From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations
Abstract
1. Introduction
2. Congenital Genetic Heart Defects
2.1. Marfan Syndrome
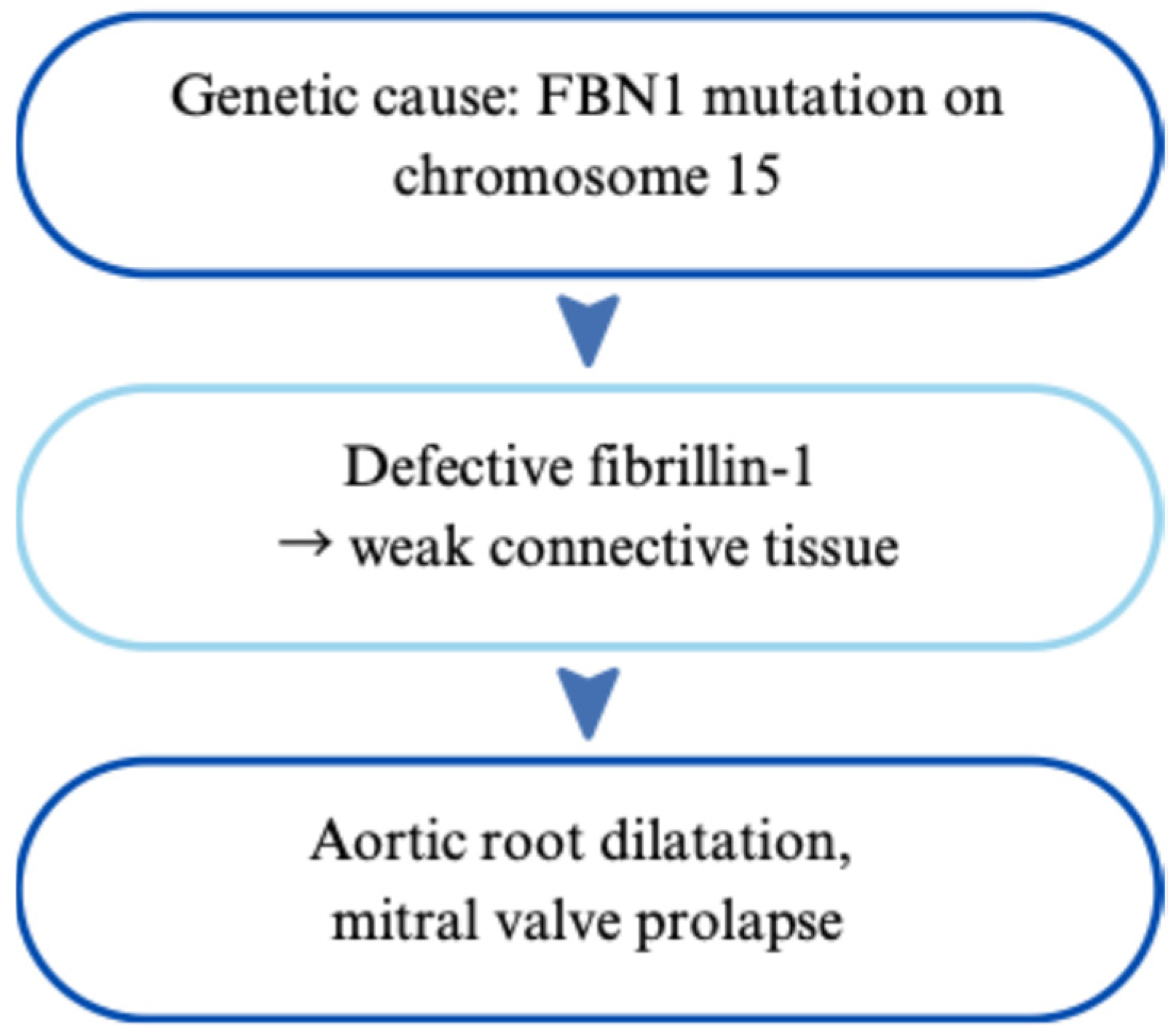
2.1.1. Genetic Basis and Phenotypic Impact of Inherited Variants
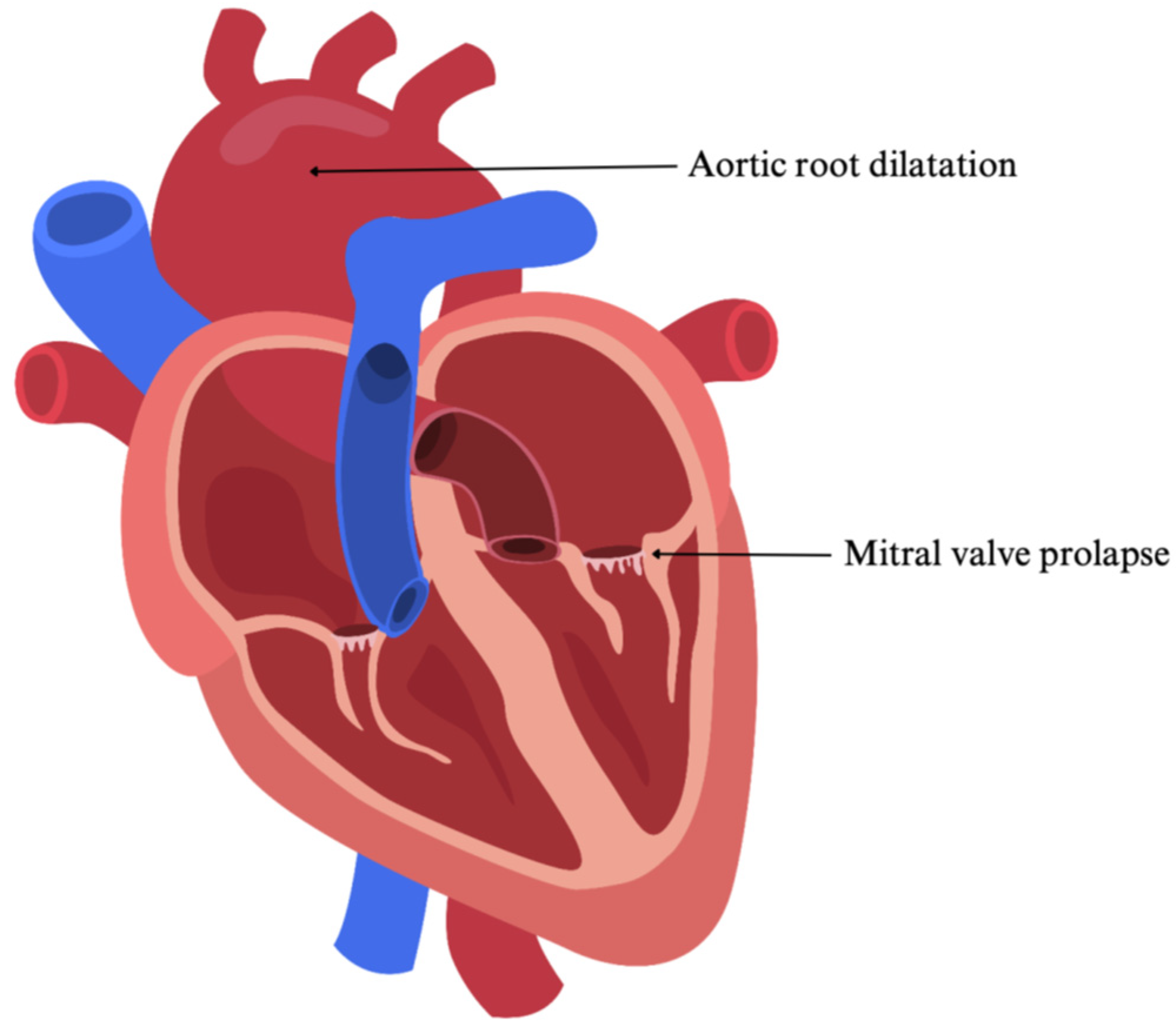
2.1.2. Impact on the Heart and Cardiovascular System
2.1.3. Symptoms and Clinical Presentation in Children
2.1.4. Diagnosis and Management
2.2. Noonan Syndrome
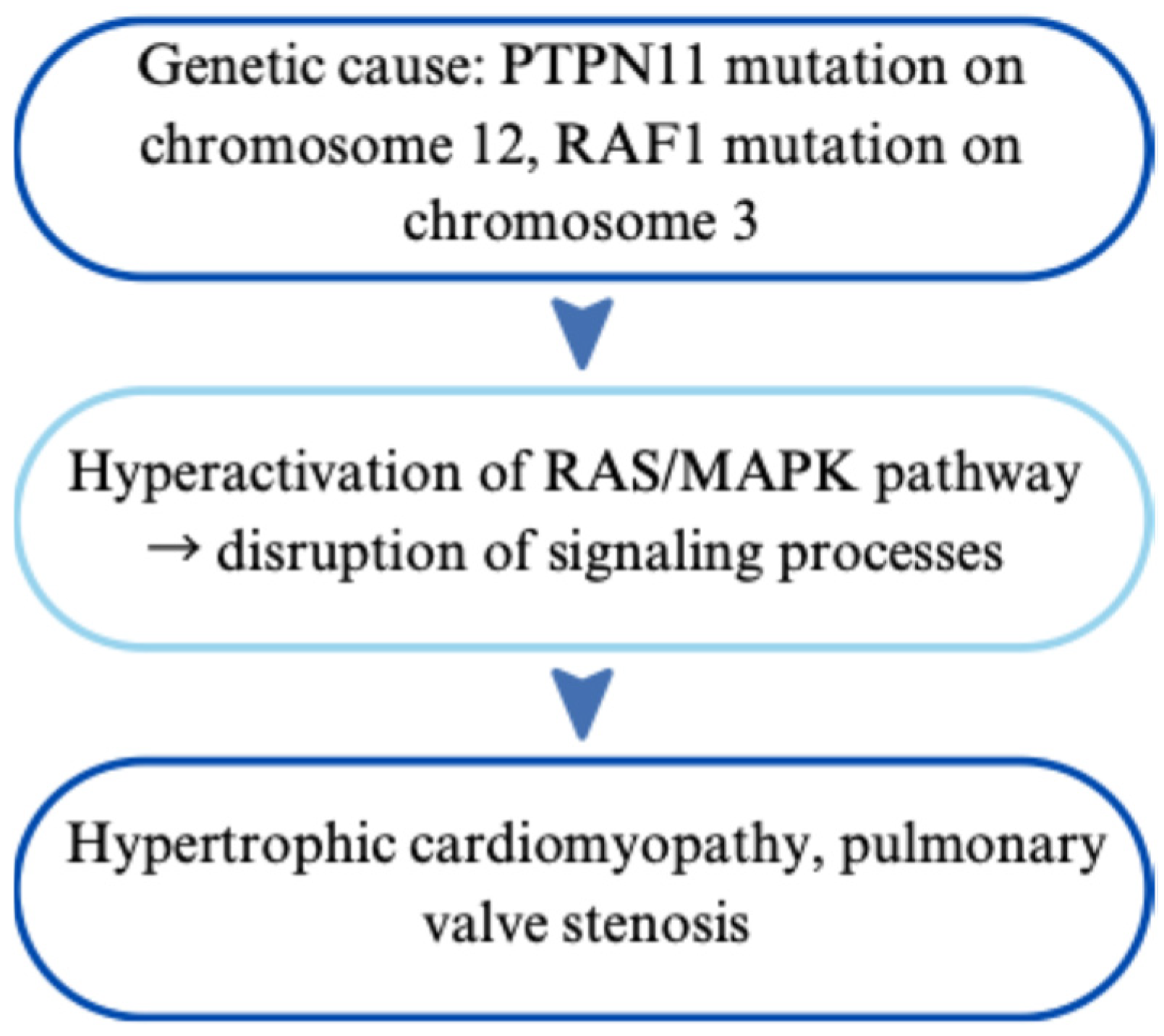
2.2.1. Genetic Basis and Phenotypic Impact of Inherited Variants
2.2.2. Impact on the Heart and Cardiovascular System
2.2.3. Symptoms and Clinical Presentation in Children
2.2.4. Diagnosis and Management
2.3. 22q11.2 Deletion Syndrome
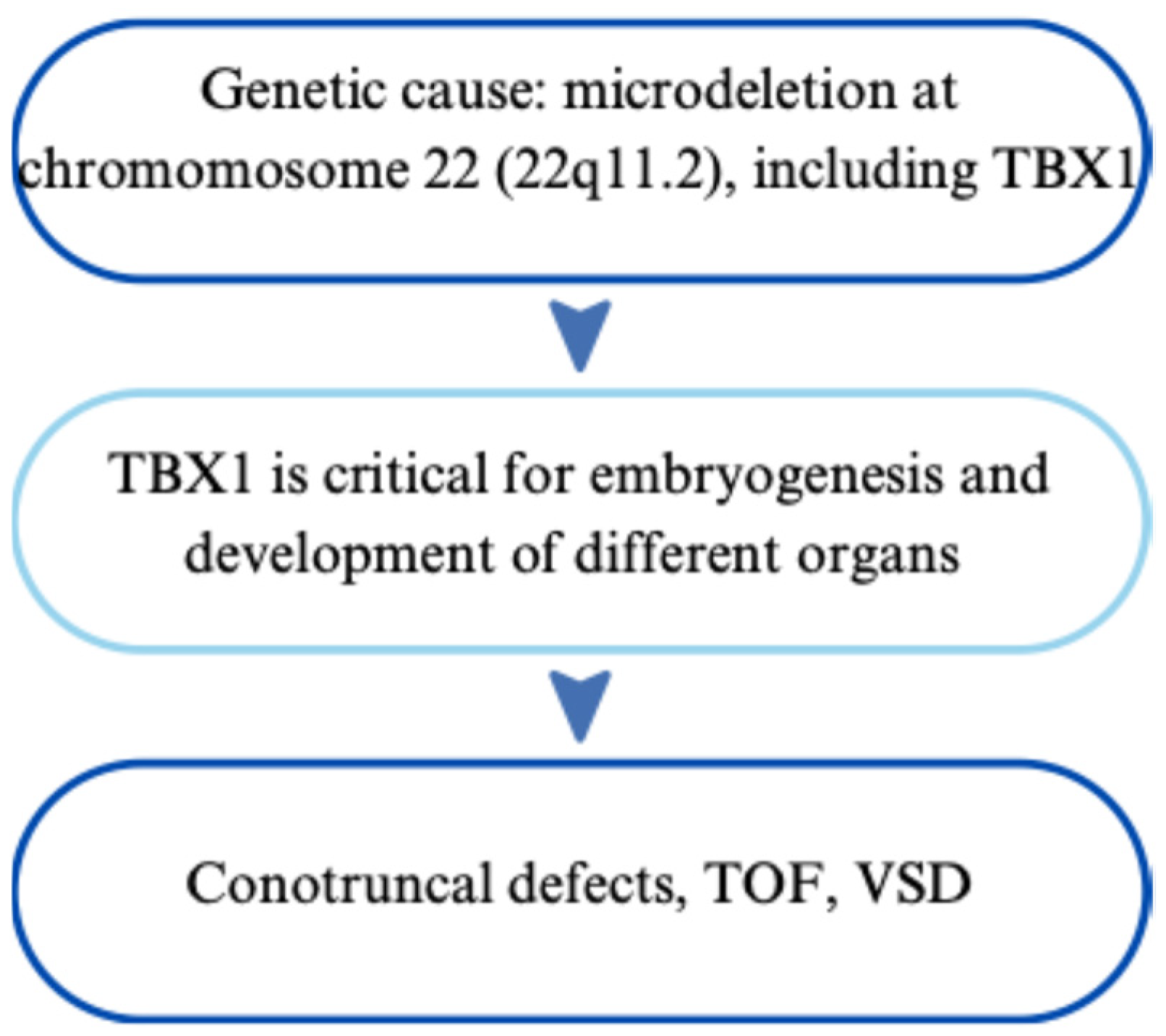
2.3.1. Genetic Basis and Phenotypic Impact of Inherited Variants
2.3.2. Impact on the Heart and Cardiovascular System
2.3.3. Symptoms and Clinical Presentation in Children
2.3.4. Diagnosis and Management
2.4. Dilated Cardiomyopathy
2.4.1. Genetic Basis and Phenotypic Impact of Inherited Variants
2.4.2. Impact on the Heart and Cardiovascular System
2.4.3. Symptoms and Clinical Presentation in Children
2.4.4. Diagnosis and Management
2.5. Hypertrophic Cardiomyopathy
2.5.1. Genetic Basis and Phenotypic Impact of Inherited Variants
2.5.2. Impact on the Heart and Cardiovascular System
2.5.3. Symptoms and Clinical Presentation in Children
2.5.4. Diagnosis and Management
2.6. Tetralogy of Fallot
2.6.1. Genetic Basis and Phenotypic Impact of Inherited Variants
2.6.2. Impact on the Heart and Cardiovascular System
2.6.3. Symptoms and Clinical Presentation in Children
2.6.4. Diagnosis and Management
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, J.; Li, Q.; Deng, L.; Xiong, J.; Cheng, Z.; Ye, C. Global, regional, and national epidemiology of congenital heart disease in children from 1990 to 2021. Front. Cardiovasc. Med. 2025, 12, 1522644. Available online: https://pubmed.ncbi.nlm.nih.gov/40454242/ (accessed on 28 April 2025). [CrossRef]
- Diab, N.S.; Barish, S.; Dong, W.; Zhao, S.; Allington, G.; Yu, X.; Kahle, K.T.; Brueckner, M.; Jin, S.C. Molecular Genetics and Complex Inheritance of Congenital Heart Disease. Genes 2021, 12, 1020. Available online: https://pubmed.ncbi.nlm.nih.gov/34209044/ (accessed on 28 April 2025). [CrossRef]
- Coelho, S.G.; Almeida, A.G. Marfan syndrome revisited: From genetics to the clinic. Rev. Port. Cardiol. (Engl. Ed.) 2020, 39, 215–226. Available online: https://pubmed.ncbi.nlm.nih.gov/32439107/ (accessed on 30 April 2025). [CrossRef]
- Wright, M.J.; Connolly, H.M. Genetics, Clinical Features, and Diagnosis of Marfan Syndrome and Related Disorders. UpToDate. [updated 1 October 2024]. Available online: https://www.uptodate.com/contents/genetics-clinical-features-and-diagnosis-of-marfan-syndrome-and-related-disorders#topicContent (accessed on 6 June 2025).
- Milewicz, D.M.; Braverman, A.C.; De Backer, J.; Morris, S.A.; Boileau, C.; Maumenee, I.H.; Jondeau, G.; Evangelista, A.; Pyeritz, R.E. Marfan syndrome. Nat. Rev. Dis. Primers. 2021, 7, 64. Available online: https://pubmed.ncbi.nlm.nih.gov/34475413/ (accessed on 5 May 2025). [CrossRef] [PubMed]
- Lazea, C.; Bucerzan, S.; Crisan, M.; Al-Khzouz, C.; Miclea, D.; Şufană, C.; Cismaru, G.; Grigorescu-Sido, P. Cardiovascular manifestations in Marfan syndrome. Med. Pharm. Rep. 2021, 94, S25–S27. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8411824/ (accessed on 5 May 2025).
- Wozniak-Mielczarek, L.; Sabiniewicz, R.; Drezek-Nojowicz, M.; Nowak, R.; Gilis-Malinowska, N.; Mielczarek, M.; Łabuc, A.; Waldoch, A.; Wierzba, J. Differences in Cardiovascular Manifestation of Marfan Syndrome Between Children and Adults. Pediatr. Cardiol. 2019, 40, 393–403. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6399167/ (accessed on 6 May 2025). [CrossRef] [PubMed]
- Muiño-Mosquera, L.; Cervi, E.; De Groote, K.; Dewals, W.; Fejzic, Z.; Kazamia, K.; Mathur, S.; Milleron, O.; Mir, T.S.; Nielsen, D.G.; et al. Management of aortic disease in children with FBN1-related Marfan syndrome. Eur. Heart J. 2024, 45, 4156–4169. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11472455/ (accessed on 7 May 2025). [CrossRef] [PubMed]
- Kemezyte, A.; Gegieckiene, R.; Burnyte, B. Genotype-phenotype spectrum and prognosis of early-onset Marfan syndrome. BMC Pediatr. 2023, 23, 539. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10612290/ (accessed on 8 May 2025). [CrossRef]
- Akram, H.; Aragon-Martin, J.A.; Chandra, A. Marfan syndrome and the eye clinic: From diagnosis to management. Ther. Adv. Rare Dis. 2021, 2, 26330040211055738. Available online: https://pubmed.ncbi.nlm.nih.gov/37181104/ (accessed on 9 May 2025). [CrossRef]
- Esfandiari, H.; Ansari, S.; Mohammad-Rabei, H.; Mets, M.B. Management Strategies of Ocular Abnormalities in Patients with Marfan Syndrome: Current Perspective. J. Ophthalmic Vis. Res. 2019, 14, 71–77. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC6388525/ (accessed on 10 May 2025). [CrossRef]
- Pollock, L.; Ridout, A.; The, J.; Nnadi, C.; Stavroulias, D.; Pitcher, A.; Blair, E.; Wordsworth, P.; Vincent, T.L. The Musculoskeletal Manifestations of Marfan Syndrome: Diagnosis, Impact, and Management. Curr. Rheumatol. Rep. 2021, 23, 81. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8626407/ (accessed on 10 May 2025). [CrossRef]
- Loeys, B.L.; Dietz, H.C.; Braverman, A.C.; Callewaert, B.L.; De Backer, J.; Devereux, R.B.; Hilforst-Hofstee, Y.; Jondeau, G.; Faivre, L.; Milewicz, D.; et al. The revised Ghent nosology for the Marfan syndrome. J. Med Genet. 2010, 47, 476–485. Available online: https://jmg.bmj.com/content/jmedgenet/47/7/476.full.pdf (accessed on 7 June 2025). [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; Committee on Selected Heritable Disorders of Connective Tissue and Disability. Marfan Syndrome and Related Hereditary Aortopathis. In Selected Heritable Disorders of Connective Tissue and Disability; Volberding, P., Spicer, C.M., Cartaxo, T., Wedge, R., Eds.; National Academies Press: Washington, DC, USA, 2022; Chapter 3. Available online: https://www.ncbi.nlm.nih.gov/books/NBK584974/ (accessed on 28 April 2025).
- Marelli, S.; Micaglio, E.; Taurino, J.; Salvi, P.; Rurali, E.; Perrucci, G.L.; Dolci, C.; Udugampolage, N.S.; Caruso, R.; Gentilini, D.; et al. Marfan Syndrome: Enhanced Diagnostic Tools and Follow-up Management Strategies. Diagnostics 2023, 13, 2284. Available online: https://pubmed.ncbi.nlm.nih.gov/37443678/ (accessed on 28 April 2025). [CrossRef]
- Isselbacher, E.M.; Preventza, O.; Hamilton Black, J., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. Available online: https://pubmed.ncbi.nlm.nih.gov/36322642/ (accessed on 28 April 2025). [CrossRef] [PubMed]
- Dahlgren, J.; Noordam, C. Growth, Endocrine Features, and Growth Hormone Treatment in Noonan Syndrome. J. Clin. Med. 2022, 11, 2034. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8999676/ (accessed on 30 April 2025). [CrossRef] [PubMed]
- Sun, L.; Xie, Y.M.; Wang, S.S.; Zhang, Z.W. Cardiovascular Abnormalities and Gene Mutations in Children with Noonan Syndrome. Front. Genet. 2022, 13, 915129. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9234298/ (accessed on 1 April 2025). [CrossRef] [PubMed]
- Saint-Laurent, C.; Mazeyrie, L.; Yart, A.; Edouard, T. Novel therapeutic perspectives in Noonan syndrome and RASopathies. Eur. J. Pediatr. 2024, 183, 1011–1019. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10951041/ (accessed on 28 April 2025). [CrossRef]
- Zenker, M.; Wolf, C.M. Cardiovascular aspects of Noonan syndrome and related disorders. Med. Genet. 2025, 37, 113–124. Available online: https://pubmed.ncbi.nlm.nih.gov/40207038/ (accessed on 30 April 2025). [CrossRef]
- Linglart, L.; Gelb, B.D. Congenital heart defects in Noonan syndrome: Diagnosis, management, and treatment. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 73–80. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7682536/ (accessed on 28 April 2025). [CrossRef]
- Hilal, N.; Chen, Z.; Chen, M.H.; Choudhury, S. RASopathies and cardiac manifestations. Front. Cardiovasc. Med. 2023, 10, 1176828. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10387527/ (accessed on 30 April 2025). [CrossRef]
- Baldo, F.; Fachin, A.; Da Re, B.; Rubinato, E.; Bobbo, M.; Barbi, E. New insights on Noonan syndrome’s clinical phenotype: A single center retrospective study. BMC Pediatr. 2022, 22, 734. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9789552/ (accessed on 15 May 2025). [CrossRef]
- Roberts, A.E. Noonan Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/20301303/ (accessed on 20 June 2025). [PubMed]
- Sleutjes, J.; Kleimeier, L.; Leenders, E.; Klein, W.; Draaisma, J. Lymphatic Abnormalities in Noonan Syndrome Spectrum Disorders: A Systematic Review. Mol. Syndromol. 2022, 13, 1–11. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8832235/ (accessed on 15 May 2025). [CrossRef] [PubMed]
- Zenker, M.; Edouard, T.; Blair, J.C.; Cappa, M. Noonan syndrome: Improving recognition and diagnosis. Arch. Dis. Child. 2022, 107, 1073–1078. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9685729/ (accessed on 1 May 2025). [CrossRef] [PubMed]
- Allen, M.J.; Sharma, S. Noonan Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/30335302/ (accessed on 19 June 2025). [PubMed]
- Wolf, C.M.; Zenker, M.; Burkitt-Wright, E.; Edouard, T.; García-Miñaúr, S.; Lebl, J.; Shaikh, G.; Tartaglia, M.; Verloes, A.; Östman-Smith, I. Management of cardiac aspects in children with Noonan syndrome—Results from a European clinical practice survey among paediatric cardiologists. Eur. J. Med. Genet. 2022, 65, 104372. Available online: https://pubmed.ncbi.nlm.nih.gov/34757052/ (accessed on 15 June 2025). [CrossRef] [PubMed]
- Kowalik, D.; Lewiński, A.; Stawerska, R. Management strategies for optimal linear growth in Noonan syndrome (NS) children: Minireview and case series of three patients with NS due to PTPN11 mutation and confirmed growth hormone deficiency. Endokrynol. Pol. 2024, 75, 592–603. Available online: https://pubmed.ncbi.nlm.nih.gov/40091322/ (accessed on 15 June 2025). [CrossRef]
- Szczawińska-Popłonyk, A.; Schwartzmann, E.; Chmara, Z.; Głukowska, A.; Krysa, T.; Majchrzycki, M.; Olejnicki, M.; Ostrowska, P.; Babik, J. Chromosome 22q11.2 Deletion Syndrome: A Comprehensive Review of Molecular Genetics in the Context of Multidisciplinary Clinical Approach. Int. J. Mol. Sci. 2023, 24, 8317. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10179617/ (accessed on 22 June 2025). [CrossRef]
- Biggs, S.E.; Gilchrist, B.; May, K.R. Chromosome 22q11.2 Deletion (DiGeorge Syndrome): Immunologic Features, Diagnosis, and Management. Curr. Allergy Asthma Rep. 2023, 23, 213–222. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9999075/ (accessed on 16 July 2025). [CrossRef]
- McDonald-McGinn, D.M.; Hain, H.S.; Emanuel, B.S.; Zackai, E.H. 22q11.2 Deletion Syndrome. In GeneReviews®; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. Available online: https://pubmed.ncbi.nlm.nih.gov/20301696/ (accessed on 26 June 2025). [PubMed]
- Zhao, Y.; Wang, Y.; Shi, L.; McDonald-McGinn, D.M.; Crowley, T.B.; McGinn, D.E.; Tran, O.T.; Miller, D.; Lin, J.R.; Zackai, E.; et al. Chromatin regulators in the TBX1 network confer risk for conotruncal heart defects in 22q11.2DS. NPJ Genom. Med. 2023, 8, 17. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10354062/ (accessed on 16 July 2025).
- Putotto, C.; Unolt, M.; Lambiase, C.; Marchetti, F.; Anaclerio, S.; Favoriti, A.; Tancredi, G.; Mastromoro, G.; Pugnaloni, F.; Liberati, N.; et al. Cardiac function in adolescents and young adults with 22q11.2 deletion syndrome without congenital heart disease. Eur. J. Med. Genet. 2023, 66, 104651. Available online: https://pubmed.ncbi.nlm.nih.gov/36404488/ (accessed on 16 July 2025).
- Diniz, B.L.; Deconte, D.; Gadelha, K.A.; Glaeser, A.B.; Guaraná, B.B.; de Moura, A.Á.; Rosa, R.F.M.; Zen, P.R.G. Congenital Heart Defects and 22q11.2 Deletion Syndrome: A 20-Year Update and New Insights to Aid Clinical Diagnosis. J. Pediatr. Genet. 2023, 12, 113–122. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10118709/ (accessed on 1 July 2025). [CrossRef]
- Putotto, C.; Pulvirenti, F.; Pugnaloni, F.; Isufi, I.; Unolt, M.; Anaclerio, S.; Caputo, V.; Bernardini, L.; Messina, E.; Moretti, C.; et al. Clinical Risk Factors for Aortic Root Dilation in Patients with 22q11.2 Deletion Syndrome: A Longitudinal Single-Center Study. Genes 2022, 13, 2334. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9778342/ (accessed on 16 July 2025). [CrossRef]
- Pavlicek, J.; Klaskova, E.; Kapralova, S.; Palatova, A.M.; Piegzova, A.; Spacek, R.; Gruszka, T. Major heart defects: The diagnostic evaluations of first-year-olds. BMC Pediatr. 2021, 21, 528. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8630885/ (accessed on 18 February 2025). [CrossRef] [PubMed]
- Funato, N. Craniofacial Phenotypes and Genetics of DiGeorge Syndrome. J. Dev. Biol. 2022, 10, 18. Available online: https://pubmed.ncbi.nlm.nih.gov/35645294/ (accessed on 18 February 2025). [CrossRef] [PubMed]
- Agarwal, M.; Kumar, V.; Dwivedi, A. Diagnosis of 22q11.2 deletion syndrome in children with congenital heart diseases and facial dysmorphisms. Med. J. Armed Forces India. 2023, 79, S196–S201. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10746800/ (accessed on 18 June 2025). [CrossRef] [PubMed]
- Yu, H.H.; Chien, Y.H.; Lu, M.Y.; Hu, Y.C.; Lee, J.H.; Wang, L.C.; Lin, Y.T.; Yang, Y.H.; Chiang, B.L. Clinical and Immunological Defects and Outcomes in Patients with Chromosome 22q11.2 Deletion Syndrome. J. Clin. Immunol. 2022, 42, 1721–1729. [Google Scholar] [CrossRef]
- Rojnueangit, K.; Khetkham, T.; Onsod, P.; Chareonsirisuthigul, T. Clinical Features to Predict 22q11.2 Deletion Syndrome Proven by Molecular Genetic Testing. J. Pediatr. Genet. 2020, 11, 22–27. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8847066/ (accessed on 1 April 2025). [CrossRef]
- Óskarsdóttir, S.; Boot, E.; Crowley, T.B.; Loo, J.C.Y.; Arganbright, J.M.; Armando, M.; Baylis, A.L.; Breetvelt, E.J.; Castelein, R.M.; Chadehumbe, M.; et al. Updated clinical practice recommendations for managing children with 22q11.2 deletion syndrome. Genet. Med. 2023, 25, 100338. Available online: https://pubmed.ncbi.nlm.nih.gov/36729053/ (accessed on 16 July 2025). [CrossRef]
- Mahmaljy, H.; Yelamanchili, V.S.; Singhal, M. Dilated Cardiomyopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/28722940/ (accessed on 19 June 2025). [PubMed]
- Novelli, V.; Canonico, F.; Laborante, R.; Manzoni, M.; Arcudi, A.; Pompilio, G.; Mercuri, E.; Patti, G.; D’Amario, D. Unraveling the Genetic Heartbeat: Decoding Cardiac Involvement in Duchenne Muscular Dystrophy. Biomedicines. 2025, 13, 102. Available online: https://pubmed.ncbi.nlm.nih.gov/39857686/ (accessed on 18 February 2025). [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers. 2021, 7, 13. Available online: https://pubmed.ncbi.nlm.nih.gov/33602943/ (accessed on 18 February 2025). [CrossRef]
- Schultz, T.I.; Raucci, F.J., Jr.; Salloum, F.N. Cardiovascular Disease in Duchenne Muscular Dystrophy: Overview and Insight Into Novel Therapeutic Targets. JACC Basic. Transl. Sci. 2022, 7, 608–625. Available online: https://pubmed.ncbi.nlm.nih.gov/35818510/ (accessed on 25 February 2025). [CrossRef]
- Bakhshandeh, M.; Behroozi, S. Next-generation sequencing approach to molecular diagnosis of Iranian patients with Duchenne/Becker muscular dystrophy: Several novel variants identified. eNeurologicalSci 2023, 30, 100446. Available online: https://pubmed.ncbi.nlm.nih.gov/36845278/ (accessed on 25 February 2025). [CrossRef]
- Venugopal, V.; Pavlakis, S. Duchenne Muscular Dystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/29493971/ (accessed on 19 June 2025). [PubMed]
- Lupu, M.; Pintilie, I.M.; Teleanu, R.I.; Marin, G.G.; Vladâcenco, O.A.; Severin, E.M. Early Cardiac Dysfunction in Duchenne Muscular Dystrophy: A Case Report and Literature Update. Int. J. Mol. Sci. 2025, 26, 1685. Available online: https://pubmed.ncbi.nlm.nih.gov/40004149/ (accessed on 18 February 2025). [CrossRef]
- Johnson, R.; Otway, R.; Chin, E.; Horvat, C.; Ohanian, M.; Wilcox, J.A.L.; Su, Z.; Prestes, P.; Smolnikov, A.; Soka, M.; et al. DMD-Associated Dilated Cardiomyopathy: Genotypes, Phenotypes, and Phenocopies. Circ. Genom. Precis. Med. 2023, 16, 421–430. Available online: https://pubmed.ncbi.nlm.nih.gov/37671549/ (accessed on 25 February 2025). [CrossRef] [PubMed]
- Landfeldt, E.; Alemán, A.; Abner, S.; Zhang, R.; Werner, C.; Tomazos, I.; Lochmüller, H.; Quinlivan, R.M.; Wahbi, K. Predictors of cardiac disease in duchenne muscular dystrophy: A systematic review and evidence grading. Orphanet J. Rare Dis. 2024, 19, 359. Available online: https://pubmed.ncbi.nlm.nih.gov/39342355/ (accessed on 18 February 2025). [CrossRef] [PubMed]
- Adorisio, R.; Mencarelli, E.; Cantarutti, N.; Calvieri, C.; Amato, L.; Cicenia, M.; Silvetti, M.; D’Amico, A.; Grandinetti, M.; Drago, F.; et al. Duchenne Dilated Cardiomyopathy: Cardiac Management from Prevention to Advanced Cardiovascular Therapies. J. Clin. Med. 2020, 9, 3186. Available online: https://pubmed.ncbi.nlm.nih.gov/33019553/ (accessed on 30 April 2025). [CrossRef] [PubMed]
- Sarker, S.; Eshaque, T.B.; Soorajkumar, A.; Nassir, N.; Zehra, B.; Kanta, S.I.; Rahaman, M.A.; Islam, A.; Akter, S.; Ali, M.K.; et al. Mutational spectrum and phenotypic variability of Duchenne muscular dystrophy and related disorders in a Bangladeshi population. Sci. Rep. 2023, 13, 21547. Available online: https://pubmed.ncbi.nlm.nih.gov/38057384/ (accessed on 30 April 2025). [CrossRef]
- Gatto, F.; Benemei, S.; Piluso, G.; Bello, L. The complex landscape of DMD mutations: Moving towards personalized medicine. Front. Genet. 2024, 15, 1360224. Available online: https://pubmed.ncbi.nlm.nih.gov/38596212/ (accessed on 30 April 2025). [CrossRef]
- Jama, A.; Alshudukhi, A.A.; Burke, S.; Dong, L.; Kamau, J.K.; Morris, B.; Alkhomsi, I.A.; Finck, B.N.; Voss, A.A.; Ren, H. Exploring lipin1 as a promising therapeutic target for the treatment of Duchenne muscular dystrophy. J. Transl. Med. 2024, 22, 664. Available online: https://pubmed.ncbi.nlm.nih.gov/39014470/ (accessed on 28 April 2025). [CrossRef]
- LaPelusa, A.; Asuncion, R.M.D.; Kentris, M. Muscular Dystrophy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/32809417/ (accessed on 20 June 2025). [PubMed]
- Prakash, N.; Suthar, R.; Sihag, B.K.; Debi, U.; Kumar, R.M.; Sankhyan, N. Cardiac MRI and Echocardiography for Early Diagnosis of Cardiomyopathy Among Boys with Duchenne Muscular Dystrophy: A Cross-Sectional Study. Front. Pediatr. 2022, 10, 818608. Available online: https://pubmed.ncbi.nlm.nih.gov/35359887/ (accessed on 30 June 2025). [CrossRef]
- D’Amario, D.; Arcudi, A.; Narducci, M.L.; Novelli, V.; Canonico, F.; Parodi, A.; Dell’Era, G.; Di Francesco, M.; Laborante, R.; Borovac, J.A.; et al. Arrhythmic Risk Stratification and Sudden Cardiac Death Prevention in Duchenne Muscular Dystrophy: A Critical Appraisal. Rev. Cardiovasc. Med. 2025, 26, 27089. Available online: https://pubmed.ncbi.nlm.nih.gov/40160579/ (accessed on 30 June 2025). [CrossRef]
- Takeda, S.; Clemens, P.R.; Hoffman, E.P. Exon-Skipping in Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, S343–S358. Available online: https://pubmed.ncbi.nlm.nih.gov/34180420/ (accessed on 30 June 2025). [CrossRef]
- Arcudi, A.; Di Francesco, M.; Rodolico, D.; D’Amario, D. Angiotensin receptor-neprilysin inhibitor in symptomatic patients with Duchenne dilated cardiomyopathy: A primetime. ESC Heart Fail. 2022, 9, 3639–3642. Available online: https://pubmed.ncbi.nlm.nih.gov/35712811/ (accessed on 30 June 2025). [CrossRef]
- Zhao, M.; He, X.; Min, X.; Yang, H.; Wu, W.; Zhong, J.; Xu, H.; Chen, J. Recent Clinical Updates of Hypertrophic Cardiomyopathy and Future Therapeutic Strategies. Rev. Cardiovasc. Med. 2025, 26, 25132. Available online: https://pubmed.ncbi.nlm.nih.gov/40026515/ (accessed on 25 June 2025). [CrossRef] [PubMed]
- Yigit, G.; Kaulfuß, S.; Wollnik, B. Understanding inherited cardiomyopathies: Clinical aspects and genetic determinants. Med. Genet. 2025, 37, 103–111. Available online: https://pubmed.ncbi.nlm.nih.gov/40207042/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 2024, 45, 2727–2734. Available online: https://pubmed.ncbi.nlm.nih.gov/38984491/ (accessed on 16 July 2025). [CrossRef]
- Tudurachi, B.S.; Zăvoi, A.; Leonte, A.; Țăpoi, L.; Ureche, C.; Bîrgoan, S.G.; Chiuariu, T.; Anghel, L.; Radu, R.; Sascău, R.A.; et al. An Update on MYBPC3 Gene Mutation in Hypertrophic Cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 10510. Available online: https://pubmed.ncbi.nlm.nih.gov/37445689/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Velicki, L.; Jakovljevic, D.G.; Preveden, A.; Golubovic, M.; Bjelobrk, M.; Ilic, A.; Stojsic, S.; Barlocco, F.; Tafelmeier, M.; Okwose, N.; et al. Genetic determinants of clinical phenotype in hypertrophic cardiomyopathy. BMC Cardiovasc. Disord. 2020, 20, 516. Available online: https://pubmed.ncbi.nlm.nih.gov/33297970/ (accessed on 30 April 2025). [CrossRef]
- Kim, O.H.; Kim, J.; Kim, Y.; Lee, S.; Lee, B.H.; Kim, B.J.; Park, H.Y.; Park, M.H. Exploring novel MYH7 gene variants using in silico analyses in Korean patients with cardiomyopathy. BMC Med. Genomics 2024, 17, 225. Available online: https://pubmed.ncbi.nlm.nih.gov/39237976/ (accessed on 15 May 2025). [CrossRef]
- Bahout, M.; Severa, G.; Kamoun, E.; Bouhour, F.; Pegat, A.; Toutain, A.; Lagrange, E.; Duval, F.; Tard, C.; De la Cruz, E.; et al. MYH7-related myopathies: Clinical, myopathological and genotypic spectrum in a multicentre French cohort. J. Neurol. Neurosurg. Psychiatry 2025, 96, 453–461. Available online: https://pubmed.ncbi.nlm.nih.gov/39448255/ (accessed on 15 May 2025). [CrossRef]
- Hespe, S.; Waddell, A.; Asatryan, B.; Owens, E.; Thaxton, C.; Adduru, M.L.; Anderson, K.; Brown, E.E.; Hoffman-Andrews, L.; Jordan, E.; et al. Genes Associated with Hypertrophic Cardiomyopathy: A Reappraisal by the ClinGen Hereditary Cardiovascular Disease Gene Curation Expert Panel. J. Am. Coll. Cardiol. 2025, 85, 727–740. Available online: https://pubmed.ncbi.nlm.nih.gov/39971408/ (accessed on 10 May 2025). [CrossRef]
- Topriceanu, C.C.; Pereira, A.C.; Moon, J.C.; Captur, G.; Ho, C.Y. Meta-Analysis of Penetrance and Systematic Review on Transition to Disease in Genetic Hypertrophic Cardiomyopathy. Circulation 2024, 149, 107–123. Available online: https://pubmed.ncbi.nlm.nih.gov/37929589/ (accessed on 1 July 2025). [CrossRef] [PubMed]
- Gharibeh, L.; Smedira, N.G.; Grau, J.B. Comprehensive left ventricular outflow tract management beyond septal reduction to relieve obstruction. Asian Cardiovasc. Thorac. Ann. 2022, 30, 43–52. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC8941720/ (accessed on 2 July 2025). [PubMed]
- Verheyen, N.; Auer, J.; Bonaros, N.; Buchacher, T.; Dalos, D.; Grimm, M.; Mayr, A.; Rab, A.; Reinstadler, S.; Scherr, D.; et al. Austrian consensus statement on the diagnosis and management of hypertrophic cardiomyopathy. Wien. Klin. Wochenschr. 2024, 136, 571–597. Available online: https://pubmed.ncbi.nlm.nih.gov/39352517/ (accessed on 5 July 2025). [CrossRef] [PubMed]
- Shen, H.; Dong, S.Y.; Ren, M.S.; Wang, R. Ventricular arrhythmia and sudden cardiac death in hypertrophic cardiomyopathy: From bench to bedside. Front. Cardiovasc. Med. 2022, 9, 949294. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC9433716/ (accessed on 6 July 2025). [CrossRef]
- Basit, H.; Alahmadi, M.H.; Rout, P.; Sharma, S. Hypertrophic Cardiomyopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/28613539/ (accessed on 14 June 2025). [PubMed]
- Thomas, S.L.; Heaton, J.; Makaryus, A.N. Physiology, Cardiovascular Murmurs. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/30247833/ (accessed on 12 June 2025). [PubMed]
- Hsu, J.C.; Huang, Y.T.; Lin, L.Y. Stroke risk in hypertrophic cardiomyopathy patients with atrial fibrillation: A nationwide database study. Aging 2020, 12, 24219–24227. Available online: https://pubmed.ncbi.nlm.nih.gov/33226371/ (accessed on 13 June 2025). [CrossRef]
- Braunwald, E. Hypertrophic Cardiomyopathy: A Brief Overview. Am. J. Cardiol. 2024, 212S, S1–S3. Available online: https://pubmed.ncbi.nlm.nih.gov/38368032/ (accessed on 14 June 2025). [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2024, 149, e1239–e1311. Available online: https://pubmed.ncbi.nlm.nih.gov/38718139/ (accessed on 18 July 2025). [CrossRef]
- Georgiopoulos, G.; Figliozzi, S.; Pateras, K.; Nicoli, F.; Bampatsias, D.; Beltrami, M.; Finocchiaro, G.; Chiribiri, A.; Masci, P.G.; Olivotto, I. Comparison of Demographic, Clinical, Biochemical, and Imaging Findings in Hypertrophic Cardiomyopathy Prognosis: A Network Meta-Analysis. JACC Heart Fail. 2023, 11, 30–41. Available online: https://pubmed.ncbi.nlm.nih.gov/36599547/ (accessed on 18 July 2025). [CrossRef]
- Li, X.; Hong, S.J.; Hong, H.; Zhang, Z.Q.; Li, J. Neonatal hypertrophic cardiomyopathy with dyspnoea as the first symptom: A case report. Front. Pediatr. 2023, 11, 1295539. Available online: https://pubmed.ncbi.nlm.nih.gov/38094187/ (accessed on 22 July 2025). [CrossRef]
- Shafqat, A.; Shaik, A.; Koritala, S.; Mushtaq, A.; Sabbah, B.N.; Nahid Elshaer, A.; Baqal, O. Contemporary review on pediatric hypertrophic cardiomyopathy: Insights into detection and management. Front. Cardiovasc. Med. 2024, 10, 1277041. Available online: https://pubmed.ncbi.nlm.nih.gov/38250029/ (accessed on 20 July 2025). [CrossRef]
- Norrish, G.; Field, E.; Kaski, J.P. Childhood Hypertrophic Cardiomyopathy: A Disease of the Cardiac Sarcomere. Front. Pediatr. 2021, 9, 708679. Available online: https://pubmed.ncbi.nlm.nih.gov/34277528/ (accessed on 18 July 2025). [CrossRef]
- Nawaz, A.; Sheng, Z.; Akram, M.J.; Li, J.; Liu, L.; Yuan, Y.; Tian, J. Clinical characteristics and mortality risk factors in pediatric hypertrophic, restrictive, and rapidly progressive hypertrophic cardiomyopathy: A retrospective cohort study with follow-up. Front. Cardiovasc. Med. 2025, 12, 1541651. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11994607/ (accessed on 18 July 2025). [CrossRef]
- Leggit, J.C.; Whitaker, D. Diagnosis and Management of Hypertrophic Cardiomyopathy: Updated Guidelines From the ACC/AHA. Am. Fam. Physician 2022, 105, 207–209. Available online: https://www.aafp.org/pubs/afp/issues/2022/0200/p207.html (accessed on 16 July 2025).
- Townsend, M.; Jeewa, A.; Khoury, M.; Cunningham, C.; George, K.; Conway, J. Unique Aspects of Hypertrophic Cardiomyopathy in Children. Can. J. Cardiol. 2024, 40, 907–920. Available online: https://pubmed.ncbi.nlm.nih.gov/38244986/ (accessed on 16 July 2025). [CrossRef]
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 372–389. Available online: https://pubmed.ncbi.nlm.nih.gov/35086660/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Trasca, L.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Echocardiography in the Diagnosis of Cardiomyopathies: Current Status and Future Directions. Rev. Cardiovasc. Med. 2022, 23, 280. Available online: https://pubmed.ncbi.nlm.nih.gov/39076629/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Nealy, Z.; Kramer, C. Imaging in Hypertrophic Cardiomyopathy: Beyond Risk Stratification. Heart Fail. Clin. 2023, 19, 419–428. Available online: https://pubmed.ncbi.nlm.nih.gov/37714584/ (accessed on 17 July 2025). [CrossRef] [PubMed]
- Ireland, C.G.; Ho, C.Y. Genetic Testing in Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2024, 212S, S4–S13. Available online: https://pubmed.ncbi.nlm.nih.gov/38368035/ (accessed on 16 July 2025). [CrossRef]
- Ottaviani, A.; Mansour, D.; Molinari, L.V.; Galanti, K.; Mantini, C.; Khanji, M.Y.; Chahal, A.A.; Zimarino, M.; Renda, G.; Sciarra, L.; et al. Revisiting Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Current Practice and Novel Perspectives. J. Clin. Med. 2023, 12, 5710. Available online: https://pubmed.ncbi.nlm.nih.gov/37685777/ (accessed on 15 July 2025). [CrossRef]
- Glavaški, M.; Velicki, L.; Vučinić, N. Hypertrophic Cardiomyopathy: Genetic Foundations, Outcomes, Interconnections, and Their Modifiers. Medicina 2023, 59, 1424. Available online: https://pubmed.ncbi.nlm.nih.gov/37629714/ (accessed on 13 July 2025). [CrossRef]
- Amano, M.; Kitaoka, H.; Yoshikawa, Y.; Sakata, Y.; Dohi, K.; Tokita, Y.; Kato, T.; Matsushima, S.; Kitai, T.; Okada, A.; et al. Validation of Guideline Recommendation on Sudden Cardiac Death Prevention in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2025, 13, 1014–1026. Available online: https://pubmed.ncbi.nlm.nih.gov/40088231/ (accessed on 16 July 2025). [CrossRef]
- Aggarwal, K.; Boyapati, S.P.; Valecha, J.; Noor, A.; Kanwal, F.; Jain, R.; Kanagala, S.G. Arrhythmias and Hypertrophic Cardiomyopathy: Unravelling the Connection. Curr. Cardiol. Rev. 2024, 20, e240124226139. Available online: https://pubmed.ncbi.nlm.nih.gov/38279754/ (accessed on 12 July 2025). [CrossRef] [PubMed]
- Dicorato, M.M.; Citarelli, G.; Mangini, F.; Alemanni, R.; Albanese, M.; Cicco, S.; Greco, C.A.; Forleo, C.; Basile, P.; Carella, M.C.; et al. Integrative Approaches in the Management of Hypertrophic Cardiomyopathy: A Comprehensive Review of Current Therapeutic Modalities. Biomedicines 2025, 13, 1256. Available online: https://pubmed.ncbi.nlm.nih.gov/40427081/ (accessed on 10 July 2025). [CrossRef] [PubMed]
- Liu, C.; Zheng, F.; Gao, Y.; Wang, Z.; Zhang, X.; Tian, X. Case Report: Dual-chamber pacemaker for hypertrophic cardiomyopathy with bradyarrhythmia and idiopathic pericardial effusion: A report of two cases and literature review. Front. Cardiovasc. Med. 2025, 12, 1518000. Available online: https://pubmed.ncbi.nlm.nih.gov/40083822/ (accessed on 13 July 2025). [CrossRef] [PubMed]
- Gragnano, F.; Pelliccia, F.; Guarnaccia, N.; Niccoli, G.; De Rosa, S.; Piccolo, R.; Moscarella, E.; Fabris, E.; Montone, R.A.; Cesaro, A.; et al. Alcohol Septal Ablation in Patients with Hypertrophic Obstructive Cardiomyopathy: A Contemporary Perspective. J. Clin. Med. 2023, 12, 2810. Available online: https://pubmed.ncbi.nlm.nih.gov/37109147/ (accessed on 16 July 2025). [CrossRef]
- Torres, M.F.; Perez-Villa, F. Heart transplantation in patients with hypertrophic cardiomyopathy. Glob. Cardiol. Sci. Pract. 2018, 2018, 32. Available online: https://pubmed.ncbi.nlm.nih.gov/30393644/ (accessed on 10 July 2025). [CrossRef]
- Addepalli, A.; Guillen, M.; Dreyfuss, A.; Mantuani, D.; Nagdev, A.; Martin, D.A. Point-of-Care Ultrasound Diagnosis of Tetralogy of Fallot Causing Cyanosis: A Case Report. Clin. Pract. Cases Emerg. Med. 2022, 6, 280–283. Available online: https://pubmed.ncbi.nlm.nih.gov/36427029/ (accessed on 9 July 2025). [CrossRef]
- Horenstein, M.S.; Diaz-Frias, J.; Guillaume, M. Tetralogy of Fallot. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/30020660/ (accessed on 29 June 2025). [PubMed]
- Lindor, R.A.; Heller, K.; Hodgson, N.R.; Kishi, P.; Monas, J.; Rappaport, D.; Thomas, A.; Urumov, A.; Walker, L.E.; Majdalany, D.S. Adult Congenital Heart Disease in the Emergency Department. J. Pers. Med. 2024, 14, 66. Available online: https://pubmed.ncbi.nlm.nih.gov/38248767/ (accessed on 16 July 2025). [CrossRef]
- Yasuhara, J.; Garg, V. Genetics of congenital heart disease: A narrative review of recent advances and clinical implications. Transl. Pediatr. 2021, 10, 2366–2386. Available online: https://pubmed.ncbi.nlm.nih.gov/34733677/ (accessed on 16 July 2025). [CrossRef]
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental Risk Factors for Congenital Heart Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a037234. Available online: https://pubmed.ncbi.nlm.nih.gov/31548181/ (accessed on 17 July 2025).
- Althali, N.J.; Hentges, K.E. Genetic insights into non-syndromic Tetralogy of Fallot. Front. Physiol. 2022, 13, 1012665. Available online: https://pubmed.ncbi.nlm.nih.gov/36277185/ (accessed on 19 July 2025). [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. Available online: https://pubmed.ncbi.nlm.nih.gov/34272501/ (accessed on 20 July 2025).
- Diaz-Frias, J.; Kondamudi, N.P. Alagille Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/29939604/ (accessed on 30 June 2025). [PubMed]
- Hayes-Lattin, M.; Salmi, D. Educational Case: Tetralogy of Fallot and a Review of the Most Common Forms of Congenital Heart Disease. Acad. Pathol. 2020, 7, 2374289520934094. Available online: https://pubmed.ncbi.nlm.nih.gov/32671199/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Wilson, R.; Ross, O.; Griksaitis, M.J. Tetralogy of Fallot. BJA Educ. 2019, 19, 362–369. Available online: https://pubmed.ncbi.nlm.nih.gov/33456859/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Zhong, Z.; Wen, T. Pentalogy of Fallot: A case report. J. Int. Med. Res. 2025, 53, 3000605251334463. Available online: https://pubmed.ncbi.nlm.nih.gov/40292549/ (accessed on 16 July 2025). [PubMed]
- Milczanowski, K.; Tyczyński, P.; Kukuła, K.; Michałowska, I.; Kowalik, E.; Kowalik, I.; Mazurkiewicz, Ł.; Biernacka, E.K.; Różański, J.; Demkow, M.; et al. Pentalogy of Fallot. Kardiol. Pol. 2024, 82, 330–332. Available online: https://pubmed.ncbi.nlm.nih.gov/38348616/ (accessed on 16 July 2025). [CrossRef]
- Robyn, S.; Veronica, N.; Stephen, B.; Joanne, P. Undernutrition in young children with congenital heart disease undergoing cardiac surgery in a low-income environment. BMC Pediatr. 2024, 24, 73. Available online: https://pubmed.ncbi.nlm.nih.gov/38262979/ (accessed on 17 July 2025). [CrossRef]
- Kordopati-Zilou, K.; Sergentanis, T.; Pervanidou, P.; Sofianou-Petraki, D.; Panoulis, K.; Vlahos, N.; Eleftheriades, M. Neurodevelopmental Outcomes in Tetralogy of Fallot: A Systematic Review. Children 2022, 9, 264. Available online: https://pubmed.ncbi.nlm.nih.gov/35204984/ (accessed on 17 July 2025). [CrossRef]
- Shahjehan, R.D.; Abraham, J. Intracardiac Shunts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://pubmed.ncbi.nlm.nih.gov/32644395/ (accessed on 29 June 2025). [PubMed]
- Elizabeth Kaiser, A.; Husnain, M.A.; Fakhare Alam, L.; Kumar Murugan, S.; Kumar, R. Management of Fallot’s Uncorrected Tetralogy in Adulthood: A Narrative Review. Cureus 2024, 16, e67063. Available online: https://pubmed.ncbi.nlm.nih.gov/39286683/ (accessed on 18 February 2025).
- Vetten, Z.; Auld, B.; Han, D.Y.; Lee-Tannock, A.; Robertson, T.; Yim, D.; Brooks, P.; Hutchinson, D.; Alsweiler, J.; Gentles, T.L. Prenatal Predictors of Early Intervention in Simple Tetralogy of Fallot: A Retrospective Multi-Centre Study. Prenat. Diagn. 2025, 45, 743–751. [Google Scholar]
- Vanderlaan, R.D.; Barron, D.J. Optimal Surgical Management of Tetralogy of Fallot. CJC Pediatr. Congenit. Heart Dis. 2023, 2, 352–360. Available online: https://pubmed.ncbi.nlm.nih.gov/38161666/ (accessed on 16 July 2025). [CrossRef]
- Rasool, F.; Qureshi, A.Z.; Khan, A.; Kazmi, T.; Shah, S.A. Role of BT shunt in tetralogy of Fallot. Cardiol. Young 2024, 34, 2445–2448. Available online: https://pubmed.ncbi.nlm.nih.gov/39385596/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Luxford, J.C.; Adams, P.E.; Roberts, P.A.; Mervis, J. Right Ventricular Outflow Tract Stenting is a Safe and Effective Bridge to Definitive Repair in Symptomatic Infants with Tetralogy of Fallot1. Heart Lung Circ. 2023, 32, 638–644. Available online: https://pubmed.ncbi.nlm.nih.gov/36964005/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Akkinapally, S.; Hundalani, S.G.; Kulkarni, M.; Fernandes, C.J.; Cabrera, A.G.; Shivanna, B.; Pammi, M. Prostaglandin E1 for maintaining ductal patency in neonates with ductal-dependent cardiac lesions. Cochrane Database Syst. Rev. 2018, 2, CD011417. Available online: https://pubmed.ncbi.nlm.nih.gov/29486048/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Fan, Q.; Wang, Y.; An, Q.; Ling, Y. Right ventricular dysfunction following tetralogy of Fallot correction: Anatomical determinants and therapeutic strategies. Int. J. Surg. 2025, 111, 3979–3988. Available online: https://pubmed.ncbi.nlm.nih.gov/40171563/ (accessed on 16 July 2025). [CrossRef]
- Sun, H.Y. Prenatal diagnosis of congenital heart defects: Echocardiography. Transl. Pediatr. 2021, 10, 2210–2224. Available online: https://pubmed.ncbi.nlm.nih.gov/34584892/ (accessed on 16 July 2025). [CrossRef]
- Bucholz, E.M.; Morton, S.U.; Madriago, E.; Roberts, A.E.; Ronai, C. The Evolving Role of Genetic Evaluation in the Prenatal Diagnosis and Management of Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2024, 11, 170. Available online: https://pubmed.ncbi.nlm.nih.gov/38921669/ (accessed on 16 July 2025). [CrossRef]
| Disorder | Genes Involved | Main Cardiac Features | Diagnostic Approach | Management Highlights | References |
|---|---|---|---|---|---|
| Marfan Syndrome | Autosomal dominant; FBN1 mutations | Aortic root dilation, mitral valve prolapse | Ghent criteria; genetic testing | β-blockers, ARBs, surgical repair if >50 mm aortic dilation | [3,4,5,6,7,8,9,10,11,12,13,14,15,16] |
| Noonan Syndrome | Autosomal dominant; PTPN11, RAF1, RAS/MAPK genes | Pulmonary stenosis, hypertrophic cardiomyopathy (HCM) | Clinical features + gene panel | Cardiac surgery, β-blockers, GH therapy in short stature | [17,18,19,20,21,22,23,24,25,26,27,28,29] |
| 22q11.2 Deletion Syndrome | Microdeletion at 22q11.2; affects TBX1 | Conotruncal defects: TOF, truncus arteriosus, VSD | FISH, MLPA, CMA | Surgical correction, immune and endocrine monitoring, calcium/Vitamin D replacement | [30,31,32,33,34,35,36,37,38,39,40,41,42] |
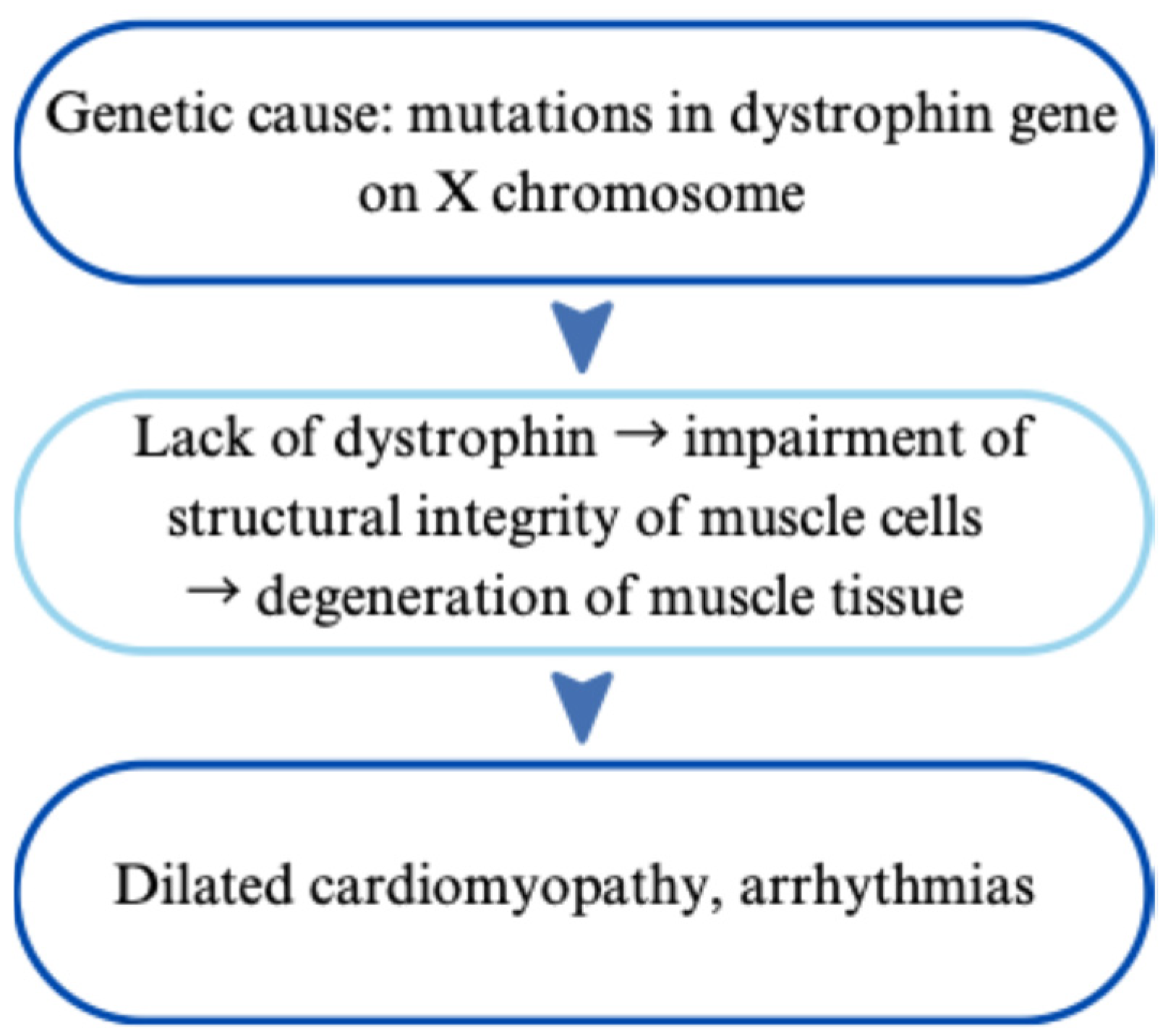
| Duchenne Muscular Dystrophy | X-linked; DMD gene mutations | Dilated cardiomyopathy (DCM), arrhythmias | Genetic testing, CMR, elevated CK | Steroids, ACE inhibitors, ARNi, ICDs, exon-skipping therapy | [43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
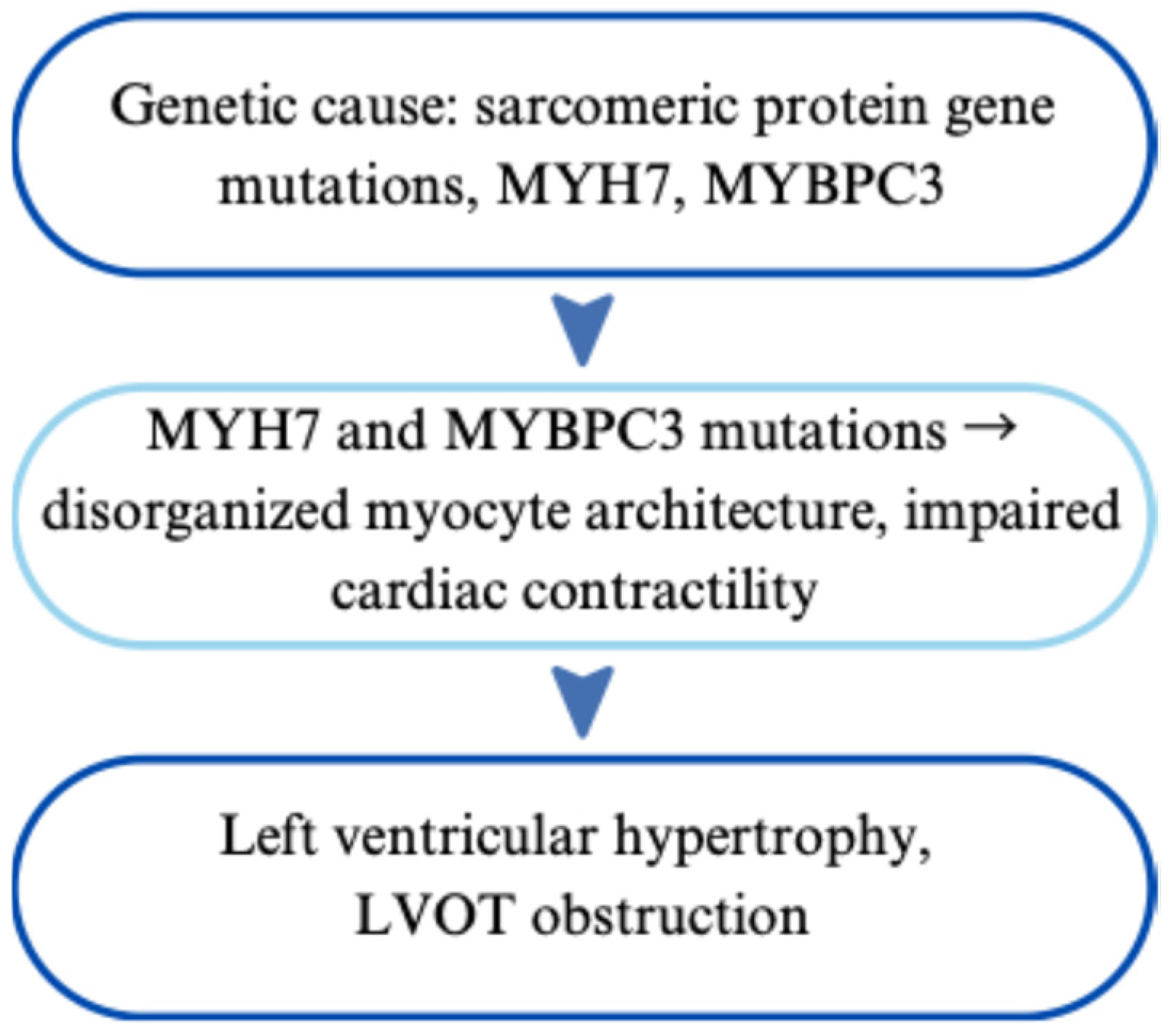
| Hypertrophic Cardiomyopathy | Mostly autosomal dominant; MYH7, MYBPC3 | Left ventricular hypertrophy, LVOT obstruction | Echocardiography, MRI, genetic testing | β-blockers, ICD for SCD prevention, septal myectomy or ablation | [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] |
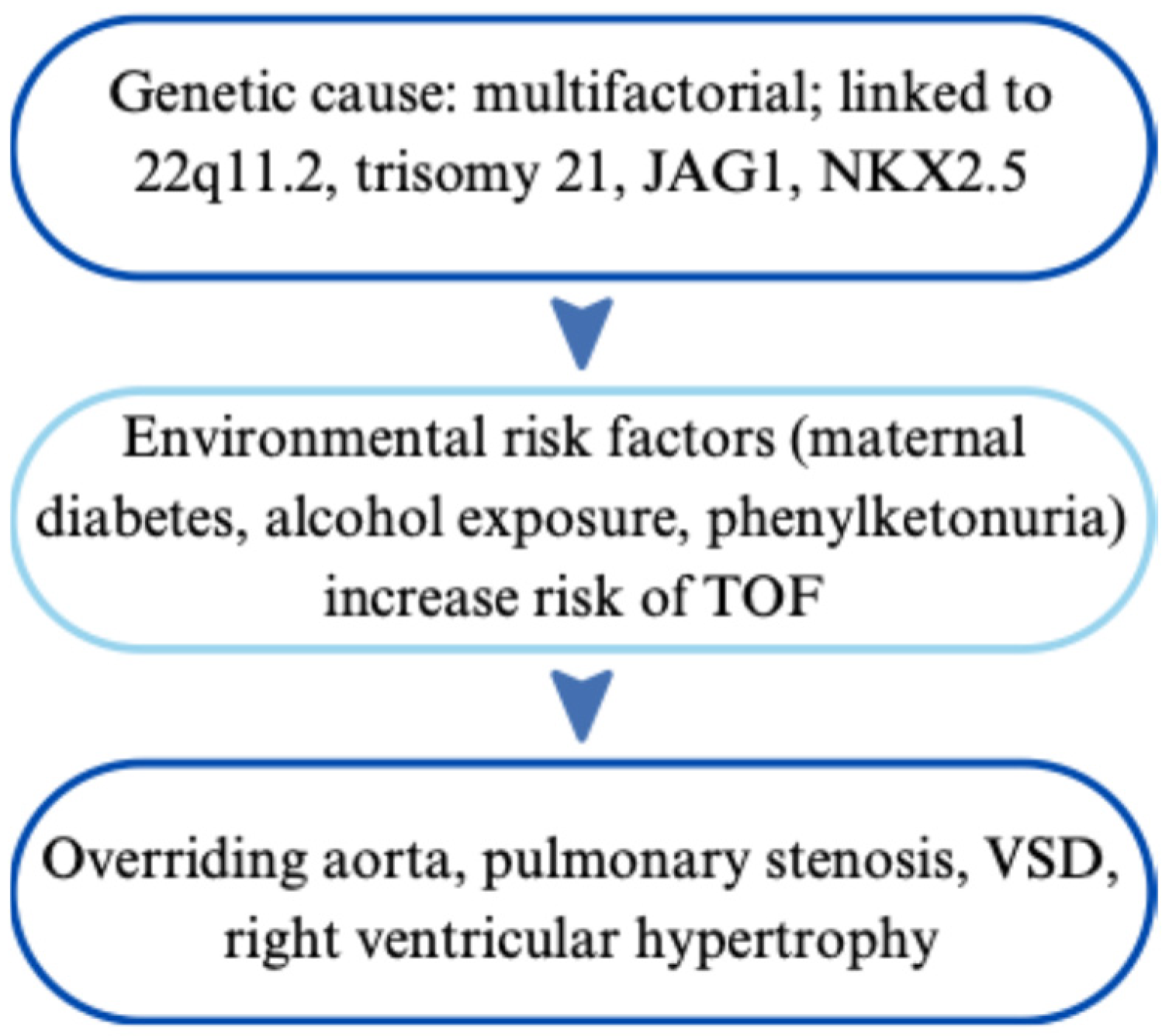
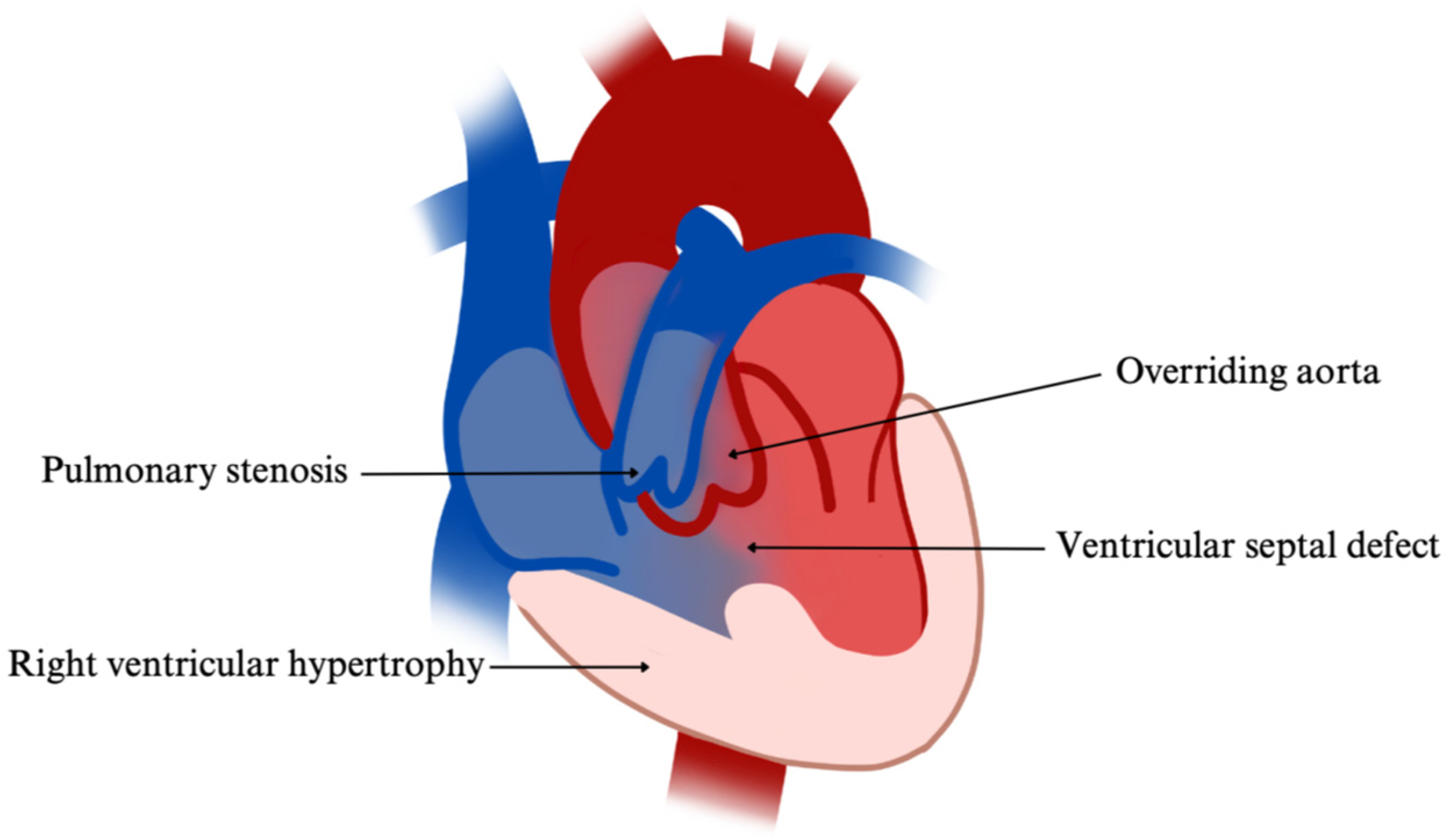
| Tetralogy of Fallot (TOF) | Multifactorial; linked to 22q11.2, JAG1, NKX2.5 | VSD, RVH, overriding aorta, pulmonary stenosis | Echo, CXR, ECG; prenatal ultrasound | Early surgical repair, prostaglandins pre-op, lifelong cardiology follow-up | [97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutmann, J.L.; Spister, A.; Baticic, L. From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations. Cardiogenetics 2025, 15, 29. https://doi.org/10.3390/cardiogenetics15040029
Gutmann JL, Spister A, Baticic L. From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations. Cardiogenetics. 2025; 15(4):29. https://doi.org/10.3390/cardiogenetics15040029
Chicago/Turabian StyleGutmann, Jule Leonie, Alina Spister, and Lara Baticic. 2025. "From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations" Cardiogenetics 15, no. 4: 29. https://doi.org/10.3390/cardiogenetics15040029
APA StyleGutmann, J. L., Spister, A., & Baticic, L. (2025). From Genetics to Phenotype: Understanding the Diverse Manifestations of Cardiovascular Genetic Diseases in Pediatric Populations. Cardiogenetics, 15(4), 29. https://doi.org/10.3390/cardiogenetics15040029






