Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review
Abstract
1. Introduction
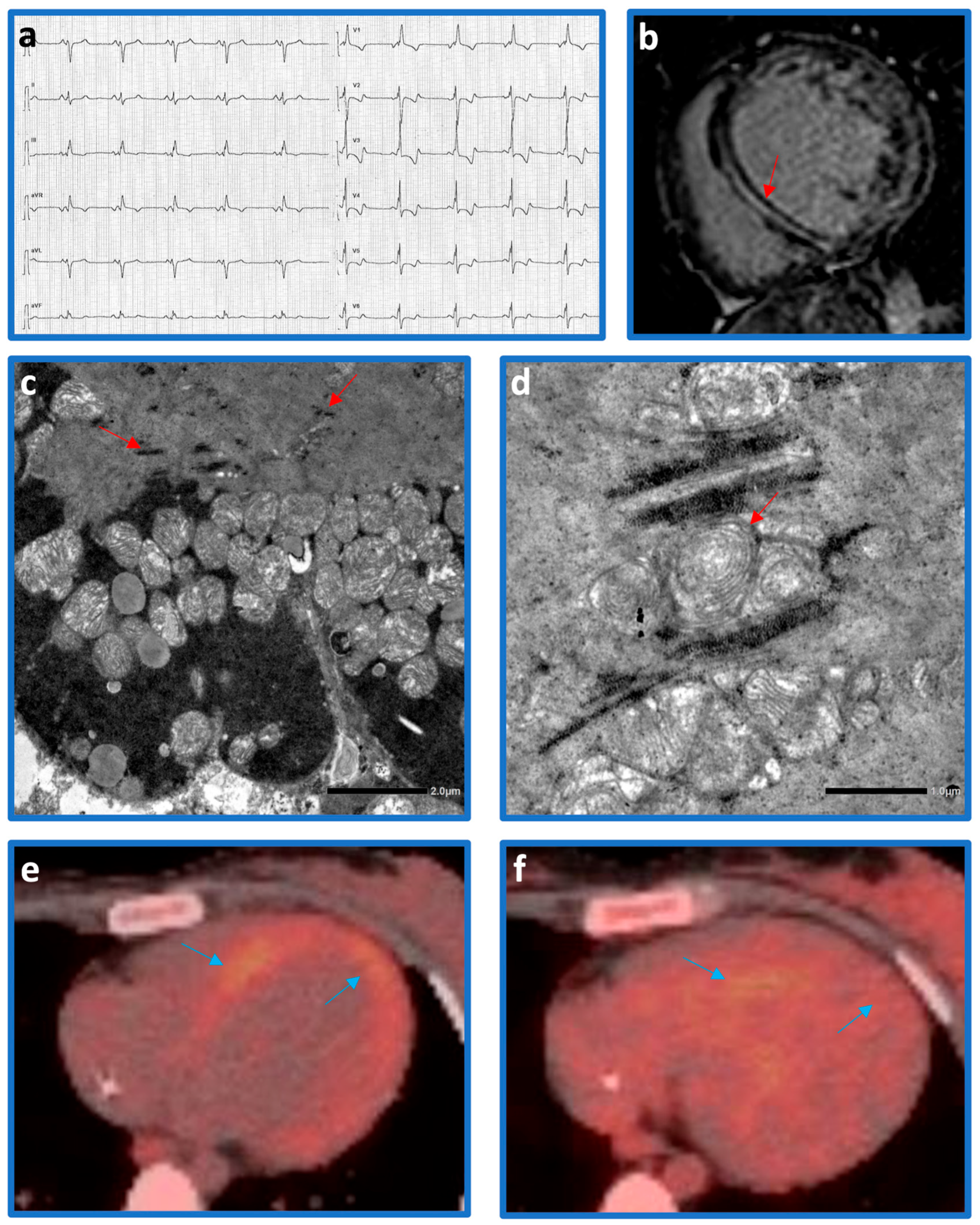
2. Clinical Case
2.1. Genetic Analysis
2.2. Follow-Up
3. Discussion
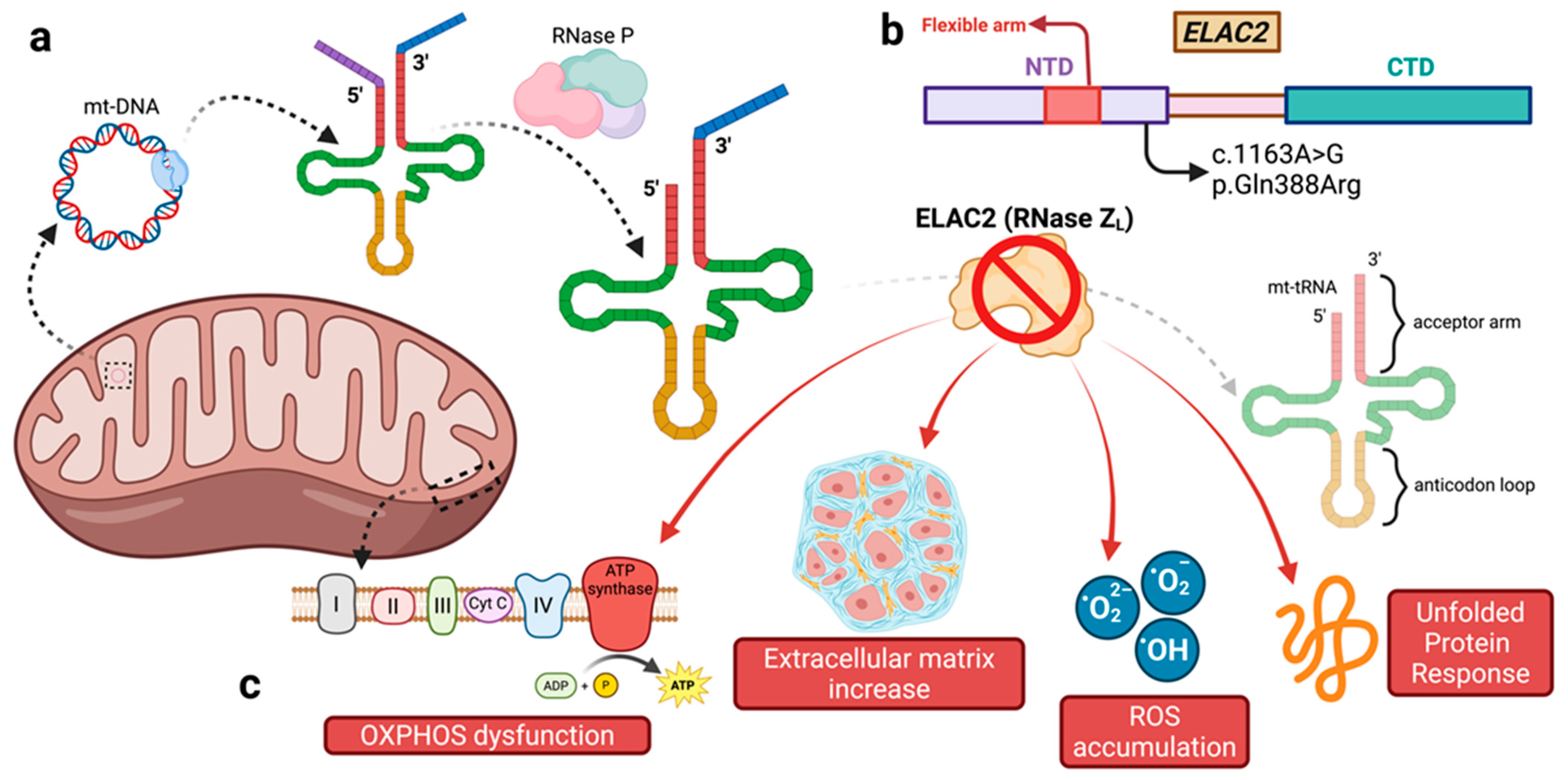
3.1. Primary Mitochondrial Disorders and Mt-tRNA Maturation
3.2. The Role of ELAC2
3.3. ELAC2 Variants, Genotype–Phenotype Correlation, Cardiac Phenotype, and Disease Course
| Study | Number of Patients Divided by Nationality | Zygosity | ELAC2 Variant | Type of Genetic Analysis | In Vitro Validation of the Variants | PolyPhen-2 In-Silico Score | Allele Frequencies (gnomAD v4.1.0) | Extra-Cardiac Features | Cardiac Phenotype | Age at Discovery of CVI | Course |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Haack et al. (2013) [22] | German (n = 2) | Compound heterozygous | c.631C > T; p.Arg211*/ c.1559C > T; p.Thr520Ile | WES | OXPHOS activities and accumulation of unprocessed mitochondrial RNA | N/A/ 1.000 | 0.0000074/ 0.00000062 | IUGR, psychomotor and growth retardation, muscular hypotonia, microcephaly, dysphagia | HCM | 3; 4 months | Death at 6 months; alive at 2.10 years |
| Arab (n = 1) | Homozygous | c.460T > C; p.Phe154Leu | WES | OXPHOS activities and accumulation of unprocessed mitochondrial RNA | 1.000 | 0.00000062 | IUGR, muscular hypotonia | HCM | 2 months | Death at 11 months | |
| Turkish (n = 2) | Homozygous | c.1267C > T; p.Leu423Phe | WES | OXPHOS activities and accumulation of unprocessed mitochondrial RNA | 1.000 | 0.00000123 | IUGR, psychomotor retardation, muscular hypotonia | HCM; HCM and later DCM | 5 months | Alive at 13 years; death at 4.9 years | |
| Akawi et al. (2016) [30] | Pakistani (n = 5) | Homozygous | c.1423 + 2 T > A | WES | Protein expression | N/A | N/A | Intellectual disability, muscle hypotonia, microcephaly | Mild septal hypertrophy in 2 subjects (known only for 2 subjects) | 2.5; 4 years (known only for 2 subjects) | Alive at 2.5–19 years |
| Shinwari et al. (2017) [29] | Arab (n = 16) | Homozygous | c.460T > C; p.Phe154Leu | WES | No | 1.000 | 0.00000062 | IUGR, developmental delay, seizures | 13 HCM; 3 DCM pericardial effusion (44%) | 2–7 months | Death at median age 4 months |
| Kim et al. (2017) [7] | Korean (n = 1) | Heterozygous | c.95C > G; p.Pro32Arg | TES | No | 0.158 | 0.00027271 | Encephalopathy, IUGR, growth retardation | Tetralogy of Fallot | 2 days | Death at 5 months |
| Paucar et al. (2018) [31] | Assyrian (n = 1) | Compound heterozygous | c.394G > A; p.Gly132Arg/c.1040C > T; p.Ser347Phe | WES | Accumulation of unprocessed mitochondrial transcripts, normal mitochondrial mRNAs, and tRNA steady-state levels | 1.000/ 0.918 | 0.00012888/ 0.00000248 | Huntingtonian disorder, hearing loss, acanthocytosis, myopathy, polyneuropathy | Mild septal hypertrophy | Not reported | Alive at 69 years |
| Saoura et al. (2019) [1] | German (n = 1) | Compound heterozygous | c.202C > T; p.Arg68Trp/c.1478C > T; p.Pro493Leu | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000/ 1.000 | 0.00000062/ 0.00000929 | Muscle weakness | HCM | Birth | Death at 3 weeks |
| Irish (n = 1) | Compound heterozygous | c.297-2_297delinsTG/c.2342G > A; p.Arg781His | NGS panel | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate (p.Arg781His variant) | N/A/ 1.000 | N/A/ 0.00089995 | Developmental delay, IUGR | HCM | 18 months | Alive at 5 years | |
| Caucasian (n = 1) | Compound heterozygous | c.2186A > G; p.Tyr729Cys/c.2342G > A; p.Arg781His | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000/ 1.000 | 0.00001301 | Rapidly progressive cardiac phenotype | HCM | 2 months | Death at 12 weeks | |
| Arab (n = 1) [proband, 4 subjects in the family with both genotype and phenotype] | Homozygous | c.460T > C; p.Phe154Leu | WES | Mild impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000 | 0.00000062 | Rapidly progressive cardiac phenotype | HCM | Neonatal | Death at 4 months | |
| Italian (n = 1) | Compound heterozygous | c.798-1G > T/c.1690C > A; p.Arg564Ser | WES | Mild impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | N/A/ 0.991 | N/A/ N/A | Developmental delay | DCM | 4 months | Death at 5 months | |
| Italian (n = 1) | Compound heterozygous | c.1979A > T; p.Lys660Ile/c.2039C > T; p.Ala680Val | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate (p.Lys660Ile variant) | 1.000/ 1.000 | 0.00000062/ N/A | Not reported | HCM | 12 months | Heart transplantation at 3.8 years | |
| Italian (n = 1) | Compound heterozygous | c.245 + 2T > A/c.1264C > G; p.Leu422Val | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate (p.Leu422Val variant) | N/A/ 1.000 | 0.00000682/ N/A | Rapidly progressive cardiac phenotype | DCM | 2 months | Death at 3 months | |
| Italian (n = 1) | Homozygous | c.1163A > G; p.Gln388Arg | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000 | 0.00000311 | Psychomotor retardation, fatigability, peripheral neuropathy | HCM | 6 months | Alive at 19 years | |
| Polish (n = 1) | Compound heterozygous | c.457delA; p.Ile153Tyrfs*6/c.2342G > A; p.Arg781His | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate (p.Arg781His variant) | N/A/ 1.000 | 0.00000062 | Developmental delay, hypotonia, gastro-intestinal dysmotility | HCM | 8 months | Heart transplantation at 10 months | |
| Arab (n = 1) | Homozygous | c.460T > C; p.Phe154Leu | WES | Mild impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000 | 0.00000062 | Mild muscular hypotonia | HCM | Birth | Death at 2.5 months | |
| African-American (n = 1) | Compound heterozygous | c.2245C > T; p.His749Tyr/ c. 297-2_297-1delinsT | WES | Impairment of kinetic parameters in vitro with pre-mt-tRNA substrate (p.His749Tyr variant) | 1.000/ N/A | 0.00001363/ N/A | Global developmental delay, hypotonia | HCM | 4 months | Heart transplantation at 10 months | |
| Saudi- Arabian (n = 1) | Homozygous | c.460T > C; p.Phe154Leu | WES | Mild impairment of kinetic parameters in vitro with pre-mt-tRNA substrate | 1.000 | 0.00000062 | Growth retardation | HCM | 5 months | Death at 5 months | |
| Brambilla et al. (2020) [13] | Italian (n = 1) | Not reported | Not reported | Not reported | No | N/A | N/A | Not reported | HCM and later DCM | Not reported | Death at 16 years |
| Mendes et al. (2022) [37] | Brazilian (n = 1) | Compound heterozygous | c.225C > G; p.Tyr75*/ c.1924G > A; p.Val642Met | WES | No | N/A/ 1.000 | 0.00000062/0.00006939 | Pseudo-hypoaldosteronism, hypertension, thrombocytosis | HCM | 6 months | Alive at 6 years |
| Cafournet et al. (2023) [32] | Pakistani (n = 2) | Compound heterozygous | c.591G > A; p.Trp197*/ c.1943C > T; p.Ala648Val | TES | Sorting Intolerant From Tolerant (SIFT) and PolyPhen-2 algorithms | N/A/ 1.000 | 0.00000929 | Growth retardation, muscle hypotonia, cerebellar ataxia, sensorineural deafness, epilepsy | Mild hypertrophy | 1 month | Alive at 15 and 13 years |
| Malian (n = 2) | Homozygous | c.2249T > C; p.Met750Thr | TES | Sorting Intolerant From Tolerant (SIFT) and PolyPhen-2 algorithms | 1.000 | 0.00000558 | IUGR, growth retardation | HCM with systolic dysfunction | 4 months | Death at 1 year; alive (age not reported) | |
| Present case | Italian (n = 1) | Homozygous | c.1163A > G; p.Gln388Arg | CES | No | 1.000 | 0.00000311 | Sensory axonal polyneuropathy | DCM | 25 years | Alive at 26 years |
3.4. The Importance of a Comprehensive Genetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saoura, M.; Powell, C.A.; Kopajtich, R.; Alahmad, A.; Al-Balool, H.H.; Albash, B.; Alfadhel, M.; Alston, C.L.; Bertini, E.; Bonnen, P.E.; et al. Mutations in ELAC2 associated with hypertrophic cardiomyopathy impair mitochondrial tRNA 3’-end processing. Hum. Mutat. 2019, 40, 1731–1748. [Google Scholar] [CrossRef]
- Protonotarios, A.; Wicks, E.; Ashworth, M.; Stephenson, E.; Guttmann, O.; Savvatis, K.; Sekhri, N.; Mohiddin, S.A.; Syrris, P.; Menezes, L.; et al. Prevalence of (18)F-fluorodeoxyglucose positron emission tomography abnormalities in patients with arrhythmogenic right ventricular cardiomyopathy. Int. J. Cardiol. 2018, 284, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Angelini, F.; Ravera, F.; Gobello, G.; Manai, R.; Bocchino, P.P.; Barreca, A.; Deaglio, S.; Pidello, S.; Raineri, C.; De Ferrari, G.M.; et al. Mycophenolic Acid for Desmoplakin-Related Cardiomyopathy: A Possible New Arrow in the Quiver. Can. J. Cardiol. 2024, 40, 2589–2591. [Google Scholar] [CrossRef]
- Scarpulla, R.C. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol. Rev. 2008, 88, 611–638. [Google Scholar] [CrossRef]
- Sanchez, M.I.G.L.; Mercer, T.R.; Davies, S.M.K.; Shearwood, A.-M.J.; Nygård, K.K.A.; Richman, T.R.; Mattick, J.S.; Rackham, O.; Filipovska, A. RNA processing in human mitochondria. Cell Cycle 2011, 10, 2904–2916. [Google Scholar] [CrossRef]
- Smeitink, J.; van den Heuvel, L.; DiMauro, S. The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2001, 2, 342–352. [Google Scholar] [CrossRef]
- Kim, S.H.; Quigley, G.J.; Suddath, F.L.; McPherson, A.; Sneden, D.; Kim, J.J.; Weinzierl, J.; Rich, A. Three-dimensional structure of yeast phenylalanine transfer RNA: Folding of the polynucleotide chain. Science 1973, 179, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.K.; Gössringer, M.; Späth, B.; Fischer, S.; Marchfelder, A. The making of tRNAs and more—RNase P and tRNase Z. Prog. Mol. Biol. Transl. Sci. 2009, 85, 319–368. [Google Scholar] [CrossRef] [PubMed]
- Holzmann, J.; Frank, P.; Löffler, E.; Bennett, K.L.; Gerner, C.; Rossmanith, W. RNase P without RNA: Identification and functional reconstitution of the human mitochondrial tRNA processing enzyme. Cell 2008, 135, 462–474. [Google Scholar] [CrossRef]
- Brzezniak, L.K.; Bijata, M.; Szczesny, R.J.; Stepien, P.P. Involvement of human ELAC2 gene product in 3’ end processing of mitochondrial tRNAs. RNA Biol. 2011, 8, 616–626. [Google Scholar] [CrossRef]
- Rossmanith, W. Localization of human RNase Z isoforms: Dual nuclear/mitochondrial targeting of the ELAC2 gene product by alternative translation initiation. PLoS ONE 2011, 6, e19152. [Google Scholar] [CrossRef]
- Bhatta, A.; Kuhle, B.; Yu, R.D.; Spanaus, L.; Ditter, K.; Bohnsack, K.E.; Hillen, H.S. Molecular basis of human nuclear and mitochondrial tRNA 3’ processing. Nat. Struct. Mol. Biol. 2025, 32, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, A.; Olivotto, I.; Favilli, S.; Spaziani, G.; Passantino, S.; Procopio, E.; Morrone, A.; Donati, M.A. Impact of cardiovascular involvement on the clinical course of paediatric mitochondrial disorders. Orphanet J. Rare Dis. 2020, 15, 196. [Google Scholar] [CrossRef]
- Xue, C.; Tian, J.; Chen, Y.; Liu, Z. Structural insights into human ELAC2 as a tRNA 3’ processing enzyme. Nucleic Acids Res. 2024, 52, 13434–13446. [Google Scholar] [CrossRef]
- Siira, S.J.; Rossetti, G.; Richman, T.R.; Perks, K.; Ermer, J.A.; Kuznetsova, I.; Hughes, L.; Shearwood, A.-M.J.; Viola, H.M.; Hool, L.C.; et al. Concerted regulation of mitochondrial and nuclear non-coding RNAs by a dual-targeted RNase Z. EMBO Rep. 2018, 19, e46198. [Google Scholar] [CrossRef]
- Stentenbach, M.; Ermer, J.A.; Rudler, D.L.; Perks, K.L.; Raven, S.A.; Lee, R.G.; McCubbin, T.; Marcellin, E.; Siira, S.J.; Rackham, O.; et al. Multi-omic profiling reveals an RNA processing rheostat that predisposes to prostate cancer. EMBO Mol. Med. 2023, 15, e17463. [Google Scholar] [CrossRef] [PubMed]
- Blinka, S.; Mishra, R.; Hsieh, A.C. ELAC2 is a functional prostate cancer risk allele. Trends Mol. Med. 2023, 29, 586–588. [Google Scholar] [CrossRef]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Richman, T.R.; Ermer, J.A.; Baker, J.; Siira, S.J.; Kile, B.T.; Linden, M.D.; Rackham, O.; Filipovska, A. Mitochondrial gene expression is required for platelet function and blood clotting. Cell Rep. 2023, 42, 113312. [Google Scholar] [CrossRef]
- Valentín Gesé, G.; Hällberg, B.M. Structural basis of 3’-tRNA maturation by the human mitochondrial RNase Z complex. EMBO J. 2024, 43, 6573–6590. [Google Scholar] [CrossRef] [PubMed]
- Kufel, J.; Tollervey, D. 3’-processing of yeast tRNATrp precedes 5’-processing. RNA 2003, 9, 202–208. [Google Scholar] [CrossRef]
- Haack, T.B.; Kopajtich, R.; Freisinger, P.; Wieland, T.; Rorbach, J.; Nicholls, T.J.; Baruffini, E.; Walther, A.; Danhauser, K.; Zimmermann, F.A.; et al. ELAC2 mutations cause a mitochondrial RNA processing defect associated with hypertrophic cardiomyopathy. Am. J. Hum. Genet. 2013, 93, 211–223. [Google Scholar] [CrossRef]
- Dubrovsky, E.B.; Dubrovskaya, V.A.; Levinger, L.; Schiffer, S.; Marchfelder, A. Drosophila RNase Z processes mitochondrial and nuclear pre-tRNA 3’ ends in vivo. Nucleic Acids Res. 2004, 32, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Migunova, E.; Theophilopoulos, J.; Mercadante, M.; Men, J.; Zhou, C.; Dubrovsky, E.B. ELAC2/RNaseZ-linked cardiac hypertrophy in Drosophila melanogaster. Dis. Model. Mech. 2021, 14, dmm048931. [Google Scholar] [CrossRef]
- Xie, X.; Dubrovsky, E.B. Knockout of Drosophila RNase ZL impairs mitochondrial transcript processing, respiration and cell cycle progression. Nucleic Acids Res. 2015, 43, 10364–10375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nandakumar, S.; Grushko, O.; Buttitta, L.A. Polyploidy in the adult Drosophila brain. eLife 2020, 9, e54385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, J.; Levichkin, I.V.; Stasinopoulos, S.; Ryan, M.T.; Hoogenraad, N.J. A mitochondrial specific stress response in mammalian cells. EMBO J. 2002, 21, 4411–4419. [Google Scholar] [CrossRef]
- Held, J.P.; Feng, G.; Saunders, B.R.; Pereira, C.V.; Burkewitz, K.; Patel, M.R. A tRNA processing enzyme is a key regulator of the mitochondrial unfolded protein response. eLife 2022, 11, e71634. [Google Scholar] [CrossRef]
- Shinwari, Z.M.A.; Almesned, A.; Alakhfash, A.; Al-Rashdan, A.M.; Faqeih, E.; Al-Humaidi, Z.; Alomrani, A.; Alghamdi, M.; Colak, D.; Alwadai, A.; et al. The Phenotype and Outcome of Infantile Cardiomyopathy Caused by a Homozygous ELAC2 Mutation. Cardiology 2017, 137, 188–192. [Google Scholar] [CrossRef]
- Akawi, N.A.; Ben-Salem, S.; Hertecant, J.; John, A.; Pramathan, T.; Kizhakkedath, P.; Ali, B.R.; Al-Gazali, L. A homozygous splicing mutation in ELAC2 suggests phenotypic variability including intellectual disability with minimal cardiac involvement. Orphanet J. Rare Dis. 2016, 11, 139. [Google Scholar] [CrossRef]
- Paucar, M.; Pajak, A.; Freyer, C.; Bergendal, Å.; Döry, M.; Laffita-Mesa, J.M.; Stranneheim, H.; Lagerstedt-Robinson, K.; Savitcheva, I.; Walker, R.H.; et al. Chorea, psychosis, acanthocytosis, and prolonged survival associated with ELAC2 mutations. Neurology 2018, 91, 710–712. [Google Scholar] [CrossRef]
- Cafournet, C.; Zanin, S.; Guimier, A.; Hully, M.; Assouline, Z.; Barcia, G.; de Lonlay, P.; Steffann, J.; Munnich, A.; Bonnefont, J.-P.; et al. Novel ELAC2 Mutations in Individuals Presenting with Variably Severe Neurological Disease in the Presence or Absence of Cardiomyopathy. Life 2023, 13, 445. [Google Scholar] [CrossRef]
- Biagini, E.; Coccolo, F.; Ferlito, M.; Perugini, E.; Rocchi, G.; Bacchi-Reggiani, L.; Lofiego, C.; Boriani, G.; Prandstraller, D.; Picchio, F.M.; et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: Prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J. Am. Coll. Cardiol. 2005, 46, 1543–1550. [Google Scholar] [CrossRef]
- Angelini, F.; Bocchino, P.P.; Dusi, V.; Pidello, S.; De Ferrari, G.M.; Raineri, C. From thick walls to clear answers: Approaches to diagnosing hypertrophic cardiomyopathy and its mimics. Eur. Heart J. Suppl. 2025, 27, i40–i46. [Google Scholar] [CrossRef] [PubMed]
- Abou Alaiwi, S.; Roston, T.M.; Marstrand, P.; Claggett, B.L.; Parikh, V.N.; Helms, A.S.; Ingles, J.; Lampert, R.; Lakdawala, N.K.; Michels, M.; et al. Left Ventricular Systolic Dysfunction in Patients Diagnosed With Hypertrophic Cardiomyopathy During Childhood: Insights From the SHaRe Registry. Circulation 2023, 148, 394–404. [Google Scholar] [CrossRef]
- Thompson, J.-L.M.; Johnson, R.; Troup, M.; Rath, E.M.; Young, P.E.; Soka, M.J.; Ohanian, M.; Tarr, I.S.; Giannoulatou, E.; Fatkin, D. Polygenic Risk in Families With Dilated Cardiomyopathy. Circ. Genom. Precis. Med. 2024, 17, e004558. [Google Scholar] [CrossRef]
- Mendes, L.C.; de Oliveira Magalhães, R.; Pereira Dos Santos, R.K.; Araújo, R.S. Pseudohypoaldosteronism associated with hypertrophic cardiomyopathy, hypertension and thrombocytosis due to mutation in the ELAC2 gene: A case report. J. Pediatr. Endocrinol. Metab. 2022, 35, 1437–1442. [Google Scholar] [CrossRef]
- Ravera, F.; Dusi, V.; Bocchino, P.P.; Gobello, G.; Giannino, G.; Melis, D.; Brach Del Prever, G.M.; Angelini, F.; Saglietto, A.; Giustetto, C.; et al. Cardiovascular Involvement in SYNE Variants: A Case Series and Narrative Review. Cardiogenetics 2025, 15, 2. [Google Scholar] [CrossRef]
- Keisling, J.; Bedoukian, E.; Burstein, D.S.; Gaynor, J.W.; Gray, C.; Krantz, I.; Izumi, K.; Leonard, J.; Lin, K.Y.; Medne, L.; et al. Diagnostic Yield of Exome Sequencing in Pediatric Cardiomyopathy. J. Pediatr. 2024, 265, 113808. [Google Scholar] [CrossRef]
- Mak, T.S.H.; Lee, Y.-K.; Tang, C.S.; Hai, J.S.H.; Ran, X.; Sham, P.-C.; Tse, H.-F. Coverage and diagnostic yield of Whole Exome Sequencing for the Evaluation of Cases with Dilated and Hypertrophic Cardiomyopathy. Sci. Rep. 2018, 8, 10846. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravera, F.; Angelini, F.; Bocchino, P.P.; Marcelli, G.; Gobello, G.; Giannino, G.; Merlino, G.; De Guidi, B.; Destefanis, A.; Brach Del Prever, G.M.; et al. Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review. Cardiogenetics 2025, 15, 20. https://doi.org/10.3390/cardiogenetics15030020
Ravera F, Angelini F, Bocchino PP, Marcelli G, Gobello G, Giannino G, Merlino G, De Guidi B, Destefanis A, Brach Del Prever GM, et al. Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review. Cardiogenetics. 2025; 15(3):20. https://doi.org/10.3390/cardiogenetics15030020
Chicago/Turabian StyleRavera, Francesco, Filippo Angelini, Pier Paolo Bocchino, Gianluca Marcelli, Giulia Gobello, Giuseppe Giannino, Guglielmo Merlino, Benedetta De Guidi, Andrea Destefanis, Giulia Margherita Brach Del Prever, and et al. 2025. "Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review" Cardiogenetics 15, no. 3: 20. https://doi.org/10.3390/cardiogenetics15030020
APA StyleRavera, F., Angelini, F., Bocchino, P. P., Marcelli, G., Gobello, G., Giannino, G., Merlino, G., De Guidi, B., Destefanis, A., Brach Del Prever, G. M., Giustetto, C., Gallone, G., Pidello, S., Barreca, A., Deaglio, S., De Ferrari, G. M., Raineri, C., & Dusi, V. (2025). Dilated Cardiomyopathy and Sensorimotor Polyneuropathy Associated with a Homozygous ELAC2 Variant: A Case Report and Literature Review. Cardiogenetics, 15(3), 20. https://doi.org/10.3390/cardiogenetics15030020






