Journal Description
Cardiogenetics
Cardiogenetics
is an international, peer-reviewed, open access journal, published quarterly online by MDPI (from Volume 10, Issue 2 - 2020).
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within ESCI (Web of Science), Scopus, Embase, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 23.1 days after submission; acceptance to publication is undertaken in 6.2 days (median values for papers published in this journal in the second half of 2025).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Impact Factor:
0.9 (2024)
Latest Articles
Digenic Contribution of Heterozygous ALPK3 and TRIM63 Variants to End-Stage Hypertrophic Cardiomyopathy in a Young Adult
Cardiogenetics 2026, 16(1), 1; https://doi.org/10.3390/cardiogenetics16010001 - 1 Jan 2026
Abstract
Hypertrophic cardiomyopathy (HCM), the most common inherited cardiac disorder, is usually caused by pathogenic variants in sarcomeric genes and is inherited in an autosomal dominant manner. Around 5% of cases are caused by variants in non-sarcomeric genes, which may involve alternative modes of
[...] Read more.
Hypertrophic cardiomyopathy (HCM), the most common inherited cardiac disorder, is usually caused by pathogenic variants in sarcomeric genes and is inherited in an autosomal dominant manner. Around 5% of cases are caused by variants in non-sarcomeric genes, which may involve alternative modes of inheritance. This study presents the first reported case of HCM associated with digenic contribution of heterozygous variants in two non-sarcomeric genes: ALPK3 and TRIM63. The patient was incidentally diagnosed with non-obstructive HCM in childhood and developed extreme myocardial hypertrophy with moderate heart failure at the age of 18. Rapid progressive left ventricular dysfunction promptly resulted in death at the age of 26. Genetic testing with an extended HCM panel identified no sarcomeric variants but revealed two truncating variants in the ALPK3 and TRIM63 genes. Whole-genome sequencing excluded any other causes of the disease. Heterozygous ALPK3 variants are typically associated with late-onset HCM, whereas TRIM63 variants are only considered pathogenic in a recessive state. This case, therefore, suggests a synergistic contribution of both variants to the development of a severe phenotype. The potential mechanisms of interaction between the protein products of ALPK3 and TRIM63 within the M-band of the sarcomere are discussed.
Full article
(This article belongs to the Section Molecular Genetics)
►
Show Figures
Open AccessReview
The Hidden Face of Danon Disease: Unique Challenges for Female Patients
by
Laura Torlai Triglia, Federico Barocelli, Enrico Ambrosini, Alberto Bettella, Filippo Luca Gurgoglione, Michele Bianconcini, Angela Guidorossi, Francesca Russo, Antonio Percesepe and Giampaolo Niccoli
Cardiogenetics 2025, 15(4), 32; https://doi.org/10.3390/cardiogenetics15040032 - 4 Dec 2025
Abstract
Danon Disease (DD) is a rare X-linked autophagic vacuolar myopathy caused by pathogenic variants in the lysosome-associated membrane protein 2 (LAMP-2) gene. Alternative splicing of the terminal exon 9 leads to the creation of three different isoforms, each with essential roles in regulating
[...] Read more.
Danon Disease (DD) is a rare X-linked autophagic vacuolar myopathy caused by pathogenic variants in the lysosome-associated membrane protein 2 (LAMP-2) gene. Alternative splicing of the terminal exon 9 leads to the creation of three different isoforms, each with essential roles in regulating autophagy. DD is characterized by cardiomyopathy, skeletal myopathy, cognitive impairment, and retinal disorders, with cardiac involvement being the primary cause of morbidity and mortality. Muscle biopsy may reveal signs of vacuolar myopathy, but the diagnosis is typically confirmed through sequencing and deletion/duplication analysis of the LAMP-2 gene using peripheral blood. Although few genotype–phenotype correlations have been described, with most being limited to isoform 2B of exon 9, the most significant prognostic indicator remains sex. The disease manifests earlier and with a more severe systemic presentation in males due to their hemizygous status, whereas in females, the typical presentation is late-onset hypertrophic or dilated cardiomyopathy, generally without extracardiac involvement. Cases of severely affected women have been described, potentially due to non-random or defective X-inactivation. The less typical and delayed clinical presentation in females can result in incorrect or missed diagnoses. The aim of this narrative review is to summarize the natural history, diagnostic criteria, management strategies, and recent advancements in the understanding of DD in women.
Full article
(This article belongs to the Section Rare Disease-Genetic Syndromes)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Sinus Bradycardia and Long QT Syndrome: Double Heterozygosity for Variants in KCNH2 and HCN4
by
Jaël S. Copier, Fenna Tuijnenburg, Karolina Andrzejczyk, Alex V. Postma, Saskia N. van der Crabben, Oussama Najih, Caroline Pham, Leander Beekman, Arie O. Verkerk, Ahmad S. Amin and Elisabeth M. Lodder
Cardiogenetics 2025, 15(4), 31; https://doi.org/10.3390/cardiogenetics15040031 - 13 Nov 2025
Abstract
Introduction: Clinical variability within families harbouring disease-causing genetic variants hampers clinical care and risk stratification. We studied a multigenerational family presenting with sinus bradycardia and long QT syndrome type 2 (LQTS2). The family harboured a pathogenic variant in KCNH2, which co-segregated
[...] Read more.
Introduction: Clinical variability within families harbouring disease-causing genetic variants hampers clinical care and risk stratification. We studied a multigenerational family presenting with sinus bradycardia and long QT syndrome type 2 (LQTS2). The family harboured a pathogenic variant in KCNH2, which co-segregated with the observed LQTS2. We studied the genetic cause of the high occurrence of sinus bradycardia in this family. Methods: Clinical data was collected, including heart rate, QT-interval, symptoms, and echocardiographic parameters. QTc was calculated using the Bazett and the Fridericia formula. Sanger sequencing of HCN4 was performed, followed by segregation analysis of the identified variant with sinus bradycardia. The biophysiological consequences of two variants, KCNH2-p.L69P (c.206T>C) and HCN4-p.R666W (c.1996C>T), were assessed by patch-clamp experiments. Therefore, a heterologous model was generated by transfection of HEK293A or CHO-k1 cells, respectively. Results: Sanger sequencing of HCN4 identified HCN4-p.R666W (c.1996C>T), which has a stronger segregation with the observed sinus bradycardia than KCNH2-p.L69P. Patch-clamp experiments revealed that KCNH2-p.L69P and HCN4-p.R666W lead to a decrease in the corresponding current densities, which explains the LQTS and sinus bradycardia observed in the patients. Carriers of both genetic variants have a more severe LQTS2 phenotype, reflected in longer QT and higher incidence of syncope. Conclusions: We identified two (likely) pathogenic variants, KCNH2-p.L69P and HCN4-p.R666W, co-segregating with LQTS2 and sinus bradycardia, respectively. Patients carrying both variants showed a more severe phenotype. These findings highlight the importance of additional genetic testing when discordant features are present, thereby enabling more accurate diagnosis, risk prediction, and management.
Full article
(This article belongs to the Section Molecular Genetics)
►▼
Show Figures

Figure 1
Open AccessCase Report
Integrating Genetic, Clinical, and Histopathological Data for Definitive Diagnosis of PRKAG2-Related Disease
by
Martina Caiazza, Emanuele Monda, Francesco Loffredo, Rossana Bussani, Vera Fico, Emanuele Bobbio, Chiara Cirillo, Anna Murredda, Immacolata Viscovo, Alessandra Scatteia, Santo Dellegrottaglie, Diego Colonna, Berardo Sarubbi, Maria Giovanna Russo, Paolo Golino, Gianfranco Sinagra and Giuseppe Limongelli
Cardiogenetics 2025, 15(4), 30; https://doi.org/10.3390/cardiogenetics15040030 - 4 Nov 2025
Cited by 1
Abstract
Background: PRKAG2-related disease is an autosomal dominant disorder caused by pathogenic variants in the PRKAG2 gene, leading to glycogen accumulation in cardiomyocytes. It is characterized by left ventricular hypertrophy (LVH), ventricular pre-excitation, and conduction disease. Due to the rarity of the condition and
[...] Read more.
Background: PRKAG2-related disease is an autosomal dominant disorder caused by pathogenic variants in the PRKAG2 gene, leading to glycogen accumulation in cardiomyocytes. It is characterized by left ventricular hypertrophy (LVH), ventricular pre-excitation, and conduction disease. Due to the rarity of the condition and the frequent occurrence of private variants, functional or pathological testing is required for definitive pathogenicity classification. Case Presentation: We describe a 22-year-old male referred for evaluation after experiencing exertional dyspnea and a syncopal episode. Family history revealed sudden cardiac deaths and conduction disease requiring pacemaker implantation. The patient exhibited mild LVH on imaging, conduction abnormalities on electrophysiological study, and a heterozygous PRKAG2 variant (c.1643C>T; p.Ser548Leu), classified as likely pathogenic according to ACMG guidelines. Cascade screening identified the variant in three family members, one of whom exhibited a positive phenotype. Endomyocardial biopsy revealed glycogen accumulation, providing histopathological confirmation of PRKAG2-related disease. Conclusions: This case underscores the importance of integrating genetic, clinical, and histopathological data in variant interpretation. Endomyocardial biopsy can provide definitive evidence to reclassify a PRKAG2 variant as pathogenic, thereby guiding management and family screening.
Full article
(This article belongs to the Section Rare Disease-Genetic Syndromes)
►▼
Show Figures

Figure 1
Open AccessReview
From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations
by
Jule Leonie Gutmann, Alina Spister and Lara Baticic
Cardiogenetics 2025, 15(4), 29; https://doi.org/10.3390/cardiogenetics15040029 - 11 Oct 2025
Abstract
Congenital genetic heart defects are major contributors to pediatric morbidity and mortality, underscoring the importance of early detection and individualized therapeutic strategies. This review aimed to summarize current knowledge on a spectrum of inherited cardiovascular disorders, with a focus on their genetic etiology,
[...] Read more.
Congenital genetic heart defects are major contributors to pediatric morbidity and mortality, underscoring the importance of early detection and individualized therapeutic strategies. This review aimed to summarize current knowledge on a spectrum of inherited cardiovascular disorders, with a focus on their genetic etiology, molecular pathogenesis, and phenotypic presentation in children. Conditions discussed include Marfan syndrome, Noonan syndrome, various cardiomyopathies, Duchenne muscular dystrophy, DiGeorge syndrome, and the tetralogy of Fallot. These six conditions were selected to represent the spectrum of pediatric cardiovascular genetic diseases, encompassing connective tissue disorders, multisystem syndromes, primary myocardial diseases, neuromuscular cardiac involvement, and structural congenital defects, thereby illustrating how distinct genotypes lead to diverse phenotypes. For each disorder, the underlying genetic mutations, associated molecular pathways, cardiovascular involvement, clinical features, and approaches to diagnosis and management are examined. Emphasis is placed on the role of timely diagnosis, genetic counseling, and personalized treatment in improving patient outcomes. The review concludes by highlighting emerging research directions and novel therapeutic interventions aimed at enhancing care for these complex pediatric conditions.
Full article
(This article belongs to the Section Inherited Heart Disease-Children)
►▼
Show Figures

Figure 1
Open AccessSystematic Review
MicroRNA and DNA Methylation Adaptation Mechanism to Endurance Training in Cardiovascular Disease: A Systematic Review
by
Jil Delhez, Jeanne Ougier, Francisco Xavier de Araujo, Raphael Martins de Abreu and Camilo Corbellini
Cardiogenetics 2025, 15(4), 28; https://doi.org/10.3390/cardiogenetics15040028 - 11 Oct 2025
Cited by 1
Abstract
Background: Regular endurance training induces physiological changes in cardiac structure and function. The precise epigenetic mechanisms by which cardiovascular adaptations are mediated are still unclear. This review seeks to clarify the role of epigenetic regulation in exercise-induced cardiovascular adaptation. Methods: This systematic review
[...] Read more.
Background: Regular endurance training induces physiological changes in cardiac structure and function. The precise epigenetic mechanisms by which cardiovascular adaptations are mediated are still unclear. This review seeks to clarify the role of epigenetic regulation in exercise-induced cardiovascular adaptation. Methods: This systematic review was conducted in accordance with the PRISMA guidelines up to 30 April 2025, using the databases PubMed, VHL, and LILACS Plus. Studies were included if they focused on microRNA expression and DNA methylation in individuals with cardiovascular disease who underwent endurance training. Results: Six articles, including 384 participants with heart failure, coronary artery disease, and hypertension, were included in the final analysis. Changes in DNA methylation and microRNA expression of specific genes involved in cardiovascular structural and functional adaptation were observed. Significant improvements were found in body composition, VO2peak, systolic and diastolic blood pressure, and left ventricular function and structure. Conclusions: Endurance training has a positive impact on epigenetic mechanisms related to cardiovascular structural and functional adaptation. A clear causal link between epigenetic modifications and clinical outcomes remains to be established.
Full article
(This article belongs to the Section Cardiovascular Genetics in Clinical Practice)
►▼
Show Figures
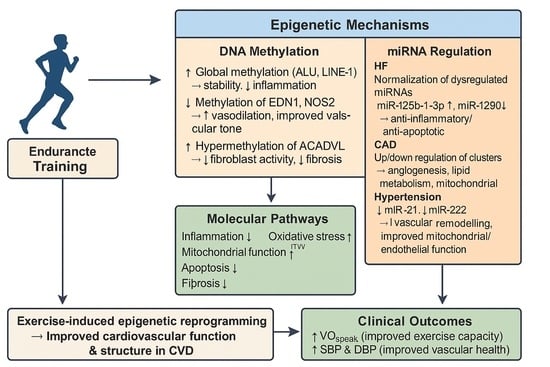
Graphical abstract
Open AccessReview
Polygenic Risk Scores and Coronary Artery Disease
by
Salman Ansari, Suvasini Lakshmanan and Matthew J. Budoff
Cardiogenetics 2025, 15(4), 27; https://doi.org/10.3390/cardiogenetics15040027 - 26 Sep 2025
Cited by 1
Abstract
Background: Polygenic risk scores (PRSs) aggregate the effects of many common genetic variants and are being investigated as tools to refine coronary artery disease (CAD) risk prediction beyond traditional clinical models. Methods and Results: We review the development of PRS from early unweighted
[...] Read more.
Background: Polygenic risk scores (PRSs) aggregate the effects of many common genetic variants and are being investigated as tools to refine coronary artery disease (CAD) risk prediction beyond traditional clinical models. Methods and Results: We review the development of PRS from early unweighted scores to contemporary genome-wide models and summarize evidence from major studies. We identified key studies through PubMed searches using the terms “polygenic risk score,” “genetic risk prediction,” and “coronary artery disease,” supplemented by citation chaining of highly cited articles and recent reviews. Large cohorts, such as the UK Biobank, show that individuals in the highest PRS percentiles have a 3–5-fold higher risk of CAD, and may gain the greatest benefit from statin therapy. PRS can also reclassify younger adults at borderline or intermediate risk and may complement coronary artery calcium (CAC) scoring. Conclusions: PRSs hold promise for lifetime risk stratification and targeted prevention in CAD but are limited by ancestry bias in GWAS, underrepresentation of diverse populations, inconsistency in individual estimates, and lack of standardized reporting. Future research should focus on expanding multi-ancestry databases, standardizing methods, prospective validation, and effective communication strategies to support equitable and evidence-based clinical use.
Full article
(This article belongs to the Section Cardiovascular Genetics in Clinical Practice)
►▼
Show Figures

Figure 1
Open AccessReview
Ethical Considerations Regarding Advanced Heart Failure Therapies in Patients Affected by Dystrophinopathies
by
Marco Spagnolin, Luca Fazzini, Amedeo Terzi, Attilio Iacovoni, Raffaele Abete, Ottavio Zucchetti, Michele Senni and Mauro Gori
Cardiogenetics 2025, 15(3), 26; https://doi.org/10.3390/cardiogenetics15030026 - 22 Sep 2025
Abstract
Dystrophinopathies, including Duchenne and Becker muscular dystrophies (DMD and BMD), are inherited neuromuscular disorders frequently complicated by progressive cardiac involvement, ultimately leading to advanced heart failure. While heart transplantation and long-term left ventricular assist device (LVAD) therapy represent potential therapeutic options, their application
[...] Read more.
Dystrophinopathies, including Duchenne and Becker muscular dystrophies (DMD and BMD), are inherited neuromuscular disorders frequently complicated by progressive cardiac involvement, ultimately leading to advanced heart failure. While heart transplantation and long-term left ventricular assist device (LVAD) therapy represent potential therapeutic options, their application in this population raises significant ethical challenges. This review explores the ethical implications surrounding the allocation of scarce medical resources, particularly in patients with limited life expectancy and multisystem disease, as in DMD. Decisions regarding eligibility for heart transplantation must balance individual benefit, considering the impact of excluding other potential recipients. LVAD therapy, although more accessible, still demands careful patient selection due to high perioperative risk and postoperative complications. The review emphasizes the need for transparent, multidisciplinary decision-making processes that respect patient autonomy while ensuring equitable and rational distribution of healthcare resources. Ultimately, while advanced therapies may be feasible in selected cases, particularly in BMD, ethical deliberation remains central to determining their appropriateness in the context of dystrophinopathies.
Full article
(This article belongs to the Section Rare Disease-Neuromuscular Diseases)
►▼
Show Figures

Figure 1
Open AccessArticle
Genetic Profile of Pediatric-Onset Cardiac Channelopathies
by
Sara Giovani, Adelaide Ballerini, Alessia Gozzini, Michele Di Lorenzo, Davide Mei, Silvia Passantino, Mattia Zampieri, Alessia Tomberli, Alberto Marchi, Giovanni Battista Calabri, Gaia Spaziani, Giulio Porcedda, Elena Bennati, Silvia Favilli, Iacopo Olivotto and Francesca Girolami
Cardiogenetics 2025, 15(3), 25; https://doi.org/10.3390/cardiogenetics15030025 - 12 Sep 2025
Abstract
This study investigates the genetic background of pediatric-onset cardiac channelopathies, a rare group of genetic disorders causing arrhythmias and sometimes sudden death, whose genetic background remains partially unknown. The research analyzed 59 pediatric patients (<18 years of age) diagnosed with different channelopathies (LQTS,
[...] Read more.
This study investigates the genetic background of pediatric-onset cardiac channelopathies, a rare group of genetic disorders causing arrhythmias and sometimes sudden death, whose genetic background remains partially unknown. The research analyzed 59 pediatric patients (<18 years of age) diagnosed with different channelopathies (LQTS, BrS, CPVT, SQTS, and conduction disorders), along with 40 of their family members, using Next-Generation Sequencing (NGS) after genetic counseling. A causative genetic variant was found in 47% of cases, mainly in the KCNQ1 (42%), RYR2 (16%), CACNA1C (10%), and SCN5A (10%) genes. Notably, a de novo large deletion in KCNH2 was detected in an LQTS patient, and a pathogenic CALM1 variant was identified in a child. A compound heterozygous KCNQ1 was consistent with Jervell and Lange-Nielsen syndrome. In light of these data, genetic testing is crucial for diagnosis, prognosis, and treatment planning; cascade screening allowed early risk identification and preventive interventions for family members. Expanding NGS technologies and research on new candidate genes may enhance personalized therapies in the future.
Full article
(This article belongs to the Section Molecular Genetics)
►▼
Show Figures

Figure 1
Open AccessCase Report
TNNC1 Gene Mutation in Ebstein’s Anomaly and Left Ventricular Hypertrabeculation: A Case Report of a New Causative Mutation?
by
Irene Raso, Claudia Chillemi, Giorgia Prontera, Arianna Laoreti, Elisa Cattaneo, Valeria Calcaterra, Gian Vincenzo Zuccotti and Savina Mannarino
Cardiogenetics 2025, 15(3), 24; https://doi.org/10.3390/cardiogenetics15030024 - 26 Aug 2025
Abstract
Background: Ebstein’s anomaly (EA) is a rare congenital heart defect characterized by failure of tricuspid valve delamination during embryogenesis. Left ventricular (LV) hypertrabeculation results from incomplete myocardial compaction during fetal development. EA is associated with LV hypertrabeculation in 0.14% of cases, and EA
[...] Read more.
Background: Ebstein’s anomaly (EA) is a rare congenital heart defect characterized by failure of tricuspid valve delamination during embryogenesis. Left ventricular (LV) hypertrabeculation results from incomplete myocardial compaction during fetal development. EA is associated with LV hypertrabeculation in 0.14% of cases, and EA is the most common congenital heart disease in LV hypertrabeculation (up to 29%), suggesting a shared embryogenetic pathway. Case Report: We describe a female patient prenatally diagnosed with EA and a large ventricular septal defect. Postnatal echocardiography confirmed EA with moderate regurgitation and revealed previously unnoticed left ventricular excessive trabeculations. Whole exome sequencing revealed a heterozygous never-described variant of unknown significance in the TNNC1 gene. Discussion: The genetic link between EA and LV hypertrabeculation remains unclear, though variants in sarcomeric or cytoskeletal genes like MYH7, TPM1, and NKX2.5—essential for cardiac development—have been implicated. A developmental hypothesis suggests that aberrant contraction during endocardial-to-mesenchymal and epicardial-to-mesenchymal transformation (5th–8th gestational weeks) may affect valve delamination and ventricular compaction via parallel signaling pathways. TNNC1 encodes troponin C1, a subunit of the troponin complex involved in muscle contraction. Its mutations are known to alter calcium sensitivity and impair cardiac contractility. Conclusions: EA and LV hypertrabeculation patients diagnosed in infancy have a greater risk of negative outcomes. Early, especially prenatal, diagnosis is crucial. Genetic analysis can provide fundamental insight into cardiac development. This new and rare variant of TNNC1 gene supports the hypothesis that early cardiomyocytes dysfunction disrupts both valve delamination and left ventricular compaction and that the two diseases share a common genetic pathway related to cardiomyocyte contraction.
Full article
(This article belongs to the Section Inherited Heart Disease-Children)
►▼
Show Figures

Figure 1
Open AccessArticle
Association Between Angiotensinogen Gene M235T and Renin–Angiotensin System Insertion/Deletion Variants and Risk of Cardiovascular Disease in North African and Middle Eastern Populations: A Systematic Review and Meta-Analysis
by
Rajaa El Mansouri, Hind Dehbi and Rachida Habbal
Cardiogenetics 2025, 15(3), 23; https://doi.org/10.3390/cardiogenetics15030023 - 8 Aug 2025
Abstract
►▼
Show Figures
Background: The renin–angiotensin system (RAS) is pivotal in regulating cardiovascular function, while cardio-genomics offers insights into genetic factors influencing cardiovascular disease (CVD) susceptibility. Aim: This study investigates the relationship between the angiotensin-converting enzyme insertion/deletion variant (ACE I/D) and the angiotensinogen gene M235T variant
[...] Read more.
Background: The renin–angiotensin system (RAS) is pivotal in regulating cardiovascular function, while cardio-genomics offers insights into genetic factors influencing cardiovascular disease (CVD) susceptibility. Aim: This study investigates the relationship between the angiotensin-converting enzyme insertion/deletion variant (ACE I/D) and the angiotensinogen gene M235T variant (AGT M235T) in Mediterranean, North African, and Middle Eastern populations. Methods: A systematic review and meta-analysis, encompassing studies until December 2023, were conducted utilizing the PubMed and Scopus databases. The study followed the PICO checklist to enroll in the review process. The meta-analysis results were obtained using CMA software V2. Results: An analysis of 12 studies (2984 participants) for ACE I/D and 7 studies (2275 participants) for AGT M235T revealed significant associations between these gene variants and increased CVD risk in Mediterranean and North African populations. Conclusions: These findings underscore the utility of cardio-genomics in delineating CVD susceptibility among these groups, emphasizing targeted interventions and personalized treatment strategies
Full article

Figure 1
Open AccessEditor’s ChoiceReview
Desmosomal Versus Non-Desmosomal Arrhythmogenic Cardiomyopathies: A State-of-the-Art Review
by
Kristian Galanti, Lorena Iezzi, Maria Luana Rizzuto, Daniele Falco, Giada Negri, Hoang Nhat Pham, Davide Mansour, Roberta Giansante, Liborio Stuppia, Lorenzo Mazzocchetti, Sabina Gallina, Cesare Mantini, Mohammed Y. Khanji, C. Anwar A. Chahal and Fabrizio Ricci
Cardiogenetics 2025, 15(3), 22; https://doi.org/10.3390/cardiogenetics15030022 - 1 Aug 2025
Cited by 1
Abstract
Arrhythmogenic cardiomyopathies (ACMs) are a phenotypically and etiologically heterogeneous group of myocardial disorders characterized by fibrotic or fibro-fatty replacement of ventricular myocardium, electrical instability, and an elevated risk of sudden cardiac death. Initially identified as a right ventricular disease, ACMs are now recognized
[...] Read more.
Arrhythmogenic cardiomyopathies (ACMs) are a phenotypically and etiologically heterogeneous group of myocardial disorders characterized by fibrotic or fibro-fatty replacement of ventricular myocardium, electrical instability, and an elevated risk of sudden cardiac death. Initially identified as a right ventricular disease, ACMs are now recognized to include biventricular and left-dominant forms. Genetic causes account for a substantial proportion of cases and include desmosomal variants, non-desmosomal variants, and familial gene-elusive forms with no identifiable pathogenic mutation. Nongenetic etiologies, including post-inflammatory, autoimmune, and infiltrative mechanisms, may mimic the phenotype. In many patients, the disease remains idiopathic despite comprehensive evaluation. Cardiac magnetic resonance imaging has emerged as a key tool for identifying non-ischemic scar patterns and for distinguishing arrhythmogenic phenotypes from other cardiomyopathies. Emerging classifications propose the unifying concept of scarring cardiomyopathies based on shared structural substrates, although global consensus is evolving. Risk stratification remains challenging, particularly in patients without overt systolic dysfunction or identifiable genetic markers. Advances in tissue phenotyping, multi-omics, and artificial intelligence hold promise for improved prognostic assessment and individualized therapy.
Full article
(This article belongs to the Section Cardiovascular Genetics in Clinical Practice)
►▼
Show Figures
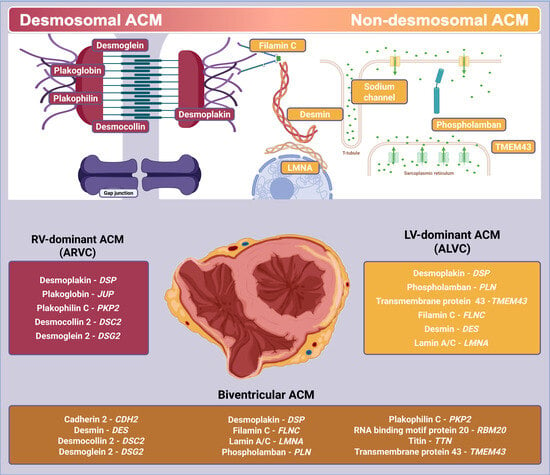
Graphical abstract
Open AccessFeature PaperArticle
How to Enhance Diagnosis in Fabry Disease: The Power of Information
by
Maria Chiara Meucci, Rosa Lillo, Margherita Calcagnino, Giampaolo Tocci, Eustachio Agricola, Federico Biondi, Claudio Di Brango, Vincenzo Guido, Valentina Parisi, Francesca Giordana, Veronica Melita, Mariaelena Lombardi, Angela Beatrice Scardovi, Li Van Stella Truong, Francesca Musella, Francesco di Spigno, Benedetta Matrone, Ivana Pariggiano, Paolo Calabrò, Roberto Spoladore, Stefania Luceri, Stefano Carugo, Francesca Graziani and Francesco Burzottaadd
Show full author list
remove
Hide full author list
Cardiogenetics 2025, 15(3), 21; https://doi.org/10.3390/cardiogenetics15030021 - 31 Jul 2025
Abstract
Background: Cardiac involvement is common in Fabry disease (FD) and typically manifests with left ventricular hypertrophy (LVH). Patients with FD are frequently misdiagnosed, and this is mainly related to the lack of disease awareness among clinicians. The aim of this study was to
[...] Read more.
Background: Cardiac involvement is common in Fabry disease (FD) and typically manifests with left ventricular hypertrophy (LVH). Patients with FD are frequently misdiagnosed, and this is mainly related to the lack of disease awareness among clinicians. The aim of this study was to determine whether providing a targeted educational intervention on FD may enhance FD diagnosis. Methods. This research was designed as a single-arm before-and-after intervention study and evaluated the impact of providing a specific training on FD to cardiologists from different Italian centers, without experience in rare diseases. In the 12-month period after the educational intervention, the rate of FD screening and diagnosis was assessed and compared with those conducted in the two years preceding the study initiation. Results: Fifteen cardiologists participated to this study, receiving a theoretical and practical training on FD. In the two previous two years, they conducted 12 FD screening (6/year), and they did not detect any cases of FD. After the training, they performed 45 FD screenings, with an eight-fold rise in the annual screening rate. The screened population (age: 61 ± 11 years, men: 82%) was mainly composed of patients with unexplained LVH (n = 43). There were four new FD diagnoses and, among of them, three had a late-onset GLA variant. After the cascade genetic screening, 11 affected relatives and 8 heterozygous carriers were also detected. Conclusions: A targeted educational intervention for cardiologists allowed the identification of four new families with FD. Enhancing FD awareness is helpful to reduce the diagnostic and therapeutic delay.
Full article
(This article belongs to the Section Education in Cardiogenetics)
►▼
Show Figures

Figure 1
Open AccessReview
Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review
by
Francesco Ravera, Filippo Angelini, Pier Paolo Bocchino, Gianluca Marcelli, Giulia Gobello, Giuseppe Giannino, Guglielmo Merlino, Benedetta De Guidi, Andrea Destefanis, Giulia Margherita Brach Del Prever, Carla Giustetto, Guglielmo Gallone, Stefano Pidello, Antonella Barreca, Silvia Deaglio, Gaetano Maria De Ferrari, Claudia Raineri and Veronica Dusi
Cardiogenetics 2025, 15(3), 20; https://doi.org/10.3390/cardiogenetics15030020 - 31 Jul 2025
Abstract
Variants in ELAC2, a gene encoding the mitochondrial RNase Z enzyme essential for mitochondrial tRNA processing, have been associated with severe pediatric-onset mitochondrial dysfunction, primarily presenting with developmental delay, hypertrophic cardiomyopathy (HCM), and lactic-acidosis. We hereby report the case of a 25-year-old
[...] Read more.
Variants in ELAC2, a gene encoding the mitochondrial RNase Z enzyme essential for mitochondrial tRNA processing, have been associated with severe pediatric-onset mitochondrial dysfunction, primarily presenting with developmental delay, hypertrophic cardiomyopathy (HCM), and lactic-acidosis. We hereby report the case of a 25-year-old young woman presenting with dilated cardiomyopathy (DCM) and peripheral sensorimotor polyneuropathy, harboring a homozygous variant in ELAC2. The same variant has been reported only once so far in a case of severe infantile-onset form of HCM and mitochondrial respiratory chain dysfunction, with in vitro data showing a moderate reduction in the RNase Z activity and supporting the current classification as C4 according to the American College of Medical Genetics (ACMG) criteria (PS3, PM2, PM3, PP4). Our extensive clinical, imaging, histological, and genetic investigations support a causal link between the identified variant and the patient’s phenotype, despite the fact that the latter might be considered atypical according to the current state of knowledge. A detailed review of the existing literature on ELAC2-related disease is also provided, highlighting the molecular mechanisms underlying tRNA maturation, mitochondrial dysfunction, and the variable phenotypic expression. Our case further expands the clinical spectrum of ELAC2-related cardiomyopathies to include a relatively late onset in young adulthood and underscores the importance of comprehensive genetic testing in unexplained cardiomyopathies with multisystem involvement.
Full article
(This article belongs to the Section Rare Disease-Genetic Syndromes)
►▼
Show Figures

Figure 1
Open AccessArticle
Integrated Deep Learning Framework for Cardiac Risk Stratification and Complication Analysis in Leigh’s Disease
by
Md Aminul Islam, Jayasree Varadarajan, Md Abu Sufian, Bhupesh Kumar Mishra and Md Ruhul Amin Rasel
Cardiogenetics 2025, 15(3), 19; https://doi.org/10.3390/cardiogenetics15030019 - 15 Jul 2025
Cited by 1
Abstract
►▼
Show Figures
Background: Leigh’s Disease is a rare mitochondrial disorder primarily affecting the central nervous system, with frequent secondary cardiac manifestations such as hypertrophic and dilated cardiomyopathies. Early detection of cardiac complications is crucial for patient management, but manual interpretation of cardiac MRI is labour-intensive
[...] Read more.
Background: Leigh’s Disease is a rare mitochondrial disorder primarily affecting the central nervous system, with frequent secondary cardiac manifestations such as hypertrophic and dilated cardiomyopathies. Early detection of cardiac complications is crucial for patient management, but manual interpretation of cardiac MRI is labour-intensive and subject to inter-observer variability. Methodology: We propose an integrated deep learning framework using cardiac MRI to automate the detection of cardiac abnormalities associated with Leigh’s Disease. Four CNN architectures—Inceptionv3, a custom 3-layer CNN, DenseNet169, and EfficientNetB2—were trained on preprocessed MRI data (224 × 224 pixels), including left ventricular segmentation, contrast enhancement, and gamma correction. Morphological features (area, aspect ratio, and extent) were also extracted to aid interpretability. Results: EfficientNetB2 achieved the highest test accuracy (99.2%) and generalization performance, followed by DenseNet169 (98.4%), 3-layer CNN (95.6%), and InceptionV3 (94.2%). Statistical morphological analysis revealed significant differences in cardiac structure between Leigh’s and non-Leigh’s cases, particularly in area (212,097 vs. 2247 pixels) and extent (0.995 vs. 0.183). The framework was validated using ROC (AUC = 1.00), Brier Score (0.000), and cross-validation (mean sensitivity = 1.000, std = 0.000). Feature embedding visualisation using PCA, t-SNE, and UMAP confirmed class separability. Grad-CAM heatmaps localised relevant myocardial regions, supporting model interpretability. Conclusions: Our deep learning-based framework demonstrated high diagnostic accuracy and interpretability in detecting Leigh’s disease-related cardiac complications. Integrating morphological analysis and explainable AI provides a robust and scalable tool for early-stage detection and clinical decision support in rare diseases.
Full article

Figure 1
Open AccessReview
Systematic Review of Pharmacogenetics of Immunosuppressants in Heart Transplantation
by
Juan Eduardo Megías-Vericat, Tomás Palanques-Pastor, Mireya Fernández-Sánchez, Eduardo Guerrero-Hurtado, Mayte Gil-Candel, Antonio Solana-Altabella, Octavio Ballesta-López, María Centelles-Oria, Javier García-Pellicer and José Luis Poveda-Andrés
Cardiogenetics 2025, 15(2), 18; https://doi.org/10.3390/cardiogenetics15020018 - 17 Jun 2025
Cited by 1
Abstract
The standard immunosuppressive treatments in heart transplantation are calcineurin inhibitors, corticosteroids, and antimetabolite agents or inhibitors of the mammalian target of rapamycin. Pharmacogenetic studies show the impact on clinical course of genetic variability in genes that encode transporters, metabolizers, or molecular targets of
[...] Read more.
The standard immunosuppressive treatments in heart transplantation are calcineurin inhibitors, corticosteroids, and antimetabolite agents or inhibitors of the mammalian target of rapamycin. Pharmacogenetic studies show the impact on clinical course of genetic variability in genes that encode transporters, metabolizers, or molecular targets of immunosuppressants. The aim of this systematic review is to elucidate the role that pharmacogenetics of immunosuppressant drugs plays in clinical outcomes upon heart transplantation. PubMed, EMBASE, the Cochrane Central Register, and the Database of Abstracts of Reviews of Effects were searched without restrictions. The 64 studies analyzed followed these criteria: (1) were based on clinical data on heart transplantation patients; (2) analyzed the associations between polymorphisms and clinical response; (3) analyzed the impact of polymorphisms on immunosuppressant safety. CYP3A4/5 variants were associated with higher doses of tacrolimus, whereas POR*28 variants with lower doses—ABCB1, ABCC2, SLCO1B1, and SLC13A1—contribute to interindividual variability in drug absorption, distribution, and toxicity. An ABCC2 polymorphism (rs717620) was related to higher risk of graft rejection in pediatrics. Variations in HLA-G, TNF-α and TGF-β genes influence transplant rejection risk and immune response. Implementing pharmacogenetic screening of polymorphisms could enhance therapeutic outcomes by improving drug efficacy, reducing toxicity, and ultimately increasing heart graft survival rates. Strong evidence supports genotyping for CYP3A5 and TPMT, but further research is required for transporter genes and cytokine polymorphisms.
Full article
(This article belongs to the Section Molecular Genetics)
►▼
Show Figures

Figure 1
Open AccessReview
Brugada Syndrome: Channelopathy and/or Cardiomyopathy
by
Michele Ciabatti, Pasquale Notarstefano, Chiara Zocchi, Giacomo Virgili, Fulvio Bellocci, Iacopo Olivotto and Maurizio Pieroni
Cardiogenetics 2025, 15(2), 17; https://doi.org/10.3390/cardiogenetics15020017 - 13 Jun 2025
Abstract
Brugada syndrome (BrS) has been traditionally considered a pure electrical disorder without an underlying structural substrate. However, early ECG studies showed the presence of depolarization abnormalities in this condition, while many studies based on advanced imaging have suggested the presence of subtle structural
[...] Read more.
Brugada syndrome (BrS) has been traditionally considered a pure electrical disorder without an underlying structural substrate. However, early ECG studies showed the presence of depolarization abnormalities in this condition, while many studies based on advanced imaging have suggested the presence of subtle structural alterations. On the other hand, electrophysiological study (EPS) and electroanatomic mapping (EAM) techniques have provided important data regarding right ventricular functional and structural arrhythmic substrate. More recently, histology and immunology shed light on the possible role of fibrotic and inflammatory substrates in BrS. Notably, a significant overlap between electro anatomical and structural features in BrS and arrhythmogenic cardiomyopathy has been proposed. In this review, we summarized the physio pathological pathways and substrate underlying BrS. A deeper knowledge of the structural abnormalities involved in the pathogenesis of this disease could improve our diagnostic and prognostic approach, while novel findings regarding the role of inflammation and immune activation could potentially lead to new therapeutic strategies for BrS.
Full article
(This article belongs to the Section Rare Disease-Genetic Syndromes)
►▼
Show Figures

Figure 1
Open AccessEditor’s ChoiceArticle
Cardiac Involvement in Patients with MELAS-Related mtDNA 3243A>G Variant
by
Aino-Maija Vuorinen, Lauri Lehmonen, Mari Auranen, Sini Weckström, Sari Kivistö, Miia Holmström and Tiina Heliö
Cardiogenetics 2025, 15(2), 16; https://doi.org/10.3390/cardiogenetics15020016 - 6 Jun 2025
Cited by 1
Abstract
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is a rare disease with variable clinical manifestations. MELAS is most often caused by the human mitochondrial DNA (mtDNA) m.3243A>G variant. We describe cardiac magnetic resonance (CMR) imaging findings and clinical features of
[...] Read more.
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) syndrome is a rare disease with variable clinical manifestations. MELAS is most often caused by the human mitochondrial DNA (mtDNA) m.3243A>G variant. We describe cardiac magnetic resonance (CMR) imaging findings and clinical features of 22 subjects with the m.3243A>G mutation and endeavored to discover the role of CMR in MELAS cardiomyopathy diagnostics. The clinical symptoms, ECG findings, and laboratory tests were retrospectively collected from the electronic medical record. Ten subjects (46%) had cardiac symptoms, and eighteen subjects (82%) had some clinical symptoms or signs of MELAS. Seventeen subjects (77%) showed cardiac findings compatible with MELAS. An ECG showed a short PR interval in six subjects (27%). Two patients had a first-degree atrioventricular block. Repolarization changes in the ECG were observed in thirteen subjects (59%), whereas left ventricular hypertrophy voltage criteria were only observed in one subject. Patients with ECG abnormalities had a strong link between proBNP value and cardiac tissue composition (T1 relaxation, p < 0.02) and showed decreased CMR-based strain (p < 0.025). The CMR findings are heterogeneous in subjects with m.3243A>G. Cardiac MELAS may include left ventricular hypertrophy, which mimics sarcomericcardiomyopathy but maypredispose individuals to severe heart failure episodes triggered by acute critical situations. CMR may be used to clarify ECG findings. This study indicates that the genetic testing of MELAS should be considered in new cases of HCM or sudden heart failure phenotypes of unknown etiology.
Full article
Open AccessReview
Novel Perspectives on Genetic Evaluation in Early-Onset Atrial Fibrillation: Clinical Implications and Future Directions
by
Angelo Laconi, Tatiana Fancello, Giuliana Solinas and Gavino Casu
Cardiogenetics 2025, 15(2), 15; https://doi.org/10.3390/cardiogenetics15020015 - 30 May 2025
Abstract
Background: Early-onset atrial fibrillation (AF) exhibits distinct clinical and genetic profiles compared to AF in older adults. The increasing detection of AF among younger patients—often in the absence of traditional risk factors—has raised interest in the genetic determinants underlying the condition. This review
[...] Read more.
Background: Early-onset atrial fibrillation (AF) exhibits distinct clinical and genetic profiles compared to AF in older adults. The increasing detection of AF among younger patients—often in the absence of traditional risk factors—has raised interest in the genetic determinants underlying the condition. This review aims to synthesize current evidence on the genetic architecture of early-onset AF, assess the clinical utility of genetic testing, and discuss future directions for integrating genetic insights into personalized management strategies. Methods: We conducted a comprehensive analysis of recent studies, including genome-wide association studies and targeted sequencing efforts, that examined rare pathogenic variants and polygenic risk scores in early-onset AF. The review also considers emerging data on atrial cardiomyopathy and evaluates current guideline recommendations for genetic testing. Results: Data indicate that rare variants, particularly in genes such as TTN, LMNA, and KCNQ1, play a significant role in early-onset AF, with evidence suggesting an association between these mutations and adverse clinical outcomes. Polygenic risk scores further complement traditional risk factors, providing a more nuanced risk stratification. Despite these advances, challenges remain in the interpretation of variants of uncertain significance, cost-effectiveness, and the need for interdisciplinary collaboration in clinical implementation. Conclusions: Integrating genetic evaluation into the diagnostic and management framework of early-onset AF holds promise for improved risk stratification and personalized therapy. Future large-scale, multi-ethnic studies and ongoing refinement of genetic risk models are essential to overcome current limitations and enhance the clinical applicability of genetic testing in this rapidly evolving field.
Full article
(This article belongs to the Section Cardiovascular Genetics in Clinical Practice)
►▼
Show Figures
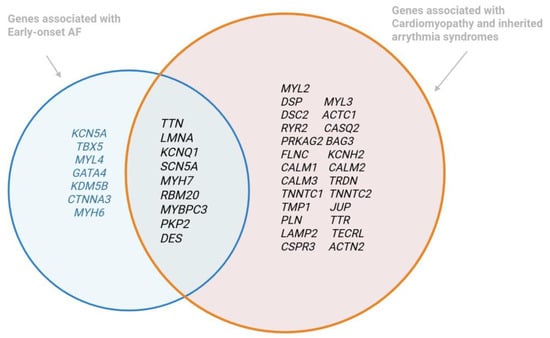
Figure 1
Open AccessSystematic Review
Pathophysiological Bases and Clinical Uses of Metalloproteases in Cardiovascular Disease: A Scoping Review
by
Laura Manuela Olarte Bermúdez, Camila Karduss Preciado, Julián Manuel Espitia Ángel, Ana María Santos Granados, Julio Cesar Martínez Lozano, Carlos Alberto Pacheco Cuentas and Diana Marcela Díaz Quijano
Cardiogenetics 2025, 15(2), 14; https://doi.org/10.3390/cardiogenetics15020014 - 29 May 2025
Abstract
(1) Objective: Cardiovascular diseases (CVD) are one of the main entities responsible for the progressive increase in morbidity and mortality worldwide. Some of the biomarkers involved in these processes are matrix metalloproteases (MMPs) and disintegrants and metalloproteases (ADAMS), produced by multiple tissues and
[...] Read more.
(1) Objective: Cardiovascular diseases (CVD) are one of the main entities responsible for the progressive increase in morbidity and mortality worldwide. Some of the biomarkers involved in these processes are matrix metalloproteases (MMPs) and disintegrants and metalloproteases (ADAMS), produced by multiple tissues and whose main function is the excessive degradation of the extracellular matrix (ECM). The aim of this study is to describe the existing literature on the role of MMP in the pathophysiology of CVD and its usefulness in clinical practice for the diagnostic and therapeutic approach. (2) Methods: A systematic exploratory review of the literature was carried out according to the guidelines of the Joanna Briggs Institute. The information was collected from the PubMed/Medline and Embase databases, using the search strategy “cardiovascular disease” AND “Metalloprotease”. (3) Results: Thirty eight papers that mainly mention 17 types of MMPs were included. Pathologies such as atherosclerosis, coagulation diseases, atrial fibrillation, ischemic heart disease, heart failure, hypertension, dyslipidemias, congenital cyanotic heart disease and Takotsubo cardiomyopathy were identified. (4) Conclusions: The stimulation or inhibition of these biomolecules could generate positive and/or negative effects, which impact the development and prognosis of the disease. Furthermore, they can be potential biomarkers for new diagnostic and even therapeutic approaches in the future.
Full article
(This article belongs to the Topic Biomarkers in Cardiovascular Disease—Chances and Risks, 2nd Volume)
►▼
Show Figures
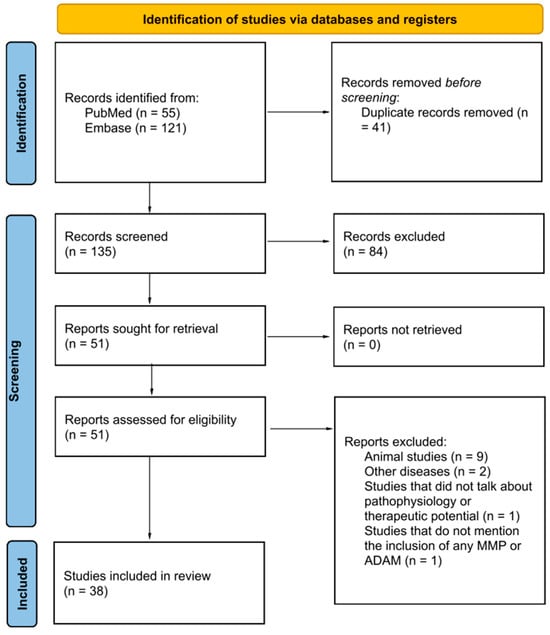
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Cardiogenetics, Hearts, JCDD, JCM
Adult Congenital Heart Disease: Advances in Diagnosis, Surgery, and Lifelong Care
Topic Editors: Satoshi Akagi, Jin Young SongDeadline: 30 June 2026

Conferences
Special Issues
Special Issue in
Cardiogenetics
Gene Therapy in Cardiovascular Genetics
Guest Editors: Matteo Vatta, Valeria NovelliDeadline: 30 April 2026
Special Issue in
Cardiogenetics
Genetic Insights into Sudden Cardiac Death: From Risk Stratification to Precision Prevention
Guest Editors: Giuseppe Limongelli, Lia Crotti, Emanuele Bobbio, Saskia Nanette Van Der CrabbenDeadline: 31 August 2026
Special Issue in
Cardiogenetics
Contemporary and Future Approaches to Inherited Cardiomyopathies
Guest Editors: Michele Ciabatti, Alessia ArgiroDeadline: 20 December 2026












