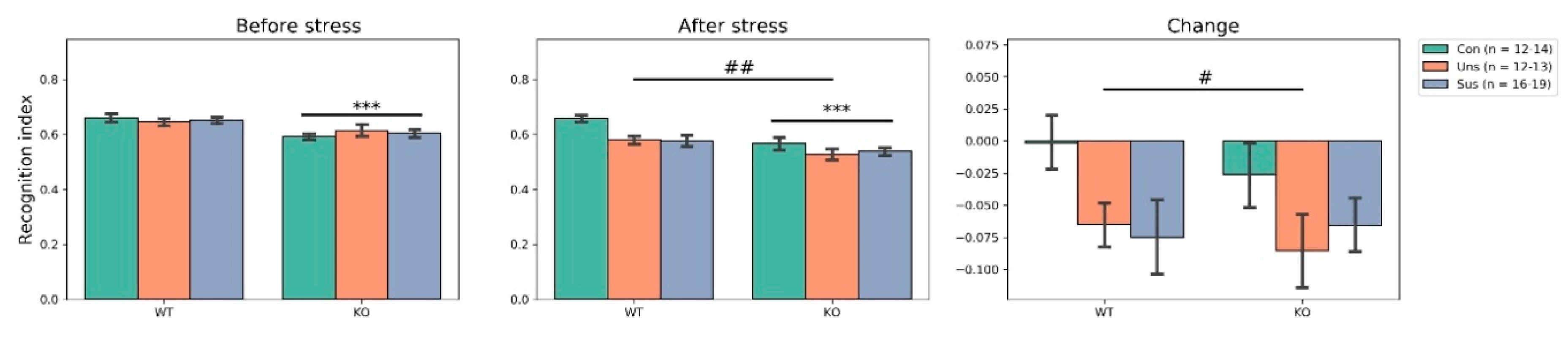
3.3. Novel Object Recognition Test (Figure 3)
3.4. Social Interaction Test (Figure 4)
3.5. Immunohistochemistry (Figure 5C)
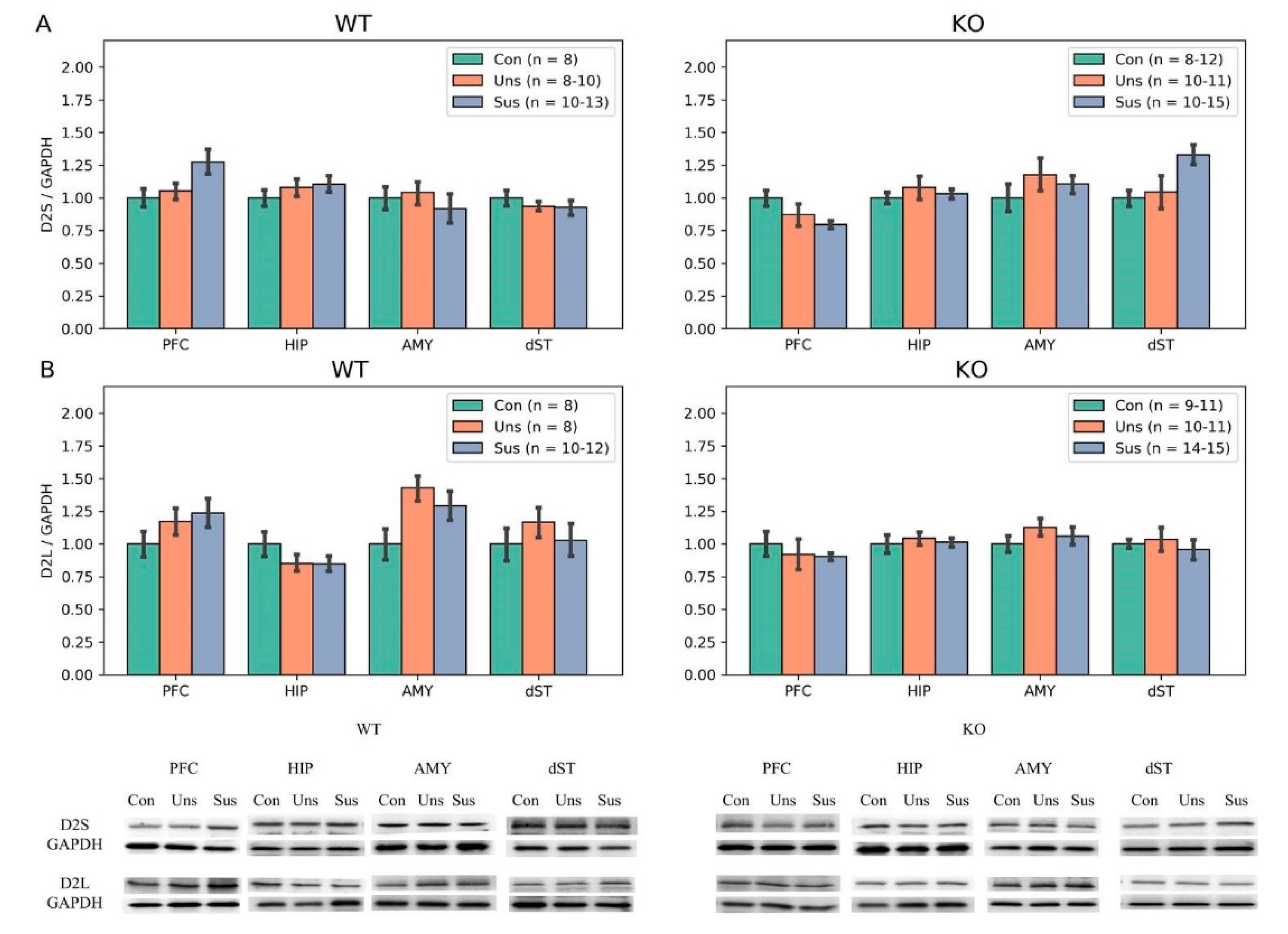
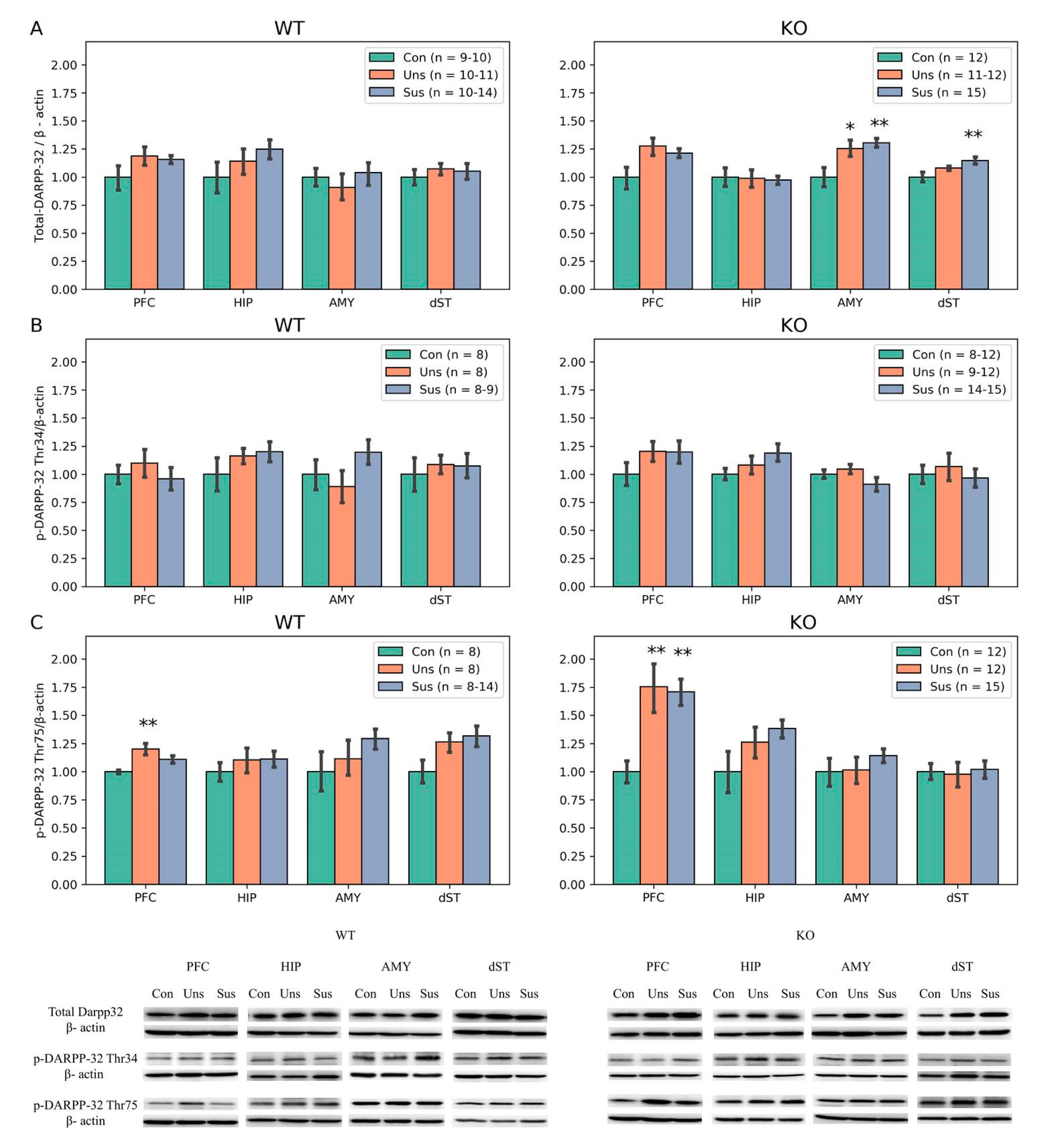
3.6. Western Blotting
4. Discussion
4.1. Effects of Stathmin 1 Gene Knockout on Behaviors
4.2. Effects of Social Defeat Stress on Stathmin Expression
4.3. Effects of Stathmin 1 Gene Knockout on Dopaminergic Markers
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Curmi, P.A.; Gavet, O.; Charbaut, E.; Ozon, S.; Lachkar-Colmerauer, S.; Manceau, V.; Siavoshian, S.; Maucuer, A.; Sobel, A. Stathmin and its phosphoprotein family: General properties, biochemical and functional interaction with tubulin. Cell Struct. Funct. 1999, 24, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Amat, J.A.; Fields, K.L.; Schubart, U.K. Distribution of phosphoprotein p19 in rat brain during ontogeny: Stage-specific expression in neurons and glia. Dev. Brain Res. 1991, 60, 205–218. [Google Scholar] [CrossRef]
- Belmont, L.D.; Mitchison, T.J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 1996, 84, 623–631. [Google Scholar] [CrossRef]
- Felkl, M.; Leube, R. Interaction assays in yeast and cultured cells confirm known and identify novel partners of the synaptic vesicle protein synaptophysin. Neuroscience 2008, 156, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Hanash, S.M.; Strahler, J.R.; Kuick, R.; Chu, E.; Nichols, D. Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J. Biol. Chem. 1988, 263, 12813–12815. [Google Scholar] [PubMed]
- Curmi, P.A.; Andersen, S.S.; Lachkar, S.; Gavet, O.; Karsenti, E.; Knossow, M.; Sobel, A. The stathmin/tubulin interaction in vitro. J. Biol. Chem. 1997, 272, 25029–25036. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Pan, Y.; Shu, H.; Ohshima, T.; Kansy, J.W.; White, C.L., III; Tamminga, C.A.; Sobel, A.; Curmi, P.A.; Mikoshiba, K. Phosphorylation of the tubulin-binding protein, stathmin, by Cdk5 and MAP kinases in the brain. J. Neurochem. 2006, 99, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Shumyatsky, G.P.; Malleret, G.; Shin, R.M.; Takizawa, S.; Tully, K.; Tsvetkov, E.; Zakharenko, S.S.; Joseph, J.; Vronskaya, S.; Yin, D.; et al. Stathmin, a gene enriched in the amygdala, controls both learned and innate fear. Cell 2005, 123, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Peschanski, M.; Hirsch, E.; Dusart, I.; Doye, V.; Marty, S.; Manceau, V.; Sobel, A. Stathmin: Cellular localization of a major phosphoprotein in the adult rat and human CNS. J. Comp. Neurol. 1993, 337, 655–668. [Google Scholar] [CrossRef]
- Brocke, B.; Lesch, K.P.; Armbruster, D.; Moser, D.A.; Müller, A.; Strobel, A.; Kirschbaum, C. Stathmin, a gene regulating neural plasticity, affects fear and anxiety processing in humans. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2010, 153, 243–251. [Google Scholar] [CrossRef]
- Paulson, L.; Martin, P.; Persson, A.; Nilsson, C.L.; Ljung, E.; Westman-Brinkmalm, A.; Eriksson, P.S.; Blennow, K.; Davidsson, P. Comparative genome-and proteome analysis of cerebral cortex from MK-801-treated rats. J. Neurosci. Res. 2003, 71, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Hattori, T.; Yamada, K.; Matsuzaki, S.; Tohyama, M. Role of the PACAP–PAC1–DISC1 and PACAP–PAC1–stathmin1 systems in schizophrenia and bipolar disorder: Novel treatment mechanisms? Pharmacogenomics 2009, 10, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Teyssier, J.-R.; Chauvet-Gelinier, J.-C.; Ragot, S.; Bonin, B. Up-regulation of leucocytes genes implicated in telomere dysfunction and cellular senescence correlates with depression and anxiety severity scores. PLoS ONE 2012, 7, e49677. [Google Scholar] [CrossRef] [PubMed]
- Ehlis, A.C.; Bauernschmitt, K.; Dresler, T.; Hahn, T.; Herrmann, M.J.; Röser, C.; Romanos, M.; Warnke, A.; Gerlach, M.; Lesch, K.P. Influence of a genetic variant of the neuronal growth associated protein Stathmin 1 on cognitive and affective control processes: An event-related potential study. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2011, 156, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.A.; Dorr, N.P.; De Gasperi, R.; Gama Sosa, M.A.; Shaughness, M.C.; Maudlin-Jeronimo, E.; Hall, A.A.; McCarron, R.M.; Ahlers, S.T. Blast exposure induces post-traumatic stress disorder-related traits in a rat model of mild traumatic brain injury. J. Neurotrauma 2012, 29, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, L.; Wang, R.; Dong, C.; Qing, Y.; Zhang, X.; Zhang, J. Stathmin genotype is associated with reexperiencing symptoms of posttraumatic stress disorder in Chinese earthquake survivors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 44, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Martel, G.; Hevi, C.; Wong, A.; Zushida, K.; Uchida, S.; Shumyatsky, G.P. Murine GRPR and stathmin control in opposite directions both cued fear extinction and neural activities of the amygdala and prefrontal cortex. PLoS ONE 2012, 7, e30942. [Google Scholar] [CrossRef] [PubMed]
- Buwalda, B.; Kole, M.H.; Veenema, A.H.; Huininga, M.; de Boer, S.F.; Korte, S.M.; Koolhaas, J.M. Long-term effects of social stress on brain and behavior: A focus on hippocampal functioning. Neurosci. Biobehav. Rev. 2005, 29, 83–97. [Google Scholar] [CrossRef]
- Hollis, F.; Kabbaj, M. Social defeat as an animal model for depression. ILAR J. 2014, 55, 221–232. [Google Scholar] [CrossRef]
- Selten, J.-P.; van der Ven, E.; Rutten, B.P.; Cantor-Graae, E. The social defeat hypothesis of schizophrenia: An update. Schizophr. Bull. 2013, 39, 1180–1186. [Google Scholar] [CrossRef]
- Selten, J.-P.; Cantor-Graae, E. Social defeat: Risk factor for schizophrenia? Br. J. Psychiatry 2005, 187, 101–102. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, S.; Kim, J.-H. Amygdala circuits for fear memory: A key role for dopamine regulation. Neuroscientist 2017, 23, 542–553. [Google Scholar] [CrossRef]
- Luo, R.; Uematsu, A.; Weitemier, A.; Aquili, L.; Koivumaa, J.; McHugh, T.J.; Johansen, J.P. A dopaminergic switch for fear to safety transitions. Nat. Commun. 2018, 9, 2483. [Google Scholar] [CrossRef]
- Bagalkot, T.R.; Jin, H.-M.; Prabhu, V.V.; Muna, S.; Cui, Y.; Yadav, B.; Chae, H.-J.; Chung, Y.-C. Chronic social defeat stress increases dopamine D2 receptor dimerization in the prefrontal cortex of adult mice. Neuroscience 2015, 311, 444–452. [Google Scholar] [CrossRef]
- Jin, H.-M.; Muna, S.S.; Bagalkot, T.; Cui, Y.; Yadav, B.; Chung, Y.-C. The effects of social defeat on behavior and dopaminergic markers in mice. Neuroscience 2015, 288, 167–177. [Google Scholar] [CrossRef]
- Prabhu, V.V.; Nguyen, T.B.; Cui, Y.; Oh, Y.-E.; Lee, K.-H.; Bagalkot, T.R.; Chung, Y.-C. Effects of social defeat stress on dopamine D2 receptor isoforms and proteins involved in intracellular trafficking. Behav. Brain Funct. 2018, 14, 16. [Google Scholar] [CrossRef]
- Golden, S.A.; Covington, H.E., III; Berton, O.; Russo, S.J. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011, 6, 1183–1191. [Google Scholar] [CrossRef]
- Krishnan, V.; Han, M.-H.; Graham, D.L.; Berton, O.; Renthal, W.; Russo, S.J.; LaPlant, Q.; Graham, A.; Lutter, M.; Lagace, D.C. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007, 131, 391–404. [Google Scholar] [CrossRef]
- McIlwrick, S.; Pohl, T.; Chen, A.; Touma, C. Late-onset cognitive impairments after early-life stress are shaped by inherited differences in stress reactivity. Front. Cell. Neurosci. 2017, 11, 9. [Google Scholar] [CrossRef]
- Dong, H.W. The Allen Reference Atlas: A Digital Color Brain Atlas of the C57Bl/6J Male Mouse; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008. [Google Scholar]
- Böer, U.; Buettner, F.F.; Schridde, A.; Klingenberg, M.; Sarikouch, S.; Haverich, A.; Wilhelmi, M. Antibody formation towards porcine tissue in patients implanted with crosslinked heart valves is directed to antigenic tissue proteins and αGal epitopes and is reduced in healthy vegetarian subjects. Xenotransplantation 2017, 24, e12288. [Google Scholar] [CrossRef]
- Gavet, O.; Ozon, S.; Manceau, V.; Lawler, S.; Curmi, P.; Sobel, A. The stathmin phosphoprotein family: Intracellular localization and effects on the microtubule network. J. Cell Sci. 1998, 111, 3333–3346. [Google Scholar]
- Mcdougall, S.A.; Der-Ghazarian, T.; Britt, C.E.; Varela, F.A.; Crawford, C.A. Postnatal manganese exposure alters the expression of D2L and D2S receptor isoforms: Relationship to PKA activity and Akt levels. Synapse 2011, 65, 583–591. [Google Scholar] [CrossRef]
- Khan, Z.U.; Mrzljak, L.; Gutierrez, A.; De La Calle, A.; Goldman-Rakic, P.S. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc. Natl. Acad. Sci. USA 1998, 95, 7731–7736. [Google Scholar] [CrossRef] [Green Version]
- Simon, P.; Dupuis, R.; Costentin, J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 1994, 61, 59–64. [Google Scholar] [CrossRef]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef]
- Lever, C.; Burton, S.; O’Keefe, J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006, 17, 111–134. [Google Scholar] [CrossRef]
- Smolinsky, A.N.; Bergner, C.L.; LaPorte, J.L.; Kalueff, A.V. Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression. In Mood and Anxiety Related Phenotypes in Mice; Springer: Berlin/Heidelberg, Germany, 2009; pp. 21–36. [Google Scholar]
- Schubart, U.K.; Yu, J.; Amat, J.A.; Wang, Z.-Q.; Hoffmann, M.K.; Edelmann, W. Normal development of mice lacking metablastin (P19), a phosphoprotein implicated in cell cycle regulation. J. Biol. Chem. 1996, 271, 14062–14066. [Google Scholar] [CrossRef]
- Han, F.; Jiang, J.; Ding, J.; Liu, H.; Xiao, B.; Shi, Y. Change of Rin1 and Stathmin in the animal model of traumatic stresses. Front. Behav. Neurosci. 2017, 11, 62. [Google Scholar] [CrossRef]
- Larsson, N.; Marklund, U.; Gradin, H.M.; Brattsand, G.; Gullberg, M. Control of microtubule dynamics by oncoprotein 18: Dissection of the regulatory role of multisite phosphorylation during mitosis. Mol. Cell. Biol. 1997, 17, 5530–5539. [Google Scholar] [CrossRef]
- Honnappa, S.; Jahnke, W.; Seelig, J.; Steinmetz, M.O. Control of intrinsically disordered stathmin by multisite phosphorylation. J. Biol. Chem. 2006, 281, 16078–16083. [Google Scholar] [CrossRef]
- Manna, T.; Thrower, D.A.; Honnappa, S.; Steinmetz, M.O.; Wilson, L. Regulation of microtubule dynamic instability in vitro by differentially phosphorylated stathmin. J. Biol. Chem. 2009, 284, 15640–15649. [Google Scholar] [CrossRef]
- Dent, E.W. Of microtubules and memory: Implications for microtubule dynamics in dendrites and spines. Mol. Biol. Cell 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Ohkawa, N.; Fujitani, K.; Tokunaga, E.; Furuya, S.; Inokuchi, K. The microtubule destabilizer stathmin mediates the development of dendritic arbors in neuronal cells. J. Cell Sci. 2007, 120, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, N.; Hashimoto, K.; Hino, T.; Migishima, R.; Yokoyama, M.; Kano, M.; Inokuchi, K. Motor discoordination of transgenic mice overexpressing a microtubule destabilizer, stathmin, specifically in Purkinje cells. Neurosci. Res. 2007, 59, 93–100. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Dong, J.; Wei, W.; Song, B.; Min, H.; Teng, W.; Chen, J. Developmental hypothyroxinaemia and hypothyroidism limit dendritic growth of cerebellar P urkinje cells in rat offspring: Involvement of microtubule-associated protein 2 (MAP 2) and stathmin. Neuropathol. Appl. Neurobiol. 2014, 40, 398–415. [Google Scholar] [CrossRef]
- Rosa, D.V.; Souza, R.P.; Souza, B.R.; Guimarães, M.M.; Carneiro, D.S.; Valvassori, S.S.; Gomez, M.V.; Quevedo, J.; Romano-Silva, M.A. DARPP-32 expression in rat brain after an inhibitory avoidance task. Neurochem. Res. 2008, 33, 2257–2262. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Tanahashi, T.; Kawai, T.; Chikahisa, S.; Katsuura, S.; Nishida, K.; Teshima-Kondo, S.; Sei, H.; Rokutan, K. Changes in behavior and gene expression induced by caloric restriction in C57BL/6 mice. Physiol. Genom. 2009, 39, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Wersinger, C.; Sidhu, A.J.B. Disruption of the interaction of α-synuclein with microtubules enhances cell surface recruitment of the dopamine transporter. Biochemistry 2005, 44, 13612–13624. [Google Scholar] [CrossRef]
- Reis, H.J.; Rosa, D.V.; Guimarães, M.M.; Souza, B.R.; Barros, A.G.; Pimenta, F.J.; Souza, R.P.; Torres, K.C.; Romano-Silva, M.A. Is DARPP-32 a potential therapeutic target? Expert Opin. Ther. Targets 2007, 11, 1649–1661. [Google Scholar] [CrossRef]
- Avgustinovichi, D.; Alekseyenko, O. [3H] SCH 23390 Binding in Various Brain Regions of C57BL/6J Mice with Repeated Experience of Victory or Social Defeat in Agonistic Interactions. Physiol. Res. 2010, 59, 455–458. [Google Scholar]
- Nishi, A.; Shuto, T. Potential for targeting dopamine/DARPP-32 signaling in neuropsychiatric and neurodegenerative disorders. Expert Opin. Ther. Targets 2017, 21, 259–272. [Google Scholar] [CrossRef]
- Greengard, P.; Allen, P.B.; Nairn, A.C.J.N. Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron 1999, 23, 435–447. [Google Scholar] [CrossRef]
- Machado-Neto, J.A.; Saad, S.T.O.; Traina, F. Stathmin 1 in normal and malignant hematopoiesis. BMB Rep. 2014, 47, 660. [Google Scholar] [CrossRef]
- Mistry, S.J.; Heng-Chun, L.; Atweh, G.F. Role for protein phosphatases in the cell-cycle-regulated phosphorylation of stathmin. Biochem. J. 1998, 334, 23–29. [Google Scholar] [CrossRef]
- Uchida, S.; Martel, G.; Pavlowsky, A.; Takizawa, S.; Hevi, C.; Watanabe, Y.; Kandel, E.R.; Alarcon, J.M.; Shumyatsky, G.P. Learning-induced and stathmin-dependent changes in microtubule stability are critical for memory and disrupted in ageing. Nat. Commun. 2014, 5, 4389. [Google Scholar] [CrossRef]




| Parameter | Group | WT | KO | pa | Group | Genotype | Genotype × Group |
|---|---|---|---|---|---|---|---|
| Social Interaction ratio | Con | 124.247 ± 12.1 | 146.994 ± 14.6 | 0.242 | F2,79 = 129.737, p < 0.001 | F1,79 = 0.443, p = 0.507 | F2,79 = 3.521, p = 0.034 |
| Uns | 156.088 ± 13.9 | 165.356 ± 17.3 | 0.689 | ||||
| Sus | 40.986 ± 6.48 | 22.175 ± 3.19 | 0.016 | ||||
| Corner ratio | Con | 0.718 ± 0.179 | 0.675 ± 0.0962 | 0.836 | F2,79 = 17.450, p < 0.001 | F1,79 = 0.942, p = 0.335 | F2,79 = 1.599, p = 0.209 |
| Uns | 0.938 ± 0.279 | 1.194 ± 0.334 | 0.562 | ||||
| Sus | 5.689 ± 1.13 | 3.808 ± 0.889 | 0.204 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.B.; Prabhu, V.V.; Piao, Y.H.; Oh, Y.E.; Zahra, R.F.; Chung, Y.-C. Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress. Brain Sci. 2019, 9, 215. https://doi.org/10.3390/brainsci9090215
Nguyen TB, Prabhu VV, Piao YH, Oh YE, Zahra RF, Chung Y-C. Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress. Brain Sciences. 2019; 9(9):215. https://doi.org/10.3390/brainsci9090215
Chicago/Turabian StyleNguyen, Thong Ba, Vishwanath Vasudev Prabhu, Yan Hong Piao, Young Eun Oh, Rami Fatima Zahra, and Young-Chul Chung. 2019. "Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress" Brain Sciences 9, no. 9: 215. https://doi.org/10.3390/brainsci9090215
APA StyleNguyen, T. B., Prabhu, V. V., Piao, Y. H., Oh, Y. E., Zahra, R. F., & Chung, Y.-C. (2019). Effects of Stathmin 1 Gene Knockout on Behaviors and Dopaminergic Markers in Mice Exposed to Social Defeat Stress. Brain Sciences, 9(9), 215. https://doi.org/10.3390/brainsci9090215





