Abstract
Dystrophinopathies, including Duchenne and Becker muscular dystrophies (DMD and BMD), are inherited neuromuscular disorders frequently complicated by progressive cardiac involvement, ultimately leading to advanced heart failure. While heart transplantation and long-term left ventricular assist device (LVAD) therapy represent potential therapeutic options, their application in this population raises significant ethical challenges. This review explores the ethical implications surrounding the allocation of scarce medical resources, particularly in patients with limited life expectancy and multisystem disease, as in DMD. Decisions regarding eligibility for heart transplantation must balance individual benefit, considering the impact of excluding other potential recipients. LVAD therapy, although more accessible, still demands careful patient selection due to high perioperative risk and postoperative complications. The review emphasizes the need for transparent, multidisciplinary decision-making processes that respect patient autonomy while ensuring equitable and rational distribution of healthcare resources. Ultimately, while advanced therapies may be feasible in selected cases, particularly in BMD, ethical deliberation remains central to determining their appropriateness in the context of dystrophinopathies.
Keywords:
Duchenne; Becker; dystrophin; dystrophinopathy; heart transplant; LVAD; heart transplant ethics 1. Introduction
Dystrophinopathies are inherited neuromuscular disorders with Duchenne and Becker muscular dystrophy (DMD and BMD) representing the most common clinical phenotypes. DMD represents the most common muscular dystrophy in children, with an annual global incidence of about one in 5000 live males [1] and an estimated global and birth prevalence of 7.1 and 19.8 per 100,000 males, respectively [2].
Similar to what is observed in skeletal muscle, dysfunction can also affect cardiomyocytes, leading patients to develop potentially life-threatening forms of heart failure. The development of cardiomyopathy is an almost universal feature of patients with DMD, typically emerging in childhood [3], whereas in BMD its incidence is more variable and tends to occur later in life [4]. In both conditions, cardiac and respiratory failure have historically represented the leading causes of death [5]. However, advances in ventilatory support have significantly improved survival, resulting in an increasing prevalence of clinically relevant cardiac involvement as this patient population ages [6,7].
Current clinical guidelines do not offer specific recommendations for the management of affected individuals, emphasizing instead the need for a personalized approach [8].
The aim of this review is to present the available therapeutic options for patients with DMD and BMD, with a particular focus on the ethical considerations that must precede their implementation.
2. Clinical Presentation
DMD is an X-linked recessive disorder caused by mutations in the dystrophin gene. While women might be carriers, men who appear healthy at birth develop muscle weakness and wasting during the very first few years of life.
Although respiratory and musculoskeletal manifestations dominate the early clinical course [9], it is cardiac involvement, particularly the development of dilated cardiomyopathy, that ultimately drives prognosis and long-term outcomes in these patients [10]. Cardiac involvement manifests as dilated cardiomyopathy which typically becomes apparent in the middle of the second decade, affecting one-third of patients by age 14 and nearly all patients over 18 years of age [3]. Remodeling leads to ventricular dilation and reduction in the ejection fraction and with the spreading of fibrosis, ventricular dysfunction, atrial arrhythmias, and ventricular arrhythmias begin to occur [11]. It is not only the left chambers that undergo dilation and dysfunction; involvement is often biventricular, as shown in Figure 1. Early clinical recognition of these cardiac anomalies may be masked by severe muscle weakness.
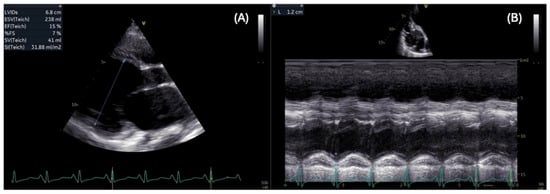
Figure 1.
Impaired biventricular function: parasternal long axis view shows severe dilation (left ventricular end systolic diameter = 68 mm) and reduced left ventricular ejection fraction = 15% (A), and apical view shows decreased tricuspid annular plane systolic excursion (TAPSE = 12 mm) in a patient with DMD (B).
Conversely, individuals with BMD have 10% to 40% of the normal dystrophin amount or have a partially functional form of the subsarcolemmal protein [12]. This leads to a wider range of age of onset, between 5 and 60 years [13] and a much broader phenotypic presentation than DMD. The extreme genotypic variability is associated with equally diverse phenotypes, not only in terms of age at symptom onset but also regarding the types of symptoms themselves. The most common neurological presentation is progressive weakness of the limb muscles, starting from the proximal segments and from the lower limbs. These neuromuscular disorders can lead to slower growth, resulting in reduced stature. Asymptomatic cardiac involvement in BMD occurs in most cases, and up to one-third of patients develop dilative cardiomyopathy with concomitant heart failure [14]. Typically, cardiac involvement follows motor symptoms, although there is no strict correlation between the severity of the involvement of the two systems. In rare cases where the disease initially presents with cardiac manifestations, dilated cardiomyopathy usually develops around the age of 20, followed by neuromuscular symptoms after a period of 1 to 6 years. As previously mentioned, the most common manifestation—and the leading cause of death—is dilation of the heart chambers, followed by arrhythmias [15].
3. Dystrophinopathies Prognosis
DMD prognosis is poor. At the age of 10–12, most patients require a wheelchair, assisted ventilation is necessary at 15–20 years old and death occurs at 20–30 years due to either cardiac or respiratory failure [9]. Other causes of death are pneumonia, aspiration, or airway obstruction. Conversely, BMD has a milder clinical course, and patients might remain in outpatient follow-up until adult life, as illustrated in Figure 2. They usually survive beyond the age of 30 and have a mean age of death in the mid-40s. The most common cause of death with BMD is heart failure due to dilated cardiomyopathy, which also causes considerable morbidity despite their milder skeletal muscle involvement [16].
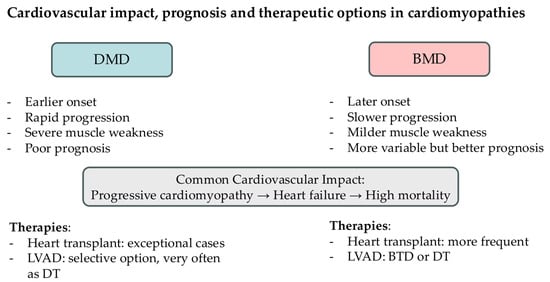
Figure 2.
Schematic representation of the cardiovascular impact, prognosis, and therapeutic options in dystrophinopathies. (DMD = Duchenne Muscular Dystrophy; BMD = Becker Muscular Dystrophy; LVAD = Left Ventricular Assist Device; DT = destination therapy; BTD = bridge to decision).
While gene therapy is gaining increasing relevance in the treatment of several genetic disorders, including degenerative neuromuscular diseases, this is not yet the case for DMD. Currently, delandistrogene moxeparvovec is the only gene therapy approved by the FDA, despite the limited evidence supporting its efficacy and the absence of randomized controlled trials [17]. Moreover, its indication is restricted to ambulatory children between the ages of 4 and 7, significantly limiting its applicability.
Whether patients with dystrophinopathies, especially DMD, can benefit from advanced heart failure therapies such as heart transplantation or long-term left ventricular assist devices (LVAD) remains largely uncertain, and clinical decision-making often relies on individual center experience. This narrative literature review aims to explore and discuss the clinical and ethical considerations surrounding the use of advanced heart failure therapies in patients with DMD and BMD.
4. Heart Transplant in DMD
The option of heart transplantation is rarely pursued in patients with DMD. Recent data indicate only 11 heart transplants performed globally in this population [18]. The first report, published in 1993 [19], described six patients, three of whom had DMD, who underwent heart transplantation. The mean age at transplantation was 22.3 years (range 12–31 years), with a mean follow-up of 2.8 years (range 10 months–5.4 years). All patients had a favorable postoperative course. A subsequent analysis in 2010 [20], also concerning patients transplanted for muscular dystrophies, reported three additional cases of DMD patients, all of whom were alive after a follow-up period of 10 years. The most recent analysis is represented by the study of Wells et al. [21], which examined the outcomes of 81 patients who underwent heart transplantation due to neuromuscular diseases. Among these, three patients with DMD were transplanted at a mean age of 15.7 years; two were still alive at the end of the follow-up, while one had died from bacteremia nearly four years post-transplant.
Additionally, there are two case reports. The first, published by Cripe et al. [22], describes a 14-year-old boy in whom the cardiac involvement was significantly more pronounced than the neuromuscular and respiratory systems, with an excellent pulmonary prognosis (the patient would not require ventilatory support for the subsequent 5–10 years). This patient was managed on an outpatient basis for the four years following transplantation. The second case [23] involves an 18-year-old patient who had undergone LVAD placement at the age of 14, at which point the cardiac involvement was already significantly predominant over the respiratory system. At the time of LVAD implantation, the patient exhibited severe LV dysfunction leading to refractory acute heart failure; the postoperative course was uneventful. Over the next four years, the patient was followed on an outpatient basis, requiring only a driveline revision due to an infection. Given the patient’s clinical stability and good condition, transplantation was considered and performed 47 months after LVAD implantation, yielding good results at three months post-surgery.
5. Heart Transplant in BMD
Unlike what happens with patients with DMD, the transplant option is much more common and well-studied in patients with BMD [24]. To date, there have been over 115 cases of heart transplantation in patients with BMD, and this option has been increasingly considered over the years: the first 2 cases were reported in 1988 [25] and 1989 [26], followed by 8 cases in the 1990s (1990–1999). Outcomes from patients transplanted in this decade appear favorable: among the five with available follow-up data, only one patient died 27 months after the transplant, while the remaining four were alive at a mean follow-up of 3.2 years, with preserved left ventricular function and no evidence of coronary artery disease. Between 2000 and 2009, 25 heart transplants were reported, with a mean patient age of 34.3 years. Finally, between 2011 and 2023, 81 additional cases were documented, with a mean age at transplant of 32.3 years and a follow-up period exceeding seven years. Studies comparing transplant outcomes in BMD patients to those of individuals undergoing transplantation for non-neuromuscular cardiac conditions have found no significant differences in survival or clinical course.
The higher frequency of heart transplantation in BMD patients compared to DMD patients depends both on a better prognosis, linked to less multisystem involvement—which prolongs the life expectancy of transplanted patients—and on a slower disease progression, which is important to ensure more time and an optimal timing for the cardiac transplant itself. In fact, based on the available data, it can be stated that—with appropriate patient selection—life expectancy and quality of life after transplantation can be comparable to those observed in patients undergoing heart transplant for other forms of cardiomyopathy.
6. Ethical Considerations
Currently, only the guidelines issued by the International Society for Heart and Lung Transplantation (ISHLT) [8] include a dedicated section on transplant options for patients with DMD and BMD. These conditions are listed among those associated with increased post-transplant morbidity and mortality. A Class I, Level B recommendation is provided, emphasizing the need for individualized assessment on a case-by-case basis. The guidelines specify that any condition likely to limit survival or quality of life following transplantation should be considered a relative contraindication. In the context of neuromuscular diseases, particular attention should be paid to respiratory muscle strength, aspiration risk, and potential for rehabilitation.
Considering this recommendation, the heart transplant option is a choice that must carefully consider the characteristics of the recipient. When a patient is being evaluated for candidacy, life expectancy in terms of both quality and quantity must be considered. This assessment applies to all medical procedures, but in the case of transplants, where treating one patient implies a selection meaning excluding another, the issue is further magnified. While candidates are numerous, organ availability is scarce. It is challenging to keep objectiveness when considering a patient with DMD, who is typically young and has good family support. However, this objectivity remains crucial in order to uphold a principle of justice, which underlies every medical decision. It is essential to limit the emotional impact due to the psychological characteristics of the patient by making choices that are as rational as possible and that can ensure the best outcome not only for the patient but for all candidates. Conversely, the implantation of an LVAD, while not without risks, does not require excluding other ill individuals from the procedure. Nevertheless, even for this therapeutic option, it is vital to carefully evaluate the patient’s characteristics and potential complications, relying on both the center’s experience and available literature.
In a survey conducted by administering questionnaires to 31 cardiology providers directly involved in the care of patients with DMD, it emerged that while 84% of participants considered LVAD as a viable option, only 52% would have considered listing a patient with DMD for heart transplantation [27].
To understand the standard clinical approach to patients with dystrophinopathies, a survey by Wall et al. [28] is informative. This study highlighted that DMD is considered an absolute or relative contraindication to listing for kidney, heart, liver, and lung transplantation in approximately 85% of patients, both in pediatric and adult populations. In contrast, BMD more frequently represents a contraindication in adults than in children (77% vs. 64%). These differences may be related both to the progressive nature of the disease, which makes the phenotype more evident in adulthood, and to a limited familiarity with patients affected by genetic disorders. Indeed, due to their often-unfavorable prognosis, such patients rarely reach adulthood; this confounding factor may lead to the perception—sometimes unjustified—that those who do survive to adulthood still have a poor prognosis. The same study also confirmed a lack of guidelines even within centers treating patients with dystrophinopathies: fewer than 10% of the surveyed centers had developed formal guidelines for listing patients with DMD and BMD.
Communicating with patients and their families remains one of the most complex and delicate aspects of advanced heart failure care. Patients and caregivers should be actively involved in the decision-making process, but only after the medical team has thoroughly evaluated the potential benefits and risks of each therapeutic option.
Based on the current discussion, it is evident that prognosis is one of the main factors influencing therapeutic decisions in patients with dystrophinopathies. For a disease like DMD, where life expectancy rarely exceeds the third decade, heart transplantation remains a marginal option. This is due to the high risk of intra- and perioperative mortality, as well as the potential for the transplanted organ to have a significantly shorter lifespan compared to what would be expected in a patient with a better overall health status.
Conversely, in patients with BMD, the prognosis is less severe, and life expectancy can extend into the sixth decade. While ethical considerations justify reservations about heart transplantation in these cases, the use of LVAD presents a different scenario. When the patient’s condition, particularly in terms of ventilatory and respiratory function, is favorable, the long-term use of a durable LVAD can be considered more frequently—even in patients with DMD.
In a disease often marked by a poor prognosis, the role of palliative care is both essential and frequently underappreciated [29]. Access to palliative services should be ensured throughout the entire course of the illness [30], from diagnosis to the terminal stages. One of the major barriers to access is the age of the patients, who are often still in the pediatric population. It is not uncommon, in fact, that patients with advanced-stage dystrophinopathies might be at the borderline between pediatric and adult age. The clinical behavior of the disease, however, reproduce that of advanced dilated cardiomyopathy, which often leads to the patient being managed by a team of intensive cardiologists who are more likely to provide care for older patients. Alongside a certain reluctance, partly related to limited experience, in providing palliative care to such young patients, there is also the challenge that, because of their age, interactions with family members are much more intense and require particular attention. This situation raises specific ethical challenges, particularly regarding the involvement of parents and families in end-of-life decision-making. Furthermore, while healthcare professionals who are closely involved in the patient’s long-term care are generally more attuned to clinical changes and thus better equipped to offer appropriate interventions to alleviate suffering, this process can be more challenging in intensive care settings. Nonetheless, this should not justify overlooking the importance of palliative care, which remains fundamental to ensuring that patients are treated with dignity at every stage of the disease and life.
Table 1 shows a comparison of key clinical features between DMD and BMD.

Table 1.
Comparison of key clinical features between DMD and BMD. (DMD = Duchenne Muscular Dystrophy; BMD = Becker Muscular Dystrophy; LVAD = Left Ventricular Assist Device).
7. LVAD in DMD
According to the ethical considerations discussed, implantation of an LVAD in patients with DMD appears to be a more viable option than heart transplantation. However, as the cardiac involvement in DMD usually presents with biventricular dysfunction, right ventricular dysfunction might limit LVAD candidacy. Moreover, experiences with LVAD implantation in this patient population remain limited.
A review by Agdamag et al. [31] reported a total of 23 cases of LVAD implants in patients with DMD documented in the literature. The largest case series was published by Perri et al. [32], which included 6 patients, of whom 3 died during a median follow-up of 21.7 months, two due to hemorrhagic complications and one due to infectious complications. Additionally, two reports [33,34] evaluated 4 patients each, with a mean age of 14 years; in one of these studies, a long-term survival rate of 75% was observed one-year post-implantation. Two other published reports described experiences with 2 cases each: Ryan et al. [35] reported two LVAD implants in patients with a mean age of 26 years, the highest reported, while Amorisio and Amodeo [36] noted a 100% survival rate at 6 months in patients with a mean age of 15 years.
Although the literature reporting cases with significant follow-up data remains limited, it appears to be more explored than in patients undergoing heart transplantation. It is important to note that this procedure still carries high risks both intraoperatively and postoperatively, with infectious complications being common given the restrictive ventilatory disorder that usually accompanies the clinical DMD scenario. Therefore, excellent anesthetic management is crucial for the appropriate care of the patient, particularly from a ventilatory perspective. In experienced centers with an experienced multidisciplinary team in managing patients with advanced heart failure, LVAD implantation as destination therapy in DMD is a feasible option [37].
Table 2 shows the outcomes of patients with DMD who underwent heart transplantation or LVAD placement.

Table 2.
Summary of reports describing advanced heart failure therapies in DMD.
8. LVAD in BMD
The option of LVAD implantation in patients with BMD seems to be less common compared to what happens in patients with DMD. According to an American registry between 2012 and 2020, only two patients with BMD received an LVAD—one as a bridge to decision and the other as destination therapy. The age at implantation was 18 years for one patient and 23.9 years for the other; in both cases, the patients were alive at the end of the follow-up, which lasted 663 days for one and 807 days for the other [38]. This small case series is complemented by a report from the United States about a 20-year-old boy who required an LVAD implantation after experiencing refractory acute heart failure unresponsive to inotropic therapy and needing mechanical circulatory support. This heart failure event occurred following a laparoscopic cholecystectomy. Although neuromuscular signs had been present since childhood, the diagnosis of BMD was made later through a muscle biopsy and subsequently confirmed by genetic analysis [39].
In addition to these cases, 6 patients were included in a Japanese trial [40] that enrolled individuals with muscular dystrophies of various etiologies: the mean age at the time of implantation was 35.3 years, and in 5 cases, the device served as a bridge to transplant, which occurred on average 53 months after LVAD implantation.
9. Limitations
Considering the primary objective of our study, the ethical aspects of advanced therapies in patients with dystrophinopathies, the scientific evidence reviewed was not based on a systematic literature review. Instead, we relied on a selection of major clinical reports and an analysis of available guidelines. This approach, while providing a focused overview, may be limited by potential publication bias and the lack of a comprehensive, quantitative synthesis of the existing literature. Consequently, our findings are primarily qualitative, driven by the authors’ ethical considerations, and may not fully represent the breadth of ethical issues across all published studies.
10. Conclusions
DMD is a degenerative neuromuscular disorder that progressively affects the myocardium, leading to cardiac dysfunction, which is a key factor in poor prognosis. Once cardiomyopathy reaches an end-stage phase, management becomes particularly challenging. Although long-term mechanical circulatory support and heart transplantation are difficult to pursue due to comorbidities and limited life expectancy, these options should not be entirely dismissed. In highly specialized tertiary centers, LVAD implantation may be considered as destination therapy, while heart transplantation should be reserved for exceptionally rare cases with a favorable overall prognosis, especially in terms of respiratory function. Conversely, BMD has a better prognosis, with a longer life expectancy and less multisystem involvement. For this reason, the heart transplant option can be considered more frequently.
Author Contributions
M.S. (Marco Spagnolin), L.F., and M.G. conceptualized the study. M.S. (Marco Spagnolin) and L.F. drafted the first version of the manuscript. M.G. provided supervision. M.S. (Marco Spagnolin), L.F., A.T., A.I., R.A., O.Z., M.S. (Michele Senni), and M.G. revised, contributed to the final draft, and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DMD | Duchenne muscular dystrophy |
| BMD | Becker muscular dystrophy |
| LVAD | Left ventricular assist device |
References
- Ellis, J.A.; Vroom, E.; Muntoni, F. 195th ENMC International Workshop: Newborn screening for Duchenne muscular dystrophy 14–16th December, 2012, Naarden, The Netherlands. Neuromuscul. Disord. 2013, 23, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Sultana, J.; Fontana, A.; Salvo, F.; Messina, S.; Trifirò, G. Global epidemiology of Duchenne muscular dystrophy: An updated systematic review and meta-analysis. Orphanet J. Rare Dis. 2020, 15, 141. [Google Scholar] [CrossRef]
- Earl, C.C.; Pyle, V.I.; Clark, S.Q.; Annamalai, K.; Torres, P.A.; Quintero, A.; Damen, F.W.; Hor, K.N.; Markham, L.W.; Soslow, J.H.; et al. Localized strain characterization of cardiomyopathy in Duchenne muscular dystrophy using novel 4D kinematic analysis of cine cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2023, 25, 14. [Google Scholar] [CrossRef]
- Ho, R.; Nguyen, M.-L.; Mather, P. Cardiomyopathy in becker muscular dystrophy: Overview. World J. Cardiol. 2016, 8, 356. [Google Scholar] [CrossRef]
- Van Ruiten, H.J.A.; Marini Bettolo, C.; Cheetham, T.; Eagle, M.; Lochmuller, H.; Straub, V.; Bushby, K.; Guglieri, M. Why are some patients with Duchenne muscular dystrophy dying young: An analysis of causes of death in North East England. Eur. J. Paediatr. Neurol. 2016, 20, 904–909. [Google Scholar] [CrossRef]
- Passamano, L.; Taglia, A.; Palladino, A.; Viggiano, E.; D’Ambrosio, P.; Scutifero, M.; Rosaria Cecio, M.; Torre, V.; DE Luca, F.; Picillo, E.; et al. Improvement of survival in Duchenne Muscular Dystrophy: Retrospective analysis of 835 patients. Acta Myol. Myopathies Cardiomyopathies Off. J. Mediterr. Soc. Myol. 2012, 31, 121–125. [Google Scholar]
- Kieny, P.; Chollet, S.; Delalande, P.; Le Fort, M.; Magot, A.; Pereon, Y.; Perrouin Verbe, B. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann. Phys. Rehabil. Med. 2013, 56, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ducharme, A.; Kittleson, M.; Bansal, N.; Stehlik, J.; Amdani, S.; Saeed, D.; Cheng, R.; Clarke, B.; Dobbels, F.; et al. International Society for Heart and Lung Transplantation Guidelines for the Evaluation and Care of Cardiac Transplant Candidates—2024. J. Heart Lung Transplant. 2024, 43, 1529–1628.e54. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Mavrogeni, S. Cardiac involvement in Duchenne and Becker muscular dystrophy. World J. Cardiol. 2015, 7, 410. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.; Burnham, T.; Ramsay, J.; Cheung, J.W.; Goyal, N.A.; Jefferies, J.L.; Donaldson, D. Electrophysiologic and cardiovascular manifestations of Duchenne and Becker muscular dystrophies. Heart Rhythm 2025, 22, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.P.; Fischbeck, K.H.; Brown, R.H.; Johnson, M.; Medori, R.; Loire, J.D.; Harris, J.B.; Waterston, R.; Brooke, M.; Specht, L.; et al. Characterization of Dystrophin in Muscle-Biopsy Specimens from Patients with Duchenne’s or Becker’s Muscular Dystrophy. N. Engl. J. Med. 1988, 318, 1363–1368. [Google Scholar] [CrossRef]
- Bradley, W.G.; Jones, M.Z.; Mussini, J.; Fawcett, P.R.W. Becker-type muscular dystrophy. Muscle Nerve 1978, 1, 111–132. [Google Scholar] [CrossRef]
- Finsterer, J.; Stöllberger, C. Cardiac involvement in Becker muscular dystrophy. Can. J. Cardiol. 2008, 24, 786–792. [Google Scholar] [CrossRef]
- Del Rio-Pertuz, G.; Morataya, C.; Parmar, K.; Dubay, S.; Argueta-Sosa, E. Dilated cardiomyopathy as the initial presentation of Becker muscular dystrophy: A systematic review of published cases. Orphanet J. Rare Dis. 2022, 17, 194. [Google Scholar] [CrossRef]
- Bushby, K.M.; Gardner-Medwin, D. The clinical, genetic and dystrophin characteristics of Becker muscular dystrophy. I. Natural history. J. Neurol. 1993, 240, 98–104. [Google Scholar] [CrossRef]
- Jolly, H.; Aartsma-Rus, A.; Bertini, E.; De Waele, L.; Haberlova, J.; Klein, A.; Niks, E.; Servais, L. Gene therapy approval for Duchenne muscular dystrophy: A European perspective. Lancet 2025, 405, 1572–1573. [Google Scholar] [CrossRef]
- Politano, L. Is Cardiac Transplantation Still a Contraindication in Patients with Muscular Dystrophy-Related End-Stage Dilated Cardiomyopathy? A Systematic Review. Int. J. Mol. Sci. 2024, 25, 5289. [Google Scholar] [CrossRef]
- Rees, W.; Schüler, S.; Hummel, M.; Hetzer, R. Heart transplantation in patients with muscular dystrophy associated with end-stage cardiomyopathy. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 1993, 12, 804–807. [Google Scholar]
- Wu, R.S.; Gupta, S.; Brown, R.N.; Yancy, C.W.; Wald, J.W.; Kaiser, P.; Kirklin, N.M.; Patel, P.C.; Markham, D.W.; Drazner, M.H.; et al. Clinical outcomes after cardiac transplantation in muscular dystrophy patients. J. Heart Lung Transplant. 2010, 29, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.; Rizwan, R.; Jefferies, J.L.; Bryant, R.; Ryan, T.D.; Lorts, A.; Chin, C.; Zafar, F.; Morales, D.L. Heart Transplantation in Muscular Dystrophy Patients: Is it a Viable Option? Circ. Heart Fail. 2020, 13, e005447. [Google Scholar] [CrossRef]
- Cripe, L.; Kinnett, K.; Uzark, K.; Eghtesady, P.; Wong, B.; Spicer, R. P1.14 Cardiac transplantation in Duchenne muscular dystrophy: A case report. Neuromuscul. Disord. 2011, 21, 645. [Google Scholar] [CrossRef]
- Piperata, A.; Bottio, T.; Toscano, G.; Avesani, M.; Vianello, A.; Gerosa, G. Is heart transplantation a real option in patients with Duchenne syndrome? Inferences from a case report. ESC Heart Fail. 2020, 7, 3198–3202. [Google Scholar] [CrossRef] [PubMed]
- Connuck, D.M.; Sleeper, L.A.; Colan, S.D.; Cox, G.F.; Towbin, J.A.; Lowe, A.M.; Wilkinson, J.D.; Orav, E.J.; Cuniberti, L.; Salbert, B.A.; et al. Characteristics and outcomes of cardiomyopathy in children with Duchenne or Becker muscular dystrophy: A comparative study from the Pediatric Cardiomyopathy Registry. Am. Heart J. 2008, 155, 998–1005. [Google Scholar] [CrossRef]
- Casazza, F.; Brambilla, G.; Salvato, A.; Morandi, L.; Gronda, E.; Bonacina, E. Dilated cardiomyopathy and successful cardiac transplantation in Becker’s muscular distrophy. Follow-up after two years. G. Ital. Cardiol. 1988, 18, 753–757. [Google Scholar] [PubMed]
- Donofrio, P.D.; Challa, V.R.; Hackshaw, B.T.; Mills, S.A.; Cordell, A.R. Cardiac Transplantation in a Patient with Muscular Dystrophy and Cardiomyopathy. Arch. Neurol. 1989, 46, 705–707. [Google Scholar] [CrossRef]
- Villa, C.; Auerbach, S.R.; Bansal, N.; Birnbaum, B.F.; Conway, J.; Esteso, P.; Gambetta, K.; Hall, E.K.; Kaufman, B.D.; Kirmani, S.; et al. Current Practices in Treating Cardiomyopathy and Heart Failure in Duchenne Muscular Dystrophy (DMD): Understanding Care Practices in Order to Optimize DMD Heart Failure Through ACTION. Pediatr. Cardiol. 2022, 43, 977–985. [Google Scholar] [CrossRef]
- Wall, A.; Lee, G.H.; Maldonado, J.; Magnus, D. Genetic disease and intellectual disability as contraindications to transplant listing in the United States: A survey of heart, kidney, liver, and lung transplant programs. Pediatr. Transplant. 2020, 24, e13837. [Google Scholar] [CrossRef]
- De Souza Leon, M.A.; Lizzi, E.A.D.S.; Viana, S.C.P.; Sobreira, C.F.D.R.; Mattiello-Sverzut, A.C. Do Patients with Duchenne Muscular Dystrophy Receive the Palliative Care Approach? Palliat Med Pract [Internet]. 22 August 2024. Available online: https://journals.viamedica.pl/palliative_medicine_in_practice/article/view/100494 (accessed on 9 July 2025).
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Colvin, M.K.; Cripe, L.; Herron, A.R.; Kennedy, A.; Kinnett, K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: Primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol. 2018, 17, 445–455. [Google Scholar] [CrossRef]
- Agdamag, A.C.; Nandar, P.P.; Tang, W.H.W. Advanced Heart Failure Therapies in Neuromuscular Diseases. Curr. Treat. Options Cardiovasc. Med. 2024, 26, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Perri, G.; Filippelli, S.; Adorisio, R.; Iacobelli, R.; Iodice, F.; Testa, G.; Paglietti, M.G.; D’Amario, D.; Massetti, M.; Amodeo, A. Left ventricular assist device as destination therapy in cardiac end-stage dystrophinopathies: Midterm results. J. Thorac. Cardiovasc. Surg. 2017, 153, 669–674. [Google Scholar] [CrossRef]
- Wittlieb-Weber, C.A.; Villa, C.R.; Conway, J.; Bock, M.J.; Gambetta, K.E.; Johnson, J.N.; Lal, A.K.; Schumacher, K.R.; Law, S.P.; Deshpande, S.R.; et al. Use of advanced heart failure therapies in Duchenne muscular dystrophy. Prog. Pediatr. Cardiol. 2019, 53, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Iodice, F.; Testa, G.; Averardi, M.; Brancaccio, G.; Amodeo, A.; Cogo, P. Implantation of a left ventricular assist device as a destination therapy in Duchenne muscular dystrophy patients with end stage cardiac failure: Management and lessons learned. Neuromuscul. Disord. 2015, 25, 19–23. [Google Scholar] [CrossRef]
- Ryan, T.D.; Jefferies, J.L.; Sawnani, H.; Wong, B.L.; Gardner, A.; Del Corral, M.; Lorts, A.; Morales, D.L.S. Implantation of the HeartMate II and HeartWare Left Ventricular Assist Devices in Patients with Duchenne Muscular Dystrophy: Lessons Learned From the First Applications. ASAIO J. 2014, 60, 246–248. [Google Scholar] [CrossRef]
- Amodeo, A.; Adorisio, R. Left ventricular assist device in Duchenne Cardiomyopathy: Can we change the natural history of cardiac disease? Int. J. Cardiol. 2012, 161, e43. [Google Scholar] [CrossRef]
- Hayes, E.A.; Nandi, D. Is there a future for the use of left ventricular assist devices in Duchenne muscular dystrophy? Pediatr. Pulmonol. 2021, 56, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Auerbach, S.R.; Bansal, N.; Buchholz, H.; Conway, J.; Esteso, P.; Kaufman, B.D.; Lal, A.K.; Law, S.P.; Lorts, A.; et al. Initial multicenter experience with ventricular assist devices in children and young adults with muscular dystrophy: An ACTION registry analysis. J. Heart Lung Transplant. 2023, 42, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Bhattal, G.; Rawitscher, D.; George, T.; Afzal, A.; Kabra, N. Durable Left Ventricular Assist Device For Dilated Cardiomyopathy From Becker’s Muscular Dystrophy. J. Card. Fail. 2023, 29, 693. [Google Scholar] [CrossRef]
- Gyoten, T.; Amiya, E.; Kinoshita, O.; Tsuji, M.; Kimura, M.; Hatano, M.; Ono, M. Clinical outcomes of continuous flow left ventricular assist device therapy as bridge to transplant strategy in muscular dystrophy: A single-center study. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 347–353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).